Abstract
Objective
Indirect acute lung injury (ALI) is associated with high morbidity/ mortality. However, the underlying pathophysiology is only marginally understood and so far no pathophysiologic based remedy exists. We hypothesized, that apoptosis of lung epithelial cells is a pathophysiological relevant process in the development of indirect ALI and that it should be accessible to a small interfering (si)RNA based therapeutic intervention in vivo.
Design
Prospective, randomized, controlled animal study.
Setting
Basic science laboratory of a university affiliated level-1 trauma center.
Subjects
Male C3H/HeN mice, 8 weeks old, n=121
Interventions
First, siRNA sequences to knock down caspase-3 expression at a RNA as well as protein level were evaluated in vitro. Then, C3H/HeN mice were subjected to hemorrhagic shock, following which they received either a caspase-3 siRNA or a control/nonsense siRNA. Subsequently, they were then subjected to polymicrobial sepsis (induced by cecal ligation and puncture).
Measurements and Main Results
Twelve and 24 hours after sepsis increased lung epithelial apoptosis, as evidenced by active caspase-3 western blotting, caspase-3-, TUNEL- and M30 immunohistochemistry, was observed. Hallmarks of ALI, such as increased concentrations of pulmonary cytokines/chemokines, lung protein leakage, myeloperoxidase activity and altered lung histology, were evident in response to these insults. The single intratracheal instillation of caspase-3 siRNA not only attenuated lung apoptosis and inflammation, but also ameliorated the development of ALI in treated mice. Most interestingly, this experimental therapeutic approach markedly improved 10 day survival of hemorrhaged-septic mice.
Conclusions
Apoptosis of lung epithelial cells is a relevant pathomechanism in the development of hemorrhage-induced indirect septic ALI, and caspase-3 appears to be a valuable therapeutic target, accessible by siRNA treatment in vivo.
Keywords: Acute Respiratory Distress Syndrome, Apoptosis, Gene Silencing, small interfering RNA, Sepsis, Hemorrhagic Shock
Introduction
The incidence of ALI and ARDS in the US has been reported to be 79 and 59 per 100,000 persons per year, respectively 1–3. Around 7% of intensive care patients will eventually develop ALI/ ARDS 4;5. The development of ALI in critical ill trauma patients contributes independently to in-hospital mortality 6. Studies suggest a lethality of 40% for ALI or ARDS 1;2 and the presence of sepsis represents one of the highest risk factors for mortality from ARDS (up to 50%) 4;7;8. In spite of these unfortunate statistics, therapeutic strategies for the treatment of ALI are still very limited and not pathophysiologically oriented. So far, lung protective ventilatory regimens 9 and intermittent prone positioning 2, are the only therapeutic options with proven efficacy.
Apoptosis represents an energy consuming, organized way of cell death. The extrinsic apoptotic pathway is triggered through the death receptors including Fas (CD95), while the intrinsic mitochondrial pathway is activated by diverse stimuli such as reactive oxygen species, radiation, and chemotherapeutic agents. Both pathways ultimately cleave caspase-3 and activated endonucleases then induce the cleavage of the DNA and the death of the cell 10. In this regard, we have previously reported, that activation of the FasL-Fas pathway in lung epithelial cells appears to be involved in the pathogenesis of indirect septic ALI 11;12. Activation of Fas in the early phase of ALI appears to trigger pulmonary apoptosis as well as lung inflammation and thus may represent mechanisms linking neutrophil recruitment and lung apoptosis 13. However, to this end it remains unclear which role lung apoptosis itself plays in the development of septic ALI and whether this potential patho-mechanism is accessible to therapeutic intervention.
Thus, we sought to investigate whether lung epithelial cell apoptosis was a crucial mechanism in the pathogenesis of shock induced septic ALI and whether it was accessible to therapeutic intervention using a clinically relevant, double-hit, animal model of hemorrhagic shock followed by polymicrobial sepsis. In as much, we tested the hypothesis that local tissue silencing of the central executioner caspase-3, by means of double stranded small interfering (si)RNAs against caspase-3 messenger RNA, in the lung post–shock, ameliorated septic ALI and beneficially affected survival.
Material and Methods
Validation of Caspase-3 siRNA Sequences in vitro
Caspase-3 and control green fluorescent protein siRNA sequences (GFP-siRNA) 14 were assembled, deprotected, duplexed and desalted by Dharmacon (Lafayette, USA). The following sequences were tested in vitro: Caspase-3 duplex 1, sense: 5’-CAACGGAAUUCGAGUCCUUUU-3’, antisense: 5`-AAGGACUCGAAUUCCGUUGUU-3´; Caspase-3 duplex 2, sense: 5’-GGAUAGUGUUUCUAAGGAAUU-3`, antisense: 5`-UUCCUUAGAAACACUAUCCUU-3`; Caspase-3 duplex 3, sense: 5`-GCACUGGAAUGUCAUCUCGUU-3´; antisense: 5`-CGAGAUGACAUUCCAGUGCUU-3`; Caspase-3 duplex 4, sense: 5`-CGCACAAGCUAGAAUUUAUUU-3`, antisense: 5`-AUAAAUUCUAGCUUGUGCGUU-3`. Control (GFP)-siRNA, sense: 5’-GGCUACGUCCAGGAGCGCACCUU-3’, antisense sequence: 5’-UGCGCUCCUGGACGUAGCCUU-3’.
