Abstract
Polymicrobial interactions are widespread in nature, and play a major role in maintaining human health and ecosystems. Whenever one organism uses metabolites produced by another organism as energy or nutrient sources, this is called cross-feeding. The ecological outcomes of cross-feeding interactions are poorly understood and potentially diverse: mutualism, competition, exploitation or commensalism. A major reason for this uncertainty is the lack of theoretical approaches linking microbial metabolism to microbial ecology. To address this issue, we explore the dynamics of a one-way interspecific cross-feeding interaction, in which food can be traded for a service (detoxification). Our results show that diverse ecological interactions (competition, mutualism, exploitation) can emerge from this simple cross-feeding interaction, and can be predicted by the metabolic, demographic and environmental parameters that govern the balance of the costs and benefits of association. In particular, our model predicts stronger mutualism for intermediate by-product toxicity because the resource-service exchange is constrained to the service being neither too vital (high toxicity impairs resource provision) nor dispensable (low toxicity reduces need for service). These results support the idea that bridging microbial ecology and metabolism is a critical step towards a better understanding of the factors governing the emergence and dynamics of polymicrobial interactions.
Keywords: mutualism, cross-feeding, microbial ecology, metabolism, modeling
Introduction
Microbial communities are widespread in nature and play a major role in shaping the world we live in, ranging from maintaining human health (Backhed et al. 2005, Flint et al. 2007, Ramsey and Whiteley 2009), to shaping our ecosystems (Stams 1994, Schink 2002). Microbial cells excrete metabolites as a result of their metabolism, and such metabolic waste sets the stage for the emergence of the complex interspecific interactions observed in these communities. Whenever these metabolites can be used by other organisms as energy or nutrient resources, this is called cross-feeding. Cross-feeding is incidental when the metabolite excreted is a waste product, and therefore non costly to produce at a basal level. In some instances, cross-feeding can be cooperative, requiring an up-front investment cost to the producer, which may or may not be paid-back by the partner species utilizing the metabolite (West et al. 2006, Bull and Harcombe 2009).
From an ecological stand-point, the functional outcomes of cross-feeding interactions are potentially diverse, spanning competition, mutualism, exploitation, or commensalism. The type of ecological interaction forged depends on the net costs and benefits emerging from the association (Bronstein 1994a, Connor 1995). The interaction is competitive if the net effect of the interaction is negative for all species, and is exploitative if a species benefits at the expense of the other species. In contrast, if the interaction is beneficial to both species, then they are mutualists. Costs and benefits to each partner are not constant, but depend on both biotic and abiotic factors (Bronstein 1994b, Herre et al. 1999, Sachs and Simms 2006). For example, spatial structure, resource availability, the number and type of other species, and environmental perturbations, are all important factors in shaping the nature of these interspecific interactions (Brockhurst et al. 2007, Hansen et al. 2007, Bull and Harcombe 2009, Shimoyama et al. 2009, Harcombe 2010, Mitri et al. 2011).
Mutually beneficial interactions are commonly found in the complex web of metabolic exchanges among species of the human microbiota (Samuel and Gordon 2006, Mahowald et al. 2009). However, the exchange of metabolites by cross-feeding can also promote exploitation. For example, a commensal bacteria might be forced to produce a metabolite at its own expense while benefiting an opportunistic bacteria (Jagmann et al. 2010). These empirical examples raise the following question: how do fundamental ecological relationships emerge from fundamental mechanistic features of interspecific interactions?
Although it is well-acknowledged that the nature of interspecific interactions is not fixed in time or space, theoretical models have traditionally focused on a single type of ecological interaction, either competition (Case and Gilpin 1974, Frank and Amarasekare 1998), mutualism (Holland et al. 2002, West et al. 2002, Foster and Wenseleers 2006), or exploitation (Frank 1996, Hochberg and van Baalen 1998). The main motivation for such models is typically to understand the evolution of traits that underlie a specific functional form of interaction (e.g., virulence, Frank 1996). However, little attention has been directed to the role that underlying mechanistic bases of interaction play in the emergence and dynamics of these ecological interactions.
Here, we aim to address this challenge by exploring the dynamics of an incidental cross-feeding interaction. Specifically, we explore a common type of incidental cross-feeding interaction where the by-product (waste) is toxic to the producer but beneficial to the cross-feeder (Marx 2009, Shimoyama et al. 2009, Hillesland and Stahl 2010). The human microbiota, with its hundred trillion microbial cells, is one place where this type of metabolic interaction is common (Egland et al. 2004, Samuel and Gordon 2006). For example, coculture of the human gut bacterium B. thetaiotaomicron and methanogen M. smithii in gnotobiotic mice revealed that the two species are involved in a one-way cross-feeding mutualism (Samuel and Gordon 2006), while also competing for nitrogen (Samuel et al. 2007). When in coculture, B. thetaiotaomicron preferentially degraded fructans, resulting in the production, from fructans fermentation, of the reducing equivalents formate and hydrogen. While formate and hydrogen inhibit the metabolism of B. thetaiotaomicron, these waste products are a source of energy for M. smithii. Thus, M. smithii facilitates B. thetaiotaomicron’s growth by removing these reducing equivalents. In turn, the methanogen benefits from the interaction by using formate and hydrogen as source of energy for methanogenesis. A similar mechanism occurs in the novel obligate mutualism experimentally evolved between the bacterium Desulfovibrio vulgaris and the methanogen Methanococcus maripaludis (Hillesland and Stahl 2010). Lactate oxidation to acetate, carbon dioxide, and hydrogen by D. vulgaris is inhibited by high hydrogen levels. The presence of M. maripaludis, however, relieves this inhibition because M. maripaludis uses hydrogen for methanogenesis and therefore helps the bacterium by keeping hydrogen at low levels. In turn, the methanogen benefits by using hydrogen as an energy source. In contrast to the B. thetaiotaomicron - M. smithii mutualism previously described, there is no evidence of interspecific competition between D. vulgaris and M. maripaludis.
