Abstract
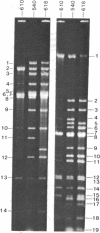
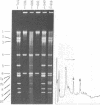
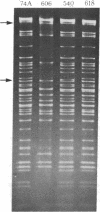
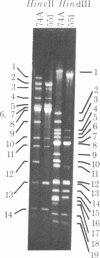
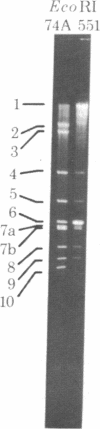
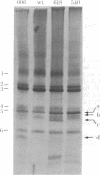
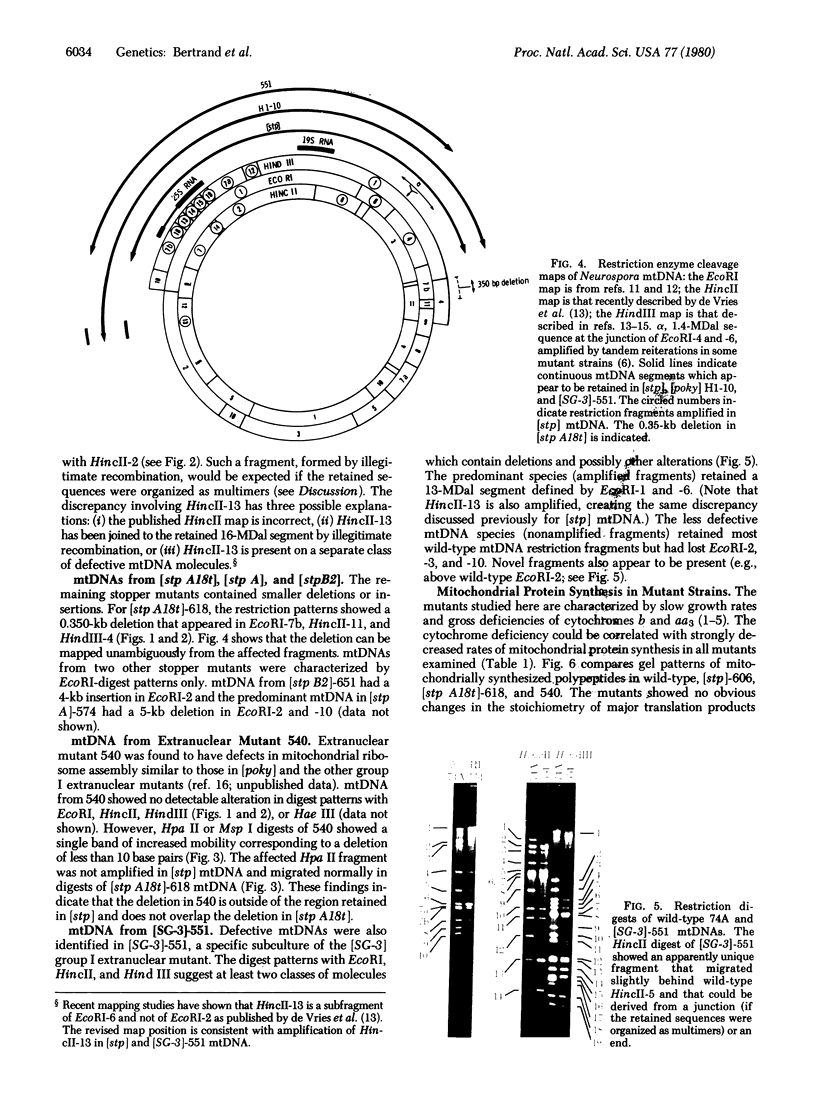
"Stoppers" are a class of Neurospora crassa extranuclear mutants characterized by gross deficiencies of cytochromes b and aa3 and an unusual growth phenotype which involves irregular periods of growth andnongrowth. In the present work, mtDNAs from all four stopper mutants were found to contain deletions or insertions detectable by restriction enzyme analysis. [stp] mtDNA consists predominantly of defective molecules which retain a 16-megadalton segment (EcoRI-1, -4, and -6) of wild-type mtDNA (40 megadaltons). The other stopper mutants show smaller alterations: [stp A18t]-618, a 0.35-kilobase deletion in EcoRI-7b; [stp B2]-651, a 4-kilobase insertion in EcoRI-2; and [stp A]-574, a 5-kilobase deletion in EcoRI-2 and -10. Based on these results, we propose that "stop-start" growth results from competition between certain defective mtDNAs which have a tendency to predominate and low concentrations of less defective mtDNA species which must be retained to sustain growth. Three additional extranuclear mutants ("nonstoppers") have also been found to contain deletions in mtDNA. Remarkably, the defective mtDNA species in two of these mutants ([poky]H1-10 and [SG-3]-551) retain different sizes (18 and 13 megadlatons, respectively) of the same region retained in [stp] mtDNA (i.e., EcoRI-1, -4, and -6). The findings suggest that production of defective mtDNAs in Neurospora is nonrandom with a preferred mechanism leading to retention of this segment. It may be significant that the retained segment contains both mitochondrial rRNA genes and most mitochondrial tRNA genes. These deletion mutants may provide a tool for genetic mapping of Neurospora mtDNA.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARLETT C. F. Induction of cytoplasmic mutations in Aspergillus nidulans. Nature. 1957 Jun 15;179(4572):1250–1251. doi: 10.1038/1791250a0. [DOI] [PubMed] [Google Scholar]
- Berg D. E. Genetic evidence for two types of gene arrangements in new lambdadv plasmid mutants. J Mol Biol. 1974 Jun 15;86(1):59–68. doi: 10.1016/s0022-2836(74)80007-0. [DOI] [PubMed] [Google Scholar]
- Bernard U., Goldthwaite C., Küntzel H. Physical map of Neurospora crassa mitochondrial DNA and its transcription unit for ribosomal RNA. Nucleic Acids Res. 1976 Nov;3(11):3101–3108. doi: 10.1093/nar/3.11.3101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertrand H., Pittenger T. H. Complementation among cytoplasmic mutants of Neurospora crassa. Mol Gen Genet. 1972;117(1):82–90. doi: 10.1007/BF00268840. [DOI] [PubMed] [Google Scholar]