Transfection of siRNA in vitro
The RAW mouse-macrophage cell line 264.7 (American Type Culture Collection, Manassas, Virginia) was grown in Falcon® tissue culture flasks (Becton Dickinson, Franklin Lakes, NY) until about 50%. Cells were resuspended in 5 ml DMEM (incl. 10% fetal calf serum (FCS), 1% 100× HEPES, but no Gentamicin). Cells were divided into 2 ml tubes (Eppendorf) at a concentration of 6×106 cells/ tube, centrifuged and were then resuspendend in 100 µl Nucleofactor solution V (Amaxa, Gaithersburg, MD) containing either 300 pmol Caspase-3 silencing RNA sequences or GFP-siRNA serving as a control. Transfection protocol T-24 (Nucleofector, Amaxa) was chosen as it was found to provide excellent transfection results (> 90%) as determined using a Cy5-tagged-siRNA-sequence, visualized using Nikon TE-2000U inverted microscope (Nikon Corporation, Tokyo, Japan) combined with good cell survival (> 85%) as determined by trypan blue exclusion. Cells were resuspended in 2 ml of DMEM (incl. 10% FCS, 1% 100× HEPES, but no antibiotics) and seeded in six well cell culture plates (Corning Incorporated, Corning, NY). Non-attached cells were removed after a 24 hour incubation period and cells were stimulated with 8 µM Camptothecin (Sigma, St. Louis, USA) for 5 hours at 37°C, 5% CO2 to activate Capase-3.
LA-4 mouse pulmonary epithelial cells (ATCC) were grown in Falcon® tissue culture flasks until about 90% confluence in FK-12 medium supplemented with 10% FCS, 0.01% Gentamicin and 1% HEPES buffered solution. Cells were then resuspended in 5 ml FK-12 (incl. 10% FCS, 1% 100× HEPES, but no Gentamicin). Cells were divided into 2 ml tubes (Eppendorf) at a concentration of 3.0×106 cells/ tube, centrifuged and resuspended in 100 µl Nucleofector solution V, containing either 300 pmol Caspase-3 silencing RNA sequences, GFP-siRNA serving as a control. Transfection protocol T-20 was chosen. Cells were then treated similarly to RAW-cells as described above.
Quantification of Caspase-3/ 18s-ribosomal mRNA in vitro and ex vivo using quantitative real-time PCR
RNA was isolated from RAW cells or lung tissue using TriPure Isolation Reagent (Boehringer, Mannheim, Germany) as described previously 11. CDNA was synthesized using iScript cDNA Synthesis Kit (BioRad) as described previously 11. Primer sequences for Caspase-3 (forward: 5’-TGGAGGCTGACTTCCTGTATGCTT -3’; reverse: 5’- ACGGGATCTGTTTCTTTGCGTGG -3’) and 18s ribosomal RNA (forward: 5’-GGACACGGACAGGATTGACAGATT-3’; reverse: 5’-AATCGCTCCACCAACTAAGAACGG-3’) were assembled by IDT, Coralville, USA.
CDNA and forward and reverse primers (150nM final concentration) in 25µl of SYBR Green QPCR Master Mix including ROX at a concentration of 1:810 as reference dye (all Stratagene, La Jolla, CA), were placed into plates and run in a Mx3000P Real-Time-PCR System (Stratagene). All samples were run in duplicate. The data were analyzed with the appropriate software (Stratagene).
Caspase-3 activity assay (in vitro)
Caspase-3 activity in RAW 264.1 cells was assessed as described previously 11 with minor modifications. Lysed cells were added to 50 µl of Caspase-3 reaction buffer (containing 100mM HEPES, 2% Sucrose, 0.2% CHAPS, 10mM Dithiothreitol (all Sigma) and 100µM Ac-DEVD-AFC fluorogenic substrate (Biomol, Plymouth Meeting, PA)) was added to the samples. Extinction was read at 400 nm excitation, 505 nm emission on a microplate fluorescence reader (FLx800, Bio-Tek Instruments Inc., Winooski, VT). Values were calculated against an AFC standard curve (Biomol) as µM cleavage of AFC/ minute/ µg total cell protein.
Animals
Male inbred C3H/HeN mice (Charles River, Wilmington, USA) were used. Experiments are in accordance with NIH guidelines and as approved by the local Animal Use Committee.
Acute Lung Injury
Acute lung injury was induced as described previously 11;12;15. Mice were anesthetized with isoflurane and catheters were inserted into both femoral arteries. Anesthesia was discontinued and blood pressure was continuously monitored through one catheter attached to a blood pressure analyzer (BPA MicroMed, Louisville, KY). When fully awake, mice were bled over a 5 to 10 minute period to a mean blood pressure of 30 (±5 mmHg) and were kept stable for 90 minutes. Immediately after hemorrhage, mice were resuscitated via the catheters with Ringer's lactate at four times the drawn blood volume. Sham-operated controls had their femoral arteries ligated and were restrained for periods of time equal to their hemorrhaged counterparts. Polymicrobial sepsis as produced by cecal ligation and puncture (CLP) was induced 24 hrs after hemorrhage as described previously 11;12;15. Mice were anesthetized with isoflurane and a 1 cm midline incision was made below the diaphragm to expose the cecum. The cecum was ligated, punctured twice with a 22-gauge needle and gently compressed. The incision was closed in layers with 6-0 Ethilon suture (Ethicon, Somerville, NJ). The animals were then resuscitated with 0.8 ml of lactated Ringer’s solution. In sham-operated controls laparotomy was performed in a similar fashion but the cecum was neither ligated nor punctured. These sham procedures still represent an insult sufficient to stimulate a minor inflammatory response.
Intratracheal Instillation
Intratracheal delivery of small interfering RNA was performed as described previously 11;12. Thirty minutes following resuscitation mice were anesthetized with isoflurane and their head was reclined. The tongue was pulled out gently and siRNA or placebo was administered in the oral cavity. Mice were maintained in the described position until aspiration was complete.
Experimental Groups and Sample Acquisition for in vivo experiments
Mice were subjected to hemorrhagic shock, received a single intratracheal instillation of 75µg of caspase-3 siRNA or control-GFP-siRNA 30 min thereafter, followed by polymicrobial sepsis 24 hrs after hemorrhage. Blood and lungs were harvested 12 or 24 hrs after the induction of sepsis. Lungs were gently flushed with 2 ml ice-cold PBS via the right ventricle. Plasma was obtained by centrifugation of blood for 10 minutes, 10,000×g at 4°C and frozen at −80°C until analysis. Lung lobes were excised, frozen immediately into liquid nitrogen and stored at −80°C. For survival animals were subjected to HEM+CLP and survival was assessed for the following 10 days on a daily basis (n=20/ group).