Based on these empirical examples, we explicitly assume that the metabolic by-product (waste product) is toxic to the producer, but beneficial to the cross-feeder (non-producer). Although there is an indirect benefit of association for both producer and cross-feeder (food in exchange for detoxification), helping a competitor also comes at a cost, due to increased competition for shared resources. Our results indicate that such a resource-service cross-feeding interaction can produce diverse stable ecological outcomes: competition, exploitation (in either direction) or mutualism. We then ask: under what conditions do the reciprocal benefits of this specific mechanism of trade outweigh the interspecific competitive costs? In other words, what factors govern the occurrence of a mutually beneficial interaction? Our model emphasizes the importance of the metabolic by-product properties in governing the outcome of the ecological interaction. In particular, we show that a more toxic and more durable by-product favour mutualism, due to the service (toxic by-product removal) being more valuable as the by-product becomes a real problem to the producer. Interestingly, our model predicts that mutualism will be stronger at intermediate by-product toxicity. This occurs because of a balance between provision of a resource and need for a service. Indeed, at high by-product toxicity, the producer is highly inhibited, and thus the provision of food to the cross-feeder (resource) is reduced. However, at low by-product toxicity, the need for help (service by detoxification) is reduced, and thus the costs of association may outweigh the benefits.
The Model
One-way by-product cross-feeding
Our model tracks the dynamics of a one-way cross-feeding interaction between two populations: a producer (A) of a by-product (E) and a non-producer (cross-feeder) (B) (see fig. 1 for a schematic representation of the model, and refer to table 1 for definitions of notation). Our model builds on the competitive Lotka-Volterra equations. In addition, we extend the competitive Lotka-Volterra model to include the explicit dynamics of the by-product (E) (Frank 1994). Specifically, we assume that the by-product (E) enhances the cross-feeder’s growth, however it inhibits the producer’s growth. Our (non-dimensional) model is defined by the following system of ordinary differential equations (see Appendix A for details on the normalization):
| (1) |
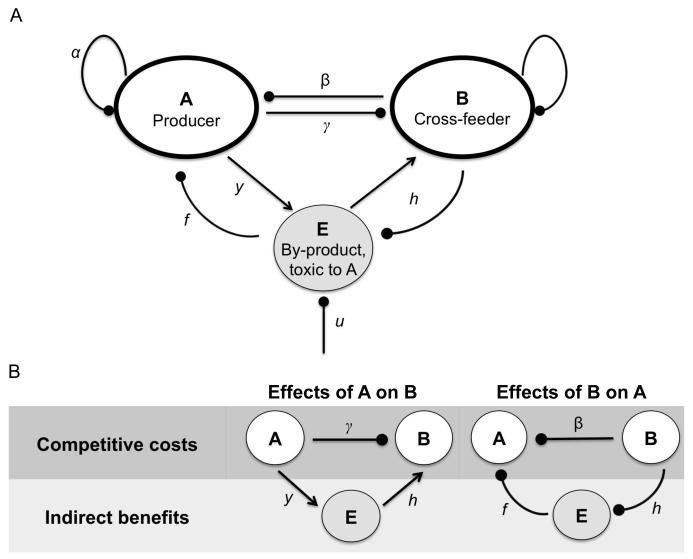
Figure 1.
Schematic diagram of the cross-feeding model (A), and illustration of the costs and benefits of association (B). A, B and E represent producer, cross-feeder and by-product, respectively. Oval arrows represent a negative effect whereas open arrows represent a positive effect upon the population or resource they are pointing towards. Arrows are labeled with the associated rate constants (see Table 1 for definitions of other notations). The non-labeled arrow represents the cross-feeder intraspecific competition, and is normalised to 1.
Table 1.
Summary of model parameters
| Symbol | Definition |
|---|---|
| A | Producer |
| B | Cross-feeder and non-producer |
| E | Metabolic by-product |
| α | Producer intraspecific competition coefficient |
| β | Cross-feeder interspecific competition coefficient |
| γ | Producer interspecific competition coefficient |
| r | Relative intrinsic growth rate of the producer to that of the cross-feeder |
| y | By-product production rate |
| h | Consumption rate of by-product |
| u | By-product decay rate |
| f | By-product toxicity rate |
| g | Cross-feeder uptake efficiency (of by-product) |
The densities of producer (A) and cross-feeder (B) are scaled to the carrying capacity of B (kb), and the individual intrinsic growth rates are scaled to the intrinsic growth rate of B (rb), thus r represents the relative intrinsic growth rate of the producer to that of the cross-feeder (r = ra/rb). Term f is the rate of the by-product toxicity on the producer, and measures the degree to which the by-product is toxic to the producer’s growth. Term h represents the cross-feeder’s by-product consumption rate, and y is the producer’s by-product production rate. Term g is a conversion constant, which can be viewed as the cross-feeder’s by-product uptake efficiency. Term u represents the by-product decay rate. Term α is the producer’s intraspecific competition coefficient, and measures the degree of competition among the population of producers relative to the competition among the population of cross-feeders (α = kb/ka, see Table A1). Term β is the cross-feeder’s interspecific competition coefficient on the producer, and γ is the producer’s interspecific competition coefficient on the cross-feeder, thus β and γ measure the competitive effect of B on A and A on B, respectively. It should be noted that we assume that both β and γ are strictly positive, as in the classic competitive Lotka-Volterra equations. Thus, any benefits of association will be due to the by-product dynamics (i.e. the metabolic interaction), and not imposed a priori to the system. Note that when f = g = h = y = u = 0, we recover the classic competitive Lotka-Volterra equations.