- Bertrand H., Pittenger T. H. Cytoplasmic Mutants Selected from Continuously Growing Cultures of NEUROSPORA CRASSA. Genetics. 1969 Mar;61(3):643–659. doi: 10.1093/genetics/61.3.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertrand H., Pittenger T. H. Isolation and classification of extranuclear mutants of Neurospora crassa. Genetics. 1972 Aug;71(4):521–533. doi: 10.1093/genetics/71.4.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertrand H., Szakacs N. A., Nargang F. E., Zagozeski C. A., Collins R. A., Harrigan J. C. The function of mitochondrial genes in Neurospora crassa. Can J Genet Cytol. 1976 Sep;18(3):397–409. doi: 10.1139/g76-049. [DOI] [PubMed] [Google Scholar]
- Borst P., Grivell L. A. The mitochondrial genome of yeast. Cell. 1978 Nov;15(3):705–723. doi: 10.1016/0092-8674(78)90257-x. [DOI] [PubMed] [Google Scholar]
- Chow L. T., Davidson N. Electron microscope study of the structures of lambdadv DNAs. J Mol Biol. 1974 Jun 15;86(1):69–89. doi: 10.1016/s0022-2836(74)80008-2. [DOI] [PubMed] [Google Scholar]
- Collins R. A., Bertrand H., LaPolla R. J., Lambowitz A. M. Mitochondrial ribosome assembly in Neurospora crassa: mutants with defects in mitochondrial ribosome assembly. Mol Gen Genet. 1979;177(1):73–84. doi: 10.1007/BF00267255. [DOI] [PubMed] [Google Scholar]
- Cummings D. J., Belcour L., Grandchamp C. Mitochondrial DNA from Podospora anserina. II. Properties of mutant DNA and multimeric circular DNA from senescent cultures. Mol Gen Genet. 1979 Mar 27;171(3):239–250. doi: 10.1007/BF00267578. [DOI] [PubMed] [Google Scholar]
- Davoli D., Ganem D., Nussbaum A. L., Fareed G., Howley P. M., Khoury G., Martin M. A. Genome Structures of reiteration mutants of simian virus 40. Virology. 1977 Mar;77(1):110–124. doi: 10.1016/0042-6822(77)90411-1. [DOI] [PubMed] [Google Scholar]
- Diacumakos E. G., Garnjobst L., Tatum E. L. A cytoplasmic character in Neurospora crassa. The role of nuclei and mitochondria. J Cell Biol. 1965 Aug;26(2):427–443. doi: 10.1083/jcb.26.2.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas M. G., Butow R. A. Variant forms of mitochondrial translation products in yeast: evidence for location of determinants on mitochondrial DNA. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1083–1086. doi: 10.1073/pnas.73.4.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faugeron-Fonty G., Culard F., Baldacci G., Goursot R., Prunell A., Bernardi G. The mitochondrial genome of wild-type yeast cells. VIII. The spontaneous cytoplasmic "petite" mutation. J Mol Biol. 1979 Nov 5;134(3):493–457. doi: 10.1016/0022-2836(79)90365-6. [DOI] [PubMed] [Google Scholar]
- Folk W. R., Fishel B. R. Tandem repetition of the origin of DNA replication in defective polyoma virus DNA's. Virology. 1975 Apr;64(2):447–453. doi: 10.1016/0042-6822(75)90122-1. [DOI] [PubMed] [Google Scholar]
- Hahn U., Lazarus C. M., Lünsdorf H., Küntzel H. Split gene for mitochondrial 24S ribosomal RNA of Neurospora crassa. Cell. 1979 May;17(1):191–200. doi: 10.1016/0092-8674(79)90307-6. [DOI] [PubMed] [Google Scholar]
- Heckman J. E., RajBhandary U. L. Organization of tRNA and rRNA genes in N. crassa mitochondria: intervening sequence in the large rRNA gene and strand distribution of the RNA genes. Cell. 1979 Jul;17(3):583–595. doi: 10.1016/0092-8674(79)90266-6. [DOI] [PubMed] [Google Scholar]