In a second set of experiments (n=4 animals/ group) with identical procedures and time points lungs were instilled in vivo with 30 µl/ g body weight of 10% buffered formaldehyde after exsanguination, excised and stored for 24 hrs in 10% formaldehyde. Afterwards, lungs were paraffin embedded and cut into 5 µm sections for immunohistochemistry or H&E staining.
In a third set of experiments mice received 0.5 mg of fluorescently labeled (Alexa Fluor® 488) albumin from bovine serum (Molecular Probes, Invitrogen Carlsbad, CA) 30 minutes prior to exsanguination. Mice were sacrificed 12 hrs after CLP. Lungs were gently flushed with 10 ml ice-cold PBS through the vascular system via the right ventricle, harvested and immediately homogenized in 1 ml of ice cold PBS and centrifuged. Supernatants were read at 480 nm on a fluorometric plate reader (FLx800, Bio-Tek Instruments Inc., Winooski, VT) and readings were calculated against a standard curve of BSA-AF488 diluted in PBS and normalized per µg lung protein. Results are expressed as a lung-plasma ratio of BSA-AF488.
Preparation of Tissue Samples for Cytokine Measurements
Preparation of lung homogenates for cytokine measurements was performed as described previously 11;12.
Protein Assay
The total amount of protein was quantified using the Bradford dye binding procedure as described previously 12.
Chemokine ELISAs
Mouse KC and MIP-2 concentrations in plasma, lung tissue homogenates, BAL and cell supernatants were determined using murine anti-cytokine antibody pairs as well as cytokine standards (both R&D Systems, Minneapolis, MN) as per the manufacture's protocol for basic sandwich ELISA. MCP-1 in cell supernatants was analyzed using mouse MCP-1 BD OptEIA™ ELISA (BD Biosciences).
Cytometric Bead Array
Mouse TNF-α, IL-6, IFN-γ and MCP-1 levels were established in plasma and lung tissue homogenates using the cytometric bead array technique (BD™ Cytometric Bead Array Mouse Inflammation Kit, BD Biosciences) as described previously 11;12.
Myeloperoxidase (MPO) Activity
Lung MPO activity was quantified as described previously 11;12.
Active Caspase-3 and β-Actin western blotting
Active Caspase-3 and β-Actin were quantified in lung tissue and cell supernatants via western blotting as described previously 12. 80µg of homogenized lung tissue or cells were electrophoresed on a 18% Tris-Glycine Gel (Invitrogen) and transferred onto a Poly-Vinyliden-Di-Fluorid membrane (Amersham, Piscataway, NJ). Membranes were incubated with polyclonal rabbit anti-mouse cleaved Caspase-3 (Asp175) antibody (Cell Signaling) at a concentration of 1:500 at 4°C overnight. Cleaved Caspase-3 (Asp175) antibody detects endogenous levels of the large fragment (17/19 kDa) of activated caspase-3 resulting from cleavage adjacent to Asp175. Membranes were incubated with ECL Rabbit IgG, HRP-linked whole antibody from donkey (Amersham) at a concentration 1:10000 for 1 hour and developed using enhanced chemiluminescence technique (ChemiGlow, Alpha Innotech, San Leandro, CA). Membranes were incubated with polyclonal rabbit anti-mouse β-Actin (Abcam, Cambridge, MA) at a concentration of 1:10000 and developed as described above. Protein expression was quantified via densitometry using FluorChem, Version 4.1.0 (Alpha Innotech) and expressed as the ratio of the integrated density values of Caspase-3 and β-Actin.
Active Caspase-3 Immunohistochemistry
Active Caspase-3 immunohistochemistry was carried out as described previously 11;12.
M-30 Immunostaining
M30 is a neo-epitope that is revealed when the intermediate filament protein cytokeratin-18 on epithelial cells is cleaved by caspase-3/-6/-9 at Asp396 16, thus indicating epithelial cell specific apoptosis. M-30 immunostaining was carried out as described previously 11;12.
TUNEL (Terminal deoxynucleotidyl Transferase Biotin-dUTP Nick End Labeling)
Quantification of TUNEL positive cells was carried out as described previously 12 using the In Situ Cell Death Detection Kit, Fluorescin (Boehringer, Mannheim, Germany).
Slides for immunohistochemistry were examined as follows: Ten high power fields (HPF) (25 µm2/ field)/ slide were randomly selected and counted in a blinded fashion (C.S.C) and the number of fluorescent cells per HPF was recorded for statistical analysis. Negative controls were performed by following the exact same protocols but omitting either the first or the second antibody.
Lung Hematoxylin and Eosin (H&E)
H&E tissue slides were histopathologically analyzed according to the following criteria as described previously 11;12: Distension of alveoli, thickening of alveolar septa, perivascular and peribronchial edema and intraalveolar cellular infiltrates. Staining (Core Research Facilities) and analysis (C.S.C.) of H&E sections were performed by a blinded observer.
Statistical Analysis
Results are presented as mean standard error of the mean (SEM). The Kolmogorov-Smirnov test (SigmaStat 3.0 [SPSS Incorporated, Chicago, IL]) was used to assess normal distribution of data prior to performing any given statistical tests. One-Way or Two-way ANOVA Analysis of Variance (ANOVA) followed by the Student-Newman-Keuls test as a post hoc test for multiple comparisons were performed to determine significant differences between experimental means or medians. Differences in survival were assessed using Kaplan-Meier, followed by LogRank test. A p-value < 0.05 was considered statistically significant.