Results
Model Analysis
A stability analysis of this model (see Appendix B for detailed Model analysis) reveals that a population of pure producers (A* = ru/(αru + fy), B* = 0, E* = ry/(αru + fy)) is locally stable if u > y(f + rgh)/(r(γ - α)) and α < γ. These results reveal that any trait that increases the production and/or accumulation of the toxic by-product will compromise the stability of pure producers, and thus facilitate the invasion of cross-feeders. In turn, a population of pure cross-feeders (A* = 0, B* = 1, E* = 0) is locally stable if β > 1, i.e. when interspecific competition of cross-feeders on producers (β) is higher than intraspecific competition within the cross-feeder population (that is normalized to 1). The model has two coexistence equilibria (see Appendix B, fig. B1). In the following analyses, we focus on parameter regimes that allow for the stable coexistence of both species (i.e., where neither single species equilibrium is stable).
Effect of association on the focal species, the producer
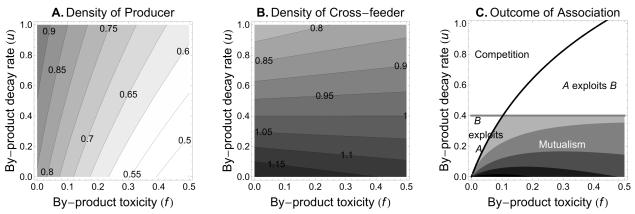
To understand how the focal species (producer) is affected by the biotic (cross-feeder) and abiotic (by-product) environment, we analyze the effect of changing parameter values on the densities of producers in the mixed community. We find that producers are favoured by lower by-product toxicity (f), lower interspecific competition from cross-feeders (β), and lower rate of by-product production (y) (fig. 2A-D). Interestingly, figure 2 reveals non-linearities in the sign of the effect of by-product consumption (h). If the by-product is weakly toxic (low f) and the cross-feeder is a moderate to strong competitor, then an increase in by-product consumption (h) decreases the density of producers in the mixed community (fig. 2A and 2C, moderate to strong competition β). In contrast, if the by-product is highly toxic (high f), and therefore a real problem to the producer, the density of producers increases with increasing by-product consumption (h) (fig. 2B and 2D). In other words, from a producer perspective, at low by-product toxicity f, the benefit of the detoxification service from the cross-feeder does not compensate for the enhanced costs of competition that result from a better-fed competitor. However, at high f the benefit from the service does compensate for the enhanced costs of competition, implying that the cross-feeder is a helper (i.e. has a net positive effect on the producer). Finally, a more durable by-product (lower u) favours the population of cross-feeders at the producers’ expense (Figures 3A and 3B).
Figure 2.
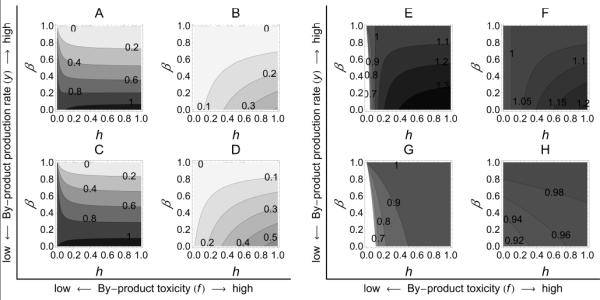
Effect of varying by-product toxicity (f) and by-product production rate (y), as well as by-product consumption rate (h) and cross-feeders’ interspecific competition (β) on the stable cross-feeding community. A-D, Effect on the producer. Contour lines in each figure represent the density of producers at equilibrium in coculture with cross-feeders (AB*), for values of β, h, y, and f. E-H, Effect on the cross-feeder. Contour lines in each figure represent the density of cross-feeders at equilibrium in coculture with producers (BA*), for values of β, h, y, and f. Darker regions indicate higher density. The parameter values used are r = 1, α = 0.9, γ = 1, g = 1, u = 0.1, y = [1; 2] and f = [0.01; 1] such that A and E, y = 2 and f = 0.01; B and F, y = 2 and f = 1; C and G, y = 1 and f = 0.01; D and H, y = 1 and f = 1.
Figure 3.
Effects of by-product durability (u) and toxicity (f) on the density of producer, density of cross-feeder, and the outcome of the association. A-B, darker regions indicate higher density. C, darker the shading stronger the mutualism. The black line represents the threshold where AB* = Aa*, and the grey line represents the threshold where BA* = Ba* (see text in Results for explanation). The parameter values used for the plots are r = 1, α = 0.9, β = 0.2, γ = 1, g = 1, h = 0.8, and y = 1.5.
Effect of association on the cross-feeder
In turn, from a cross-feeder perspective, our results suggest that the cross-feeder is favoured by higher by-product production rate (higher y), and higher by-product consumption rate (higher h) (fig. 2E-H). Interestingly, figures 2E-H and 3B reveal non-linearities in the sign of the effect of increasing interspecific competition β and toxicity f. For example, if y is low, so that there is little cross-feeding potential, increasing interspecific competition to producer (β) is beneficial to the cross-feeder (fig. 2G, H). In contrast, if y is high, so that the producer is a potentially valuable nutrient source, decreasing β may be beneficial to the cross-feeder if its by-product consumption rate is intermediate to high (fig. 2E from intermediate to high h, 2F) or detrimental if its by-product consumption rate is low (fig. 2E at low consumption h). This nonlinearity likely occurs due to a balance between the benefit from cross-feeding (higher h) and decreased interspecific competition (lower β) benefiting a potential harmer (i.e. has a net negative effect on the cross-feeder). Similarly, the effect on the cross-feeder of varying the by-product toxicity (f) depends on the nature of the interaction. Cross-feeders benefit from low f if the producer is a helper, and benefit from high f if the producer is a harmer (fig. 3B).