- Khoury G., Fareed G. C., Berry K., Martin M. A., Lee T. N., Nathans D. Characterization of a rearrangement in viral DNA: mapping of the circular simian virus 40-like DNA containing a triplication of a specific one-third of the viral genome. J Mol Biol. 1974 Aug 5;87(2):289–301. doi: 10.1016/0022-2836(74)90150-8. [DOI] [PubMed] [Google Scholar]
- Lee T. N., Brockman W. W., Nathans D. Evolutionary variants of simian virus 40: cloned substituted variants containing multiple initiation sites for DNA replication. Virology. 1975 Jul;66(1):53–69. doi: 10.1016/0042-6822(75)90178-6. [DOI] [PubMed] [Google Scholar]
- Locker J., Lewin A., Rabinowitz M. The structure and organization of mitochondrial DNA from petite yeast. Plasmid. 1979 Apr;2(2):155–181. doi: 10.1016/0147-619x(79)90036-2. [DOI] [PubMed] [Google Scholar]
- Mannella C. A., Goewert R. R., Lambowitz A. M. Characterization of variant Neurospora crassa mitochondrial DNAs which contain tandem reiterations. Cell. 1979 Dec;18(4):1197–1207. doi: 10.1016/0092-8674(79)90232-0. [DOI] [PubMed] [Google Scholar]
- Mannella C. A., Lambowitz A. M. Unidirectional gene conversion associated with two insertions in neurospora crassa mitochondrial DNA. Genetics. 1979 Nov;93(3):645–654. doi: 10.1093/genetics/93.3.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannella C. A., Lambowitz A. Interaction of wild-type and poky mitochondrial DNA in heterokaryons of Neurospora. Biochem Biophys Res Commun. 1978 Feb 14;80(3):673–679. doi: 10.1016/0006-291x(78)91621-2. [DOI] [PubMed] [Google Scholar]
- McDougall K. J., Pittenger T. H. A cytoplasmic variant of Neurospora crassa. Genetics. 1966 Aug;54(2):551–565. doi: 10.1093/genetics/54.2.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittenger T. H. SYNERGISM OF TWO CYTOPLASMICALLY INHERITED MUTANTS IN NEUROSPORA CRASSA. Proc Natl Acad Sci U S A. 1956 Oct;42(10):747–752. doi: 10.1073/pnas.42.10.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terpstra P., Holtrop M., Kroon A. M. A complete cleavage map of Neurospora crassa mtDNA obtained with endonucleases Eco RI and Bam HI. Biochim Biophys Acta. 1977 Apr 19;475(4):571–588. doi: 10.1016/0005-2787(77)90318-5. [DOI] [PubMed] [Google Scholar]
- Terpstra P., Holtrop M., Kroon A. Heterogeneous base distribution in mitochondrial DNA of Neurospora crassa. Nucleic Acids Res. 1977 Jan;4(1):129–139. doi: 10.1093/nar/4.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waalwijk C., Flavell R. A. MspI, an isoschizomer of hpaII which cleaves both unmethylated and methylated hpaII sites. Nucleic Acids Res. 1978 Sep;5(9):3231–3236. doi: 10.1093/nar/5.9.3231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vries H., de Jonge J. C., Bakker H., Meurs H., Kroon A. Laboratory of Physiological Chemistry, State University Groningen, Netherlands. Nucleic Acids Res. 1979;6(5):1791–1803. doi: 10.1093/nar/6.5.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]