Results
Evaluation of Caspase-3-siRNA sequences
The expression level of caspase-3 mRNA in RAW 264.1 cells being transfected with control-siRNA following camptothecin stimulation was set to 100% for comparative purposes. Transfection with all of the caspase-3-siRNA duplexes assessed resulted in a significant knockdown of caspase-3 mRNA (duplex 1: knockdown of 94%, duplex 2: 95%, duplex 3: 97%, duplex 4: 96%) in camptothecin activated RAW cells, when compared to nonsense siRNA. On a protein level, caspase-3 activity (µM cleaved substrate/ ng protein/ minute) in control-siRNA transfected raw cells was markedly elevated (66.9[Mean]±3.5[SEM]) after camptothecin stimulation when compared to unstimulated control-siRNA transfected raw-cells (31.6±0.3). All caspase-3-siRNA duplexes (duplex 1: 38.7±1.7, duplex 2: 41.7±1.6, duplex 3: 45.4±0.1, duplex 4: 43.4±1.7) substantially decreased caspase-3 activity when compared to control-siRNA transfected cells (66.9±3.5). Duplex 2 was then chosen for use in the subsequent in vivo studies. Of note the transfection process itself did not enhance caspase-3 mRNA or caspase-3 activity. In addition, caspase-3 siRNA was also tested on LA-4 mouse epithelial cells. LA-4 cells stimulated with camptothecin and transfected with caspase-3 siRNA (1.28[Mean]±0.25[SEM]) showed markedly less active caspase-3 protein when compared to cells transfected with GFP-siRNA (3.31±0.63). Cells not transfected (4.22±2.21) did not differ significantly from cells being transfected with PBS (6.8±3.05) or GFP-siRNA.
In vivo silencing of caspase-3 markedly decreases lung apoptosis following hemorrhagic shock and sepsis
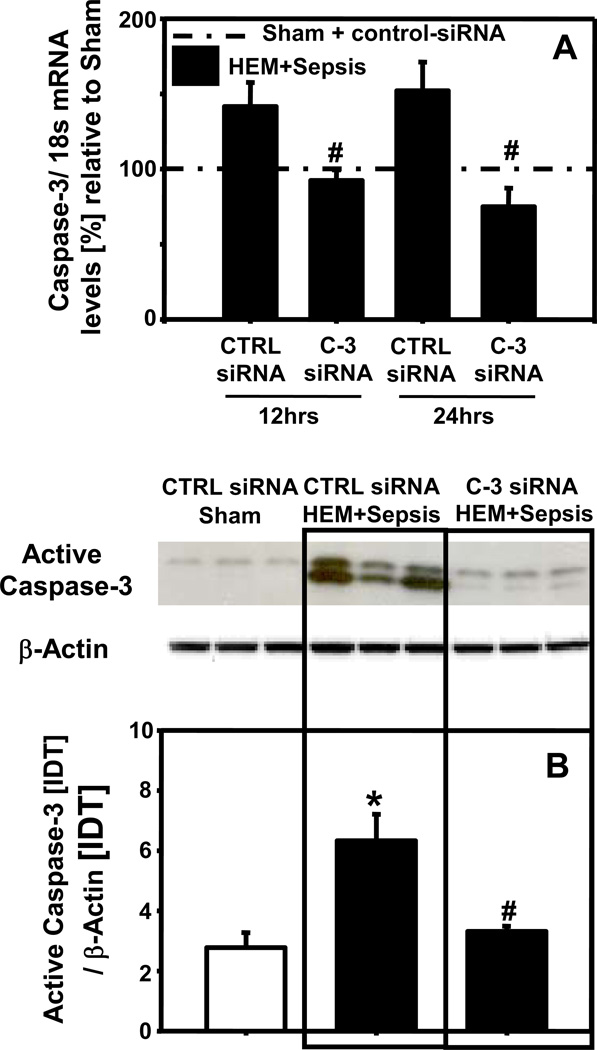
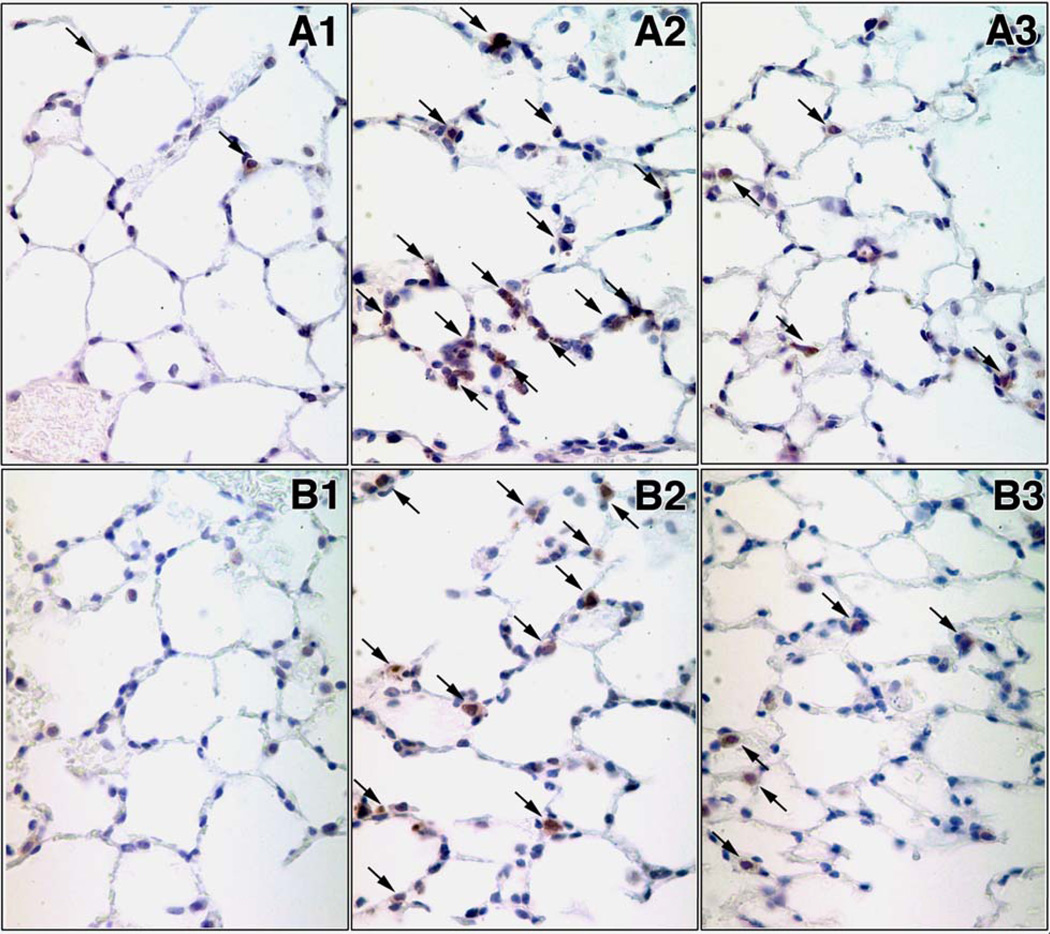
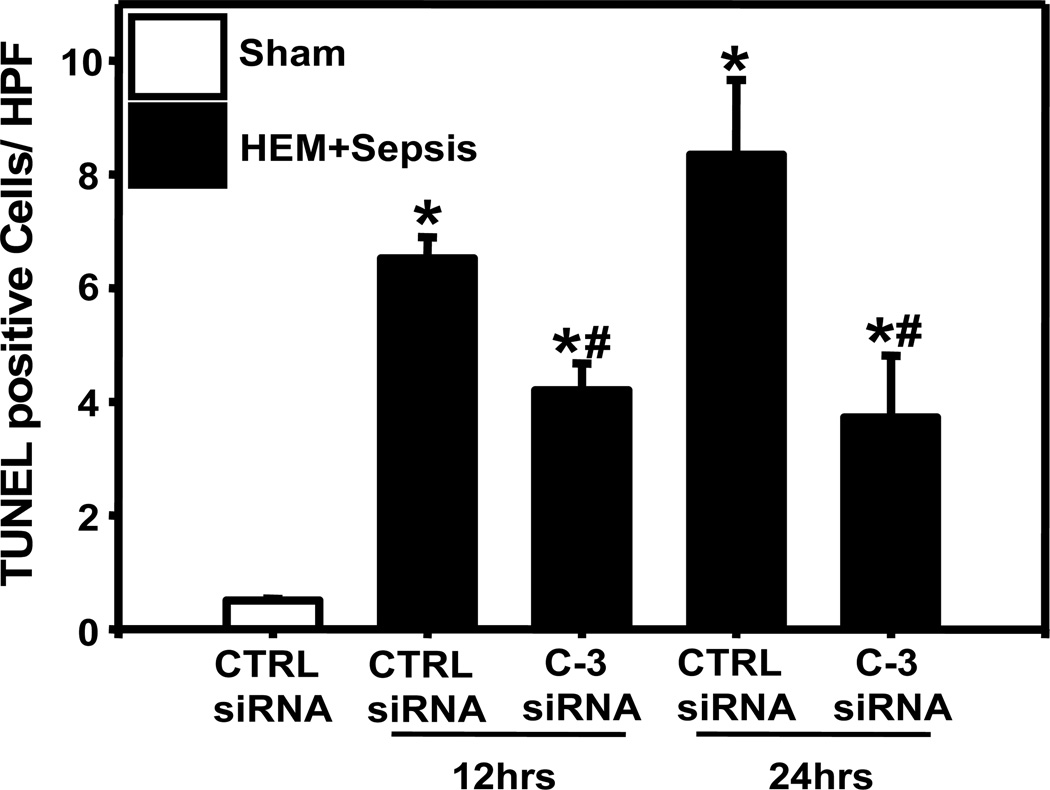