The nonlinearity in the sign of the effect of changing parameter values, as hereby described, is likely because varying the value of the parameter will affect the nature of the partner (i.e. whether the partner is a helper or a harmer). In the next sections, we describe, first, the range of ecological interactions that arise from our model and second, explore how the metabolic and environmental factors influence the nature of the resource-service interaction between producer and cross-feeder.
One-way cross-feeding can produce diverse ecological interactions
This one-way cross-feeding interaction where food is traded for detoxification can produce diverse ecological interactions (fig. 3C and 4). Generally, the cross-feeding interaction is mutually beneficial to both species if the density of A in coculture with B (AB*) is larger than the density of A alone (Aa*), and if the density of B in coculture with A (BA*) is larger than the density of B alone (Ba*) (i.e. benefits from association outweigh competitive costs endured, reciprocally). In contrast, competition occurs if both species are negatively affected by the association (AB* < Aa* and BA* < Ba*, i.e. competition outweighs benefits received, reciprocally). Three other outcomes are possible. The producer might exploit the cross-feeder, and this means that the producer density is enhanced by the association at the expense of the cross-feeder density (AB* > Aa* and BA* < Ba*). Here, the waste removal benefit to the producer outweighs its costs of association, but the food provision to the cross-feeder does not compensate its costs of association. Alternatively, the cross-feeder might exploit the producer, so that the density of producers is decreased by the interaction while the density of cross-feeders is increased (AB* < Aa* and BA* > Ba*). Here, the food provision benefit to the cross-feeder outweighs its costs of association, but the waste removal to the producer does not compensate for its costs of association. Finally, if only one of the populations benefits while the other is not affected by the interaction, then the interaction is known as commensalism.
Figure 4.
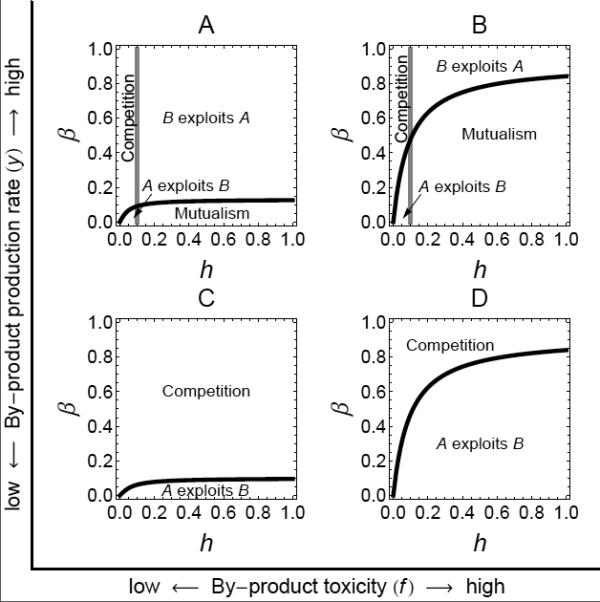
Outcome of the cross-feeding interaction for values of cross-feeder’s interspecific competition (β) and by-product consumption rate (h), as well as by-product production rate (y), and by-product toxicity (f). The black line represents the threshold where AB* = Aa*, and the grey line represents the threshold where BA* = Ba* (see text in Results for explanation). The parameter values used are r = 1, α = 0.9, γ = 1, u = 0.1, g = 1, y = [1; 2] and f = [0.01; 1] such that A, y = 2 and f = 0.01; B, y = 2 and f = 1; C, y = 1 and f = 0.01, D, y = 1 and f = 1.
Interestingly, the horizontal/vertical nature of the contour lines in fig. 3A,B may help to explain this diversity in ecological outcomes. For example, the grey horizontal line in fig. 3C represents the threshold at which the cross-feeder benefits (below) or does not benefit (above) from the association (line at BA* = Ba*), and reflects the horizontal nature of the contour lines in fig. 3B (effect on cross-feeder density). Similarly, the black oblique line in fig. 3C defines the threshold at which the producer benefits (to the right) or does not benefit (to the left) from the association (line at AB* = Aa*), and reflects the vertical nature of the contour lines in fig. 3A (effect on producer density). We now focus on the mechanisms that favour the stable mutualistic form of this association.
Mutualism (and exploitation) occurs when indirect benefits exceed competitive costs of association-Effect of interspecific competition
Mutualism can be generically explained by indirect benefits exceeding competitive costs, reciprocally (equivalent to negative interspecific competition parameters in classic Lotka-Volterra competition equations (Otto and Day 2007)). In our system, the competitive costs are defined by the interspecific competition parameters, and are separated from the indirect benefits of provision of the food resource (the effect of A on B) and the detoxification service (the effect of B on A) (fig. 1B).
We find analytically that the cross-feeder only benefits from the association (i.e. the indirect benefit of cross-feeding compensates for the competitive costs of interacting with the producer) if h > uγ/(gy-γ). This means that mutualism cannot occur when h < uγ/(gy-γ). This shows, as expected, that mutualism is favoured by lower interspecific competition γ and β (fig. 4).
Mutualism (and exploitation) occurs when indirect benefits exceed competitive costs of association-Effect of metabolic parameters
Given the specific nature of our cross-feeding interaction, varying the rate of by-product production (y) has opposite effects on producers and cross-feeders, as shown previously (Table 2). This raises the question as to what are the effects of the by-product production and consumption rates on the nature of the cross-feeding interaction?
Table 2.
Summary of the main results of the cross-feeding interaction model.
| Interspecific competition of B on A (β) |
By-product production (y) |
By- product toxicity (f) |
Consumption of by- product (h) |
By- product decay rate (u) |
Relative intrinsic growth rate (r) |
|
|---|---|---|---|---|---|---|
|
Producer density (AB*) a |
↓ | ↓ | ↓ | ↑ or ↓ | ↑ | ↑ |
|
Cross-feeder density (BA*) a |
↑ or ↓ | ↑ | ↑ or ↓ | ↑ | ↓ | ↑ or ↓ |
| Mutualism b | ↓ | ↑ | ↑ | ↑ | ↓ | ↓ |
An upward arrow means a monotonic increase with increasing parameter value, while a downward arrow means a monotonic decrease with increasing parameter value.
Qualitative switch in ecological outcome, i.e. either outcome does not change or switches to mutualism present (upward arrow), or outcome does not change or switches to mutualism absent (downward arrow).
Our results suggest that mutualism is favoured by higher by-product production rate (higher y) (fig. 4 and fig. C1A). As shown in figure 4, an increase in y may result in a shift from the producer exploiting the cross-feeder to a mutually beneficial interaction. Our results also suggest that higher by-product consumption (higher h) favours mutualism (fig. 4 and C1), and cannot occur when h < uγ/(gy-γ) (grey line in fig. 4). Indeed, increasing the rate of by-product consumption (h) confers an indirect benefit on the producer, due to greater waste removal, but also on the cross-feeder, due to greater food consumption. However, these indirect benefits should be balanced with the competitive costs of association because increasing help to a competitor increases competitive costs, whereas helping a helper increases the feedback benefits.
Mutually beneficial food for detoxification cross-feeding is favoured by a more toxic by-product, but is stronger at intermediate toxicity
The level of by-product toxicity imposed on the producer (f) plays an important role in governing the balance between costs and benefits of association. Our results suggest that mutualism is favoured by higher by-product toxicity (higher f) (fig. 4A, 4B, and fig. C1B). This occurs because the more toxic is the producer’s waste product, the more valuable is any help of waste removal (detoxification) provided by the cross-feeder, which means that the benefit of association to the producer is further increased by an increase in by-product toxicity. Interestingly, however, our results also suggest that mutualism is stronger at intermediate levels of by-product toxicity, i.e. there is a greater positive net effect from association at intermediate f (fig. 3C). To gain more insight into this behaviour, we explore how an increase in toxicity affects the densities of producer and cross-feeder when grown alone (Aa* and Ba*, respectively), and when grown in association (AB* and BA*, respectively). The nonlinearity in the response of the strength of mutualism to toxicity arises because of two opposing effects. On the one hand, if by-product toxicity is low then the competitive costs of association to the producer outweigh the benefits of association (i.e. AB* - Aa* < 0, low f in fig. C2) because of low need for detoxification (service). On the other hand, once mutualism occurs high by-product toxicity reduces the net gain of association to both partners (high f in fig. C2). This is most likely because of strong inhibition on the producer, as shown by a decrease in density of producers with increasing by-product toxicity.
Mutually beneficial food for detoxification cross-feeding is favoured by increased by-product durability
Recent studies on public goods cooperation have revealed that the costs and benefits of cooperation are shaped by the durability of public goods (Brown and Taddei 2007, Kummerli and Brown 2010). Analogously to these models, our study focuses on two lineages and an explicit molecular intermediary - so we now ask, does the durability of our toxic by-product affect the nature of the cross-feeding interaction that occurs between the producer and cross-feeder?
In monoculture, increased by-product decay rate (larger u) is beneficial for the producer. For large u, the expression of the equilibrium density of pure producers (A* = ru/(αru + fy)) will approach A* = 1/α. Thus, the toxic effect of the by-product becomes insignificant and the population carrying capacity (equilibrium density) is only limited by its own intraspecific competition (α). However, as the by-product durability increases (lower u), the toxic by-product accumulates into the environment, and the inhibition on the producer also increases. In coculture, producers are favoured by a more fragile by-product, while cross-feeders are favoured by a more durable by-product (fig. 3A and 3B). But what is the effect of the by-product durability on the nature of the interaction? Our results reveal that mutualism is favoured by a more durable by-product (low u) (fig. 3C and fig. C1D). We find analytically that mutualism cannot occur when u > h(gy - γ)/γ. This result suggests that when the toxic by-product is fragile (and therefore less able to accumulate) the indirect benefits of the cross-feeding association are low, because the reciprocal benefits of trading food in exchange for waste product detoxification are decreased significantly.
Discussion
Polymicrobial interactions are common in nature, and their ecological outcomes are potentially diverse. However, the link between microbial metabolism and microbial ecology is still poorly understood. In this study, we address this challenge by mapping metabolism to ecology for a simple form of polymicrobial interaction. More specifically, we explore the dynamics of a fundamental form of cross-feeding interaction where food (resource) is traded for detoxification (service). We found that a one-to-many relationship between mechanism of interaction and ecological outcome is possible. This means that diverse ecological interactions (mutualism, competition, exploitation) may emerge from a single mechanism of trade. This strongly suggests that it is not possible to predict exactly what the ecological outcome of a certain interaction is from knowledge of the metabolic interaction alone.
Our results are based on a specific mechanism of trade (detoxification for food), however, the basic principles we underline will follow for any mechanism of trade (or help to a partner) that is balanced against a direct competitive interaction (harm to a partner). This balance generates a variety of possible ecological outcomes, by allowing the net effects in both directions to switch from negative (competition outweighs help received) to positive (help outweighs competitive costs endured). It should be noted that the identification of a microbial service relationship does not alone demonstrate net help, it is important that the service provided is set against the competitive costs, to give a proper accounting of the net effects of the interaction. To the best of our knowledge, this is the first time that the occurrence of the four diverse ecological interactions (mutualism, exploitation in either direction, and competition) from a simple mechanistic interaction between two species has been demonstrated theoretically.