Lung caspase-3 mRNA was substantially increased at 12 and 24 hrs in response to HEM+Sepsis in animals having received control-siRNA. Caspase-3 mRNA levels were markedly decreased in animals being treated with caspase-3-siRNA when compared to control siRNA treated animals (Fig. 1A). On a protein level lung active caspase-3 was also significantly enhanced at 12 hrs following the insult and caspase-3 silencing markedly reduced lung caspase-3 activation after HEM+Sepsis (Fig. 1B). The number of active caspase-3 positive cells in the lung was also increased at 12 hrs following HEM+Sepsis in control siRNA treated animals (Fig.2A2) when compared to sham (Fig.2A1) and decreased in HEM+Sepsis mice that received caspase-3 siRNA (Fig.2A3). Furthermore, M30 staining of the lungs showed substantial epithelial cell apoptosis at 12 hrs following HEM+Sepsis when animals received control-siRNA (Fig. 2B2) when compared to sham (Fig. 2B1). Following caspase-3 silencing lung epithelial cells were markedly protected from caspase dependent programmed cell death (Fig. 2B3). This was further confirmed by assessing the number of TUNEL positive cells per high power field, which were markedly increased at 12 and 24 hrs in response to the insult in control-siRNA treated mice and substantially decreased following caspase-3 silencing (Fig. 3).
Figure 1.
Pulmonary caspase-3 mRNA levels in mice having received control (CTRL) or caspase-3 (C-3) siRNA 12 hrs and 24 hrs following hemorrhagic shock and sepsis (HEM+Sepsis) compared to sham mice (dashed line = 100%) (Fig.1A). Integrated density (IDT) values of active caspase-3 relative to IDT values of β-actin of n=6 animals per group 12 hrs following HEM+Sepsis (Fig. 1B). Quantification via western blotting and densitometry. One Way ANOVA followed by Student Newman Keuls Test, *p<0.05 vs. corresponding sham, #p<0.05 vs. corresponding control-siRNA treatment (HEM+Sepsis).
Figure 2.
Representative active caspase-3 (Fig.2A) and M30 (cleaved cytokeratin-18) (Fig.2B) immunohistochemistry of lung tissue from sham animals (Fig.2A1 and Fig.2B1, respectively), mice having received control-siRNA (Fig.2A2 and Fig.2B2 respectively) or caspase-3 siRNA (Fig.2A3 and Fig.2B3 respectively) 12 hrs after hemorrhagic shock and sepsis. n=4 animals/ group. Original magnifications, X 400.
Figure 3.
TUNEL positive cells per high power field (HPF) in sham animals and at 12 and 24 hrs following hemorrhagic shock and sepsis after treatment with control (CTRL) or caspase-3 (C-3) siRNA. One Way ANOVA followed by Student Newman Keuls Test. n=4 animals/ group. * p< 0.05 vs. Sham, # p< 0.05 vs. corresponding control-siRNA treatment (HEM+Sepsis).
In vivo silencing of caspase-3 markedly decreases lung inflammation following hemorrhagic shock and sepsis
Lung TNF-α (Fig. 4A) and IL-6 (Fig. 4B) levels were substantially increased at 12 hrs following HEM+Sepsis in both control-siRNA and caspase-3 siRNA treated animals when compared to sham controls. However, TNF-α (Fig. 4A) and IL-6 (Fig. 4B) concentrations were markedly lower in caspase-3 siRNA treated mice when compared to control-siRNA. Interestingly, lung KC (Fig. 4C), MCP-1 (Fig. 4D) and MIP-2 (Fig. 4E) were also substantially increased in both groups at 12 hrs and at 24 hrs following the insult. Whereas no differences between caspase-3 siRNA and control siRNA treated animals were observed here at the early 12hrs time point, lung KC but not lung MCP-1 or MIP-2 was markedly decreased in response to caspase-3 silencing at 24 hrs following HEM+Sepsis. Lung IFN-γ concentrations were not markedly different between experimental groups (data not shown).
Figure 4.
Lung TNF-α (Fig. 4A), IL-6 (Fig. 4B), KC (Fig. 4C), MCP-1 (Fig. 4D), MIP-2 (Fig. 4E) [pg/mg] in mice having received control (CTRL) or caspase-3 (C-3) siRNA 12 or 24 hrs following hemorrhagic shock and sepsis (HEM+Sepsis) compared to sham animals. Quantification via ELISA or CBA. N=7–8 /group. Two Way ANOVA followed by Student Newman Keuls Test, *p<0.05 vs. corresponding sham, #p<0.05 vs. corresponding control-siRNA treatment (HEM+Sepsis), +p<0.05 vs. corresponding 24 hrs time point (identical treatment and insult)
In vivo silencing of caspase-3 markedly decreases systemic inflammation following hemorrhagic shock and sepsis
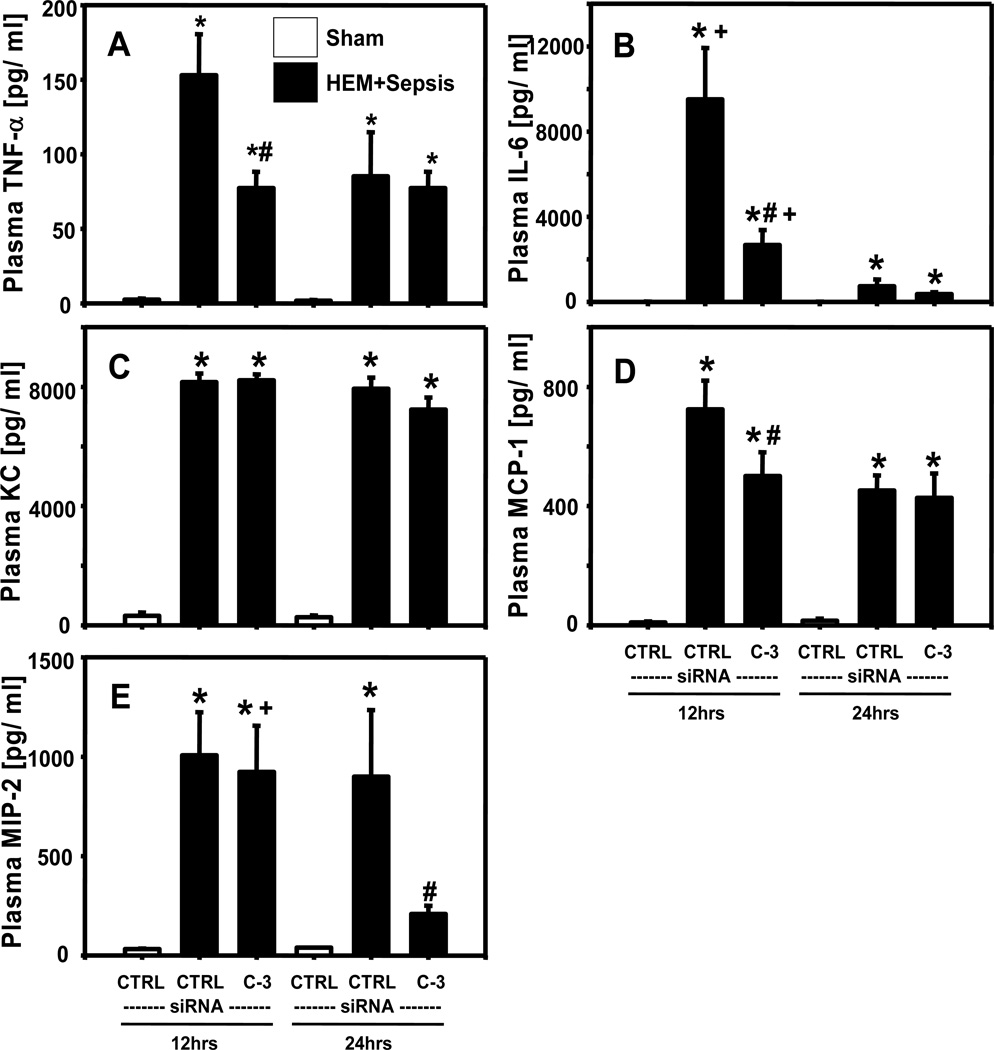
Plasma TNF-α (Fig. 5A), IL-6 (Fig. 5B), KC (Fig. 5C), MCP-1 (Fig. 5D), MIP-2 (Fig. 5E) concentrations were all substantially increased at 12 and 24 hrs following HEM+CLP when compared to sham animals. At 12 hrs after insult, plasma KC and MIP-2 did not show marked differences between caspase-3 siRNA and control siRNA treated animals, however, plasma MCP-1, TNF-α, IL-6 and IFN-γ levels were significantly reduced following caspase-3 silencing. Plasma MIP-2 was also markedly decreased at 24 hrs in double hit animals following silencing of caspase-3 when compared to mice having received control siRNA. Plasma IFN-γ concentrations were not markedly different between experimental groups (data not shown).
Figure 5.
Plasma TNF-α ( Fig. 5A), IL-6 (Fig. 5B), KC (Fig. 5C), MCP-1 (Fig. 5D), MIP-2 (Fig. 5E) [pg/ml] concentrations in mice having received control (CTRL) or caspase-3 (C-3) siRNA 12 or 24 hrs following hemorrhagic shock and sepsis (HEM+Sepsis) compared to sham animals. Quantification via ELISA or CBA. N=7–8 /group. Two Way ANOVA followed by Student Newman Keuls Test. *p<0.05 vs. corresponding sham, #p<0.05 vs. corresponding control-siRNA treatment (HEM+Sepsis), +p<0.05 vs. corresponding 24 hrs time point (identical treatment and insult)
In vivo silencing of caspase-3 markedly ameliorates hemorrhaged induced septic acute lung injury
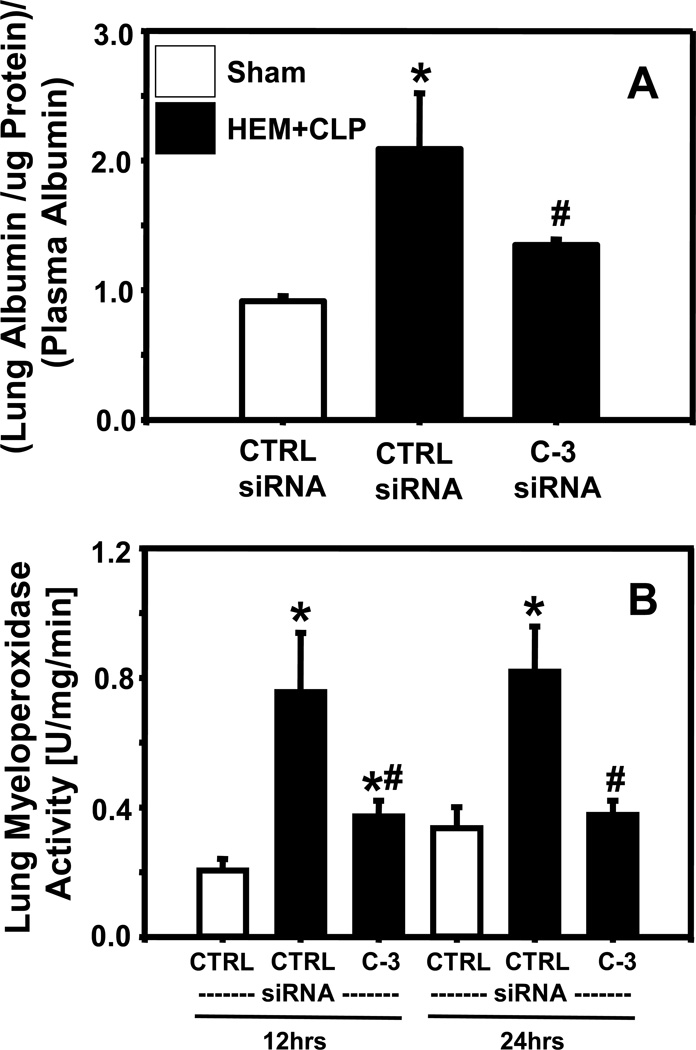
Lung vascular leak was markedly increased at 12 hrs following HEM+Sepsis when animals had received control-siRNA (Fig. 6A). Following caspase-3 silencing, lung protein leak was substantially mitigated when compared to control-siRNA animals (Fig. 6A). In addition, lung myeloperoxidase activity (Fig. 6B) was substantially enhanced at 12 and 24 hrs in response to HEM+Sepsis in animals having received control-siRNA and was markedly abrogated following caspase-3 silencing.
Figure 6.
Ratio of fluorescently labeled albumin per µg lung tissue relative to fluorescently labeled albumin in the plasma (Fig.6A) in mice having received control (CTRL) or caspase-3 (C-3) siRNA 12 hrs following hemorrhagic shock and sepsis (HEM+Sepsis). Lung myeloperoxidase activity (Fig.6B) (U)Units/ mg lung tissue/ (min)minute in mice having received control (CTRL) or caspase-3 (C-3) siRNA 12 or 24 hrs following HEM+Sepsis compared to sham animals. N=5/ group for albumin leakage, n=7–8/group for myeloperoxidase activity. One (albumin) or Two Way ANOVA (myeloperoxidase activity) followed by Student Newman Keuls Test. *p<0.05 vs. corresponding sham, #p<0.05 vs. corresponding control-siRNA treatment (HEM+Sepsis).
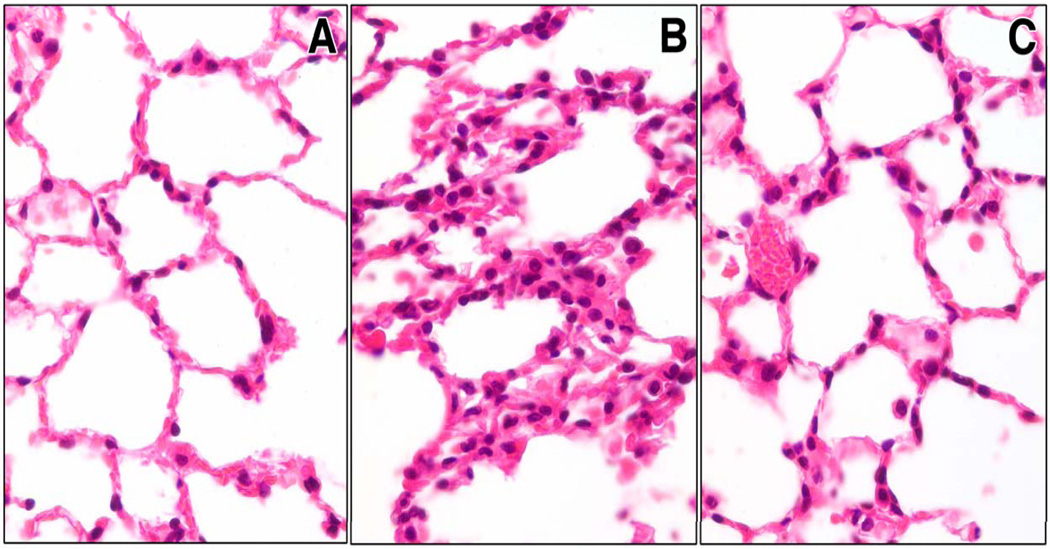
H&E prepared sections displayed lung congestion, disrupted alveolar architecture and increased numbers of neutrophils within the alveolar walls in control-siRNA treated animals 12 hrs following HEM+CLP (Fig. 7B) when compared to sham (Fig. 7A). Following caspase-3 siRNA treatment mice showed a marked protection of lung morphology following the insult (Fig. 7C).
Figure 7.
Representative H&E preparation of lung tissue slides from sham animals (Fig.7A) and mice 12 hrs after hemorrhagic shock and sepsis after having received control-siRNA (Fig.7B) or caspase-3 siRNA (Fig.7C). N=4 animals/ group. Original magnifications, X 400.
In vivo silencing of caspase-3 markedly improves survival following hemorrhagic shock and sepsis
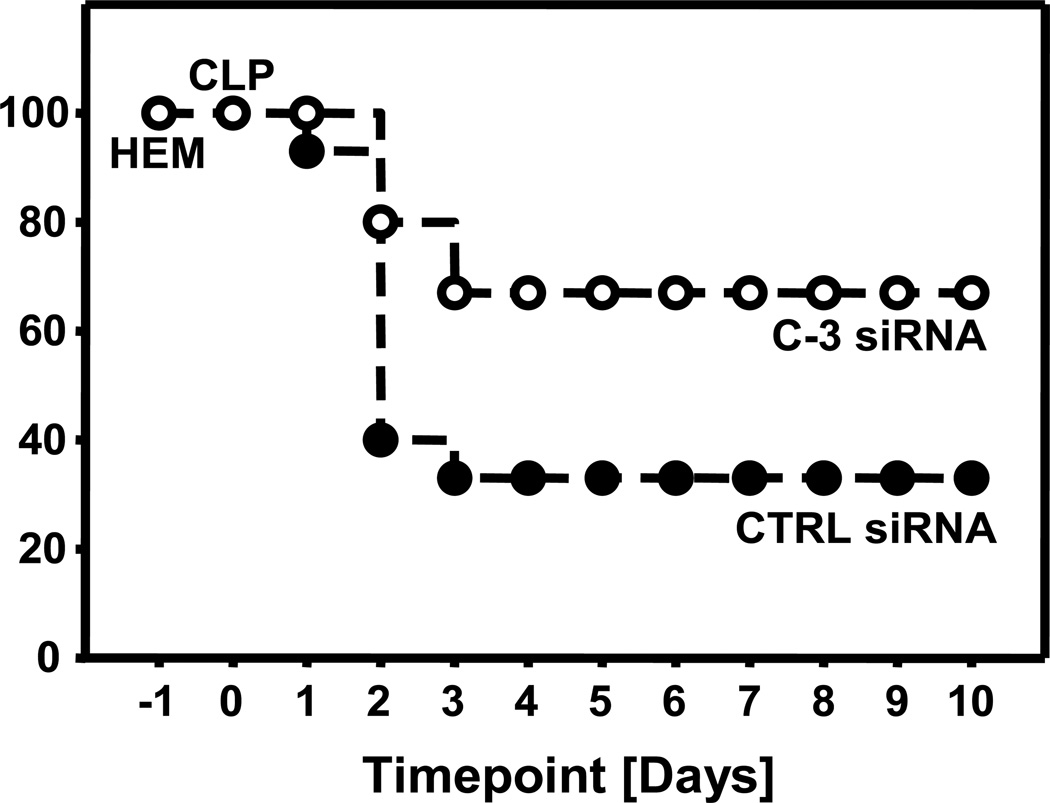
Following caspase-3 siRNA treatment 10 day survival was markedly improved following HEM+Sepsis when compared to control-siRNA treated animals (Fig. 8).
Figure 8.
Ten day survival in animals after having received control- (CTRL) or caspase-3 (C-3) siRNA following hemorrhagic shock (HEM) and subsequent sepsis 24 hrs thereafter. Kaplan-Meier, LogRank Test, n=15 animals per group, p< 0.05.
Discussion
Here we have investigated whether inhibition of apoptosis of lung epithelial cells can be exploited therapeutically during the development of indirect ALI.
Thus far, a considerable body of literature describes pulmonary apoptosis in different types of ALI 13;17–20. In this regard, during ALI, an increased concentration of the Fas death receptor and its ligand in patients’ bronchoalveolar fluid and lung tissue have been detected 21;22, particularly in the acute phase of septic ARDS 23. Furthermore, ARDS non-survivors exhibited markedly higher FasL concentrations