In addition, we also highlight the factors that favour a mutualistic interaction between the producer and the cross-feeder (our main results are summarized in Table 2). Generally, mutualism arises when an interaction is beneficial for both partners, i.e. whenever there is an “alignment of interests” (van Baalen and Jansen 2001). Hereby, such alignment of interests arises when the resource-service exchange is fair, i.e. whenever it compensates for the competitive costs of association. Interestingly, our model suggests that mutualism (common interest) is favoured in a monotonic way (i.e. shift in a specific parameter value either results in no change in the ecological outcome or always affects mutualism qualitatively in the same direction, either switch to mutualism present or mutualism absent), however, the effect on the density of producer and/or cross-feeder presents some nonlinearities (see Table 2). For example, from a producer perspective an increase in by-product toxicity (higher f) will be detrimental no matter the type of partner, but from a cross-feeder perspective the effect of increasing toxicity depends on the nature of the producer (i.e. whether the producer is a helper or competitor).
Furthermore, our model suggests that mutualism is stronger at intermediate by-product toxicity (f). An explanation for this unintuitive result is the following. At low by-product toxicity, help from the cross-feeder to the producer (i.e. service by detoxification) is low because the by-product is less of a problem to the producer. At high by-product toxicity, the producer is strongly inhibited, and thus the help it provides to the cross-feeder (i.e. resource provision) is reduced. It should be noted that this result arises because of the nature of the mechanistic cross-feeding interaction, in which there is a trade of a waste product (which is toxic for producer while food for cross-feeder) in exchange of detoxification (service). While here we focus on interspecific cross-feeding, we believe that this result is more general and relevant to the understanding of many kinds of interaction, in which the resource-service association is constrained to services being neither too vital nor dispensable. Here, we have focused on the metabolic and environmental parameters, however, demographic factors also play an important role in shaping the balance between costs and benefits of association, so to influence the nature of the resource-service interaction. Our results suggest that mutualism is favoured by lower relative growth of the producer to that of the cross-feeder, i.e. lower r (fig. C1C, and, figure C3 and Table 2 for effects on producer and cross-feeder).
Despite not having explicitly assumed a resource use trade-off, our findings suggest that a resource use trade-off in the cross-feeder (trade-off between its ability to compete for a shared energy resource - captured by the interspecific competition term β, and ability to specialize on the new resource, h) can foster mutualism in ecological time, as we found mutualism to be favoured by both declines in β and increases in h. This hypothesis will be explored in a following study. Evidence for a resource use metabolic constraint has been growing in the literature. For example, the metabolism of lactate by Aggregatibacter actinomycetemcomitans (an opportunistic pathogen) inhibits the uptake and metabolism of carbohydrates (e.g. glucose) (Brown and Whiteley 2007). When cocultured with commensal bacteria that produced lactate, this resource use trade-off was important for A. actinomycetemcomitans survival because it avoided competition with commensal bacteria for the main resource, in which A. actinomycetemcomitans is a poorer competitor (Ramsey et al. 2011). This result indicates that such metabolic constraints might be crucial for enhancing coculture infections featuring A. actinomycetemcomitans.
While our results are ecologically derived, ecological stability does not imply evolutionary stability. Now we ask, is the mutualism observed here evolutionary stable? In other words, is this by-product cooperation (Connor 1995, Sachs et al. 2004) subject to invasion by a producer cheat with reduced metabolite excretion (reduced y), and/or a cross-feeder cheat with reduced metabolite consumption (reduced h)? Cross-feeding is incidental in our model as the metabolite excreted by the producer is a waste product. This implies that a producer cheat cannot reduce y because of mechanistic constraints on the production of waste. In turn, the cross-feeder is rewarded by feeding on the producer’s waste product, and therefore, a cross-feeder cheat that forgoes this resource will have no selective advantage. Taken together, this suggests that this interspecific mutualism is robust to interspecific cheats because of mechanistic constraints imposed by the specific mechanism of trading food for detoxification.
However, many questions remain about the evolutionary trajectories of this mutualism. For example, it is tempting to speculate that this food for detoxification mutualism could evolve from a facultative association to an obligate association, for either one, or both species. Indeed, as long as there is enough by-product in the environment (from high production y), there may be selection on cross-feeder to reduce or lose its ability to catabolize the shared limiting resource (reduce β) to specialize on the metabolic by-product (increase h). This resource partitioning may then favour co-evolution in the producer of increased ‘waste’ production y (to further reduce competition).
However, this co-evolutionary scenario raises the following question: what would be the consequences of environmental perturbations? A cross-feeder with high h / low β would be relying on the producer species for food, and thus, in the absence of the latter, the cross-feeder’s ability to survive could be strongly compromised. Similarly, a producer with high y would be relying on the cross-feeder for detoxification, and in the absence of the cross-feeder, it would be drowning in its own waste. Interestingly, a theory for the evolution of dependencies has been recently proposed, suggesting that functional dependencies have evolved through adaptive gene loss of dispensable functions (Morris et al. 2012). We suggest that this specific mechanism of trading food for detoxification might be a potential microbial interaction where such a mechanism could be observed - an interesting idea for future experimental research.
Previous models have made important advances in understanding the mechanisms that favour a shift along the parasitism-mutualism continuum over evolutionary time. Models based on principles of evolutionary invasion analysis have typically aimed at understanding the long-term evolution of traits that underlie a specific functional form of interaction, e.g., virulence (Yamamura 1993, Hochberg et al. 2000, Ferdy and Godelle 2005), interspecific cooperation (Doebeli and Knowlton 1998), and partner control (Johnstone and Bshary 2002). Genetic models of coevolution have also provided important insights into how temporal and spatial variability affect fitness interactions between species, and drive fluctuations between mutualism and antagonism (Gomulkiewicz et al. 2003, Nuismer et al. 2003). The novelty of our approach lies in showing a mapping from one mechanism to many functional interactions. Specifically, our model allows us to ask: given a specific context, defined by biotic and abiotic factors, can we predict where a particular mechanistic interspecific interaction will fall on a competition-exploitation-mutualism space? Overall, we suggest that a better understanding of the metabolic, demographic and environmental parameters that govern the balance between the costs and benefits of association will help us to gain new insights into how novel multispecies associations arise, and, to predict where these interactions will fall on the competition-mutualism continuum.
Supplementary Material
Acknowledgements
We thank two anonymous referees for their helpful comments. We thank PDBC-IGC / Fundação para a Ciência e a Tecnologia, Portugal (SFRH/BD/33856/2009) (S.E.), the Rhodes Trust (C.T.), and the Wellcome Trust Grant 082273/Z/07/Z (S.P.B.) for funding.
Footnotes
Online Appendix A: Model Description and non-dimensionalization
Online Appendix B: Model Equilibria and Stability Analysis
Online Appendix C: Supplementary Figures
Literature cited
- Backhed F, Ley RE, Sonnenburg JL, Peterson DA, Gordon JI. Host-bacterial mutualism in the human intestine. Science. 2005;307:1915–20. doi: 10.1126/science.1104816. [DOI] [PubMed] [Google Scholar]
- Brockhurst MA, Buckling A, Gardner A. Cooperation peaks at intermediate disturbance. Curr Biol. 2007;17:761–5. doi: 10.1016/j.cub.2007.02.057. [DOI] [PubMed] [Google Scholar]
- Bronstein JL. Our Current Understanding of Mutualism. The Quarterly Review of Biology. 1994a;69:31–51. [Google Scholar]
- Bronstein JL. Conditional outcomes in mutualistic interactions. Trends Ecol Evol. 1994b;9:214–7. doi: 10.1016/0169-5347(94)90246-1. [DOI] [PubMed] [Google Scholar]
- Brown SA, Whiteley M. A novel exclusion mechanism for carbon resource partitioning in Aggregatibacter actinomycetemcomitans. J Bacteriol. 2007;189:6407–14. doi: 10.1128/JB.00554-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown SP, Taddei F. The durability of public goods changes the dynamics and nature of social dilemmas. PLoS One. 2007;2:e593. doi: 10.1371/journal.pone.0000593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bull JJ, Harcombe WR. Population dynamics constrain the cooperative evolution of cross-feeding. PLoS One. 2009;4:e4115. doi: 10.1371/journal.pone.0004115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Case TJ, Gilpin ME. Interference competition and niche theory. Proc Natl Acad Sci U S A. 1974;71:3073–7. doi: 10.1073/pnas.71.8.3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor RC. The Benefits of Mutualism: A Conceptual Framework. Biological Reviews. 1995;70:427–457. [Google Scholar]
- Doebeli M, Knowlton N. The evolution of interspecific mutualisms. Proc Natl Acad Sci U S A. 1998;95:8676–80. doi: 10.1073/pnas.95.15.8676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egland PG, Palmer RJ, Jr., Kolenbrander PE. Interspecies communication in Streptococcus gordonii-Veillonella atypica biofilms: signaling in flow conditions requires juxtaposition. Proc Natl Acad Sci U S A. 2004;101:16917–22. doi: 10.1073/pnas.0407457101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferdy JB, Godelle B. Diversification of transmission modes and the evolution of mutualism. Am Nat. 2005;166:613–27. doi: 10.1086/491799. [DOI] [PubMed] [Google Scholar]
- Flint HJ, Duncan SH, Scott KP, Louis P. Interactions and competition within the microbial community of the human colon: links between diet and health. Environ Microbiol. 2007;9:1101–11. doi: 10.1111/j.1462-2920.2007.01281.x. [DOI] [PubMed] [Google Scholar]
- Foster KR, Wenseleers T. A general model for the evolution of mutualisms. J Evol Biol. 2006;19:1283–93. doi: 10.1111/j.1420-9101.2005.01073.x. [DOI] [PubMed] [Google Scholar]
- Frank SA. Spatial polymorphism of bacteriocins and other allelopathic traits. Evolutionary Ecology. 1994;8:369–386. [Google Scholar]
- Frank SA. Models of parasite virulence. Q Rev Biol. 1996;71:37–78. doi: 10.1086/419267. [DOI] [PubMed] [Google Scholar]
- Frank SA, Amarasekare P. Increasing resource specialization among competitors shifts control of diversity from local to spatial processes. Ecol Lett. 1998;1:3–5. [Google Scholar]
- Gomulkiewicz R, Nuismer SL, Thompson JN. Coevolution in variable mutualisms. Am Nat. 2003;162:S80–93. doi: 10.1086/378705. [DOI] [PubMed] [Google Scholar]
- Hansen SK, Rainey PB, Haagensen JA, Molin S. Evolution of species interactions in a biofilm community. Nature. 2007;445:533–6. doi: 10.1038/nature05514. [DOI] [PubMed] [Google Scholar]
- Harcombe W. Novel cooperation experimentally evolved between species. Evolution. 2010;64:2166–72. doi: 10.1111/j.1558-5646.2010.00959.x. [DOI] [PubMed] [Google Scholar]
- Herre EA, Knowlton N, Mueller UG, Rehner SA. The evolution of mutualisms: exploring the paths between conflict and cooperation. Trends Ecol Evol. 1999;14:49–53. doi: 10.1016/s0169-5347(98)01529-8. [DOI] [PubMed] [Google Scholar]
- Hillesland KL, Stahl DA. Rapid evolution of stability and productivity at the origin of a microbial mutualism. Proc Natl Acad Sci U S A. 2010;107:2124–9. doi: 10.1073/pnas.0908456107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochberg ME, Gomulkiewicz R, Holt RD, Thompson JN. Weak sinks could cradle mutualistic symbioses – strong sources should harbour parasitic symbioses. Journal of Evolutionary Biology. 2000;13:213–222. [Google Scholar]
- Hochberg ME, Van Baalen M. Antagonistic coevolution over productivity gradients. Am Nat. 1998;152:620–34. doi: 10.1086/286194. [DOI] [PubMed] [Google Scholar]
- Holland JN, Deangelis DL, Bronstein JL. Population dynamics and mutualism: functional responses of benefits and costs. Am Nat. 2002;159:231–44. doi: 10.1086/338510. [DOI] [PubMed] [Google Scholar]
- Jagmann N, Brachvogel HP, Philipp B. Parasitic growth of Pseudomonas aeruginosa in co-culture with the chitinolytic bacterium Aeromonas hydrophila. Environ Microbiol. 2010;12:1787–802. doi: 10.1111/j.1462-2920.2010.02271.x. [DOI] [PubMed] [Google Scholar]
- Johnstone RA, Bshary R. From parasitism to mutualism: partner control in asymmetric interactions. Ecology Letters. 2002;5:634–639. [Google Scholar]
- Kummerli R, Brown SP. Molecular and regulatory properties of a public good shape the evolution of cooperation. Proc Natl Acad Sci U S A. 2010;107:18921–6. doi: 10.1073/pnas.1011154107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahowald MA, Rey FE, Seedorf H, Turnbaugh PJ, Fulton RS, Wollam A, Shah N, Wang C, Magrini V, Wilson RK, Cantarel BL, Coutinho PM, Henrissat B, Crock LW, Russell A, Verberkmoes NC, Hettich RL, Gordon JI. Characterizing a model human gut microbiota composed of members of its two dominant bacterial phyla. Proc Natl Acad Sci U S A. 2009;106:5859–64. doi: 10.1073/pnas.0901529106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marx CJ. Getting in touch with your friends. Science. 2009;324:1150–1. doi: 10.1126/science.1173088. [DOI] [PubMed] [Google Scholar]
- Mitri S, Xavier JB, Foster KR. Social evolution in multispecies biofilms. Proc Natl Acad Sci U S A. 2011;108(Suppl 2):10839–46. doi: 10.1073/pnas.1100292108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JJ, Lenski RE, Zinser ER. The Black Queen Hypothesis: Evolution of Dependencies through Adaptive Gene Loss. MBio. 2012;3 doi: 10.1128/mBio.00036-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuismer SL, Gomulkiewicz R, Morgan MT. Coevolution in temporally variable environments. Am Nat. 2003;162:195–204. doi: 10.1086/376582. [DOI] [PubMed] [Google Scholar]
- Otto SP, Day T. A biologist’s guide to mathematical modeling in ecology and evolution. Princeton University Press; Princeton, NJ: 2007. [Google Scholar]
- Ramsey MM, Rumbaugh KP, Whiteley M. Metabolite cross-feeding enhances virulence in a model polymicrobial infection. PLoS Pathog. 2011;7:e1002012. doi: 10.1371/journal.ppat.1002012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsey MM, Whiteley M. Polymicrobial interactions stimulate resistance to host innate immunity through metabolite perception. Proc Natl Acad Sci U S A. 2009;106:1578–83. doi: 10.1073/pnas.0809533106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs JL, Mueller UG, Wilcox TP, Bull JJ. The evolution of cooperation. Q.Rev.Biol. 2004;79:135–160. doi: 10.1086/383541. [DOI] [PubMed] [Google Scholar]
- Sachs JL, Simms EL. Pathways to mutualism breakdown. Trends Ecol Evol. 2006;21:585–92. doi: 10.1016/j.tree.2006.06.018. [DOI] [PubMed] [Google Scholar]
- Samuel BS, Gordon JI. A humanized gnotobiotic mouse model of host-archaeal-bacterial mutualism. Proc Natl Acad Sci U S A. 2006;103:10011–6. doi: 10.1073/pnas.0602187103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuel BS, Hansen EE, Manchester JK, Coutinho PM, Henrissat B, Fulton R, Latreille P, Kim K, Wilson RK, Gordon JI. Genomic and metabolic adaptations of Methanobrevibacter smithii to the human gut. Proc Natl Acad Sci U S A. 2007;104:10643–8. doi: 10.1073/pnas.0704189104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schink B. Synergistic interactions in the microbial world. Antonie Van Leeuwenhoek. 2002;81:257–61. doi: 10.1023/a:1020579004534. [DOI] [PubMed] [Google Scholar]
- Shimoyama T, Kato S, Ishii S, Watanabe K. Flagellum mediates symbiosis. Science. 2009;323:1574. doi: 10.1126/science.1170086. [DOI] [PubMed] [Google Scholar]
- Stams AJ. Metabolic interactions between anaerobic bacteria in methanogenic environments. Antonie Van Leeuwenhoek. 1994;66:271–94. doi: 10.1007/BF00871644. [DOI] [PubMed] [Google Scholar]
- van Baalen M, Jansen VAA. Dangerous liaisons: the ecology of private interest and common good. Oikos. 2001;95:211–224. Oikos, 95. [Google Scholar]
- West SA, Griffin AS, Gardner A, Diggle SP. Social evolution theory for microorganisms. Nat Rev Microbiol. 2006;4:597–607. doi: 10.1038/nrmicro1461. [DOI] [PubMed] [Google Scholar]
- West SA, Kiers ET, Simms EL, Denison RF. Sanctions and mutualism stability: why do rhizobia fix nitrogen? Proc Biol Sci. 2002;269:685–94. doi: 10.1098/rspb.2001.1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamura N. Vertical Transmission and Evolution of Mutualism from Parasitism. Theoretical Population Biology. 1993;44:95–109. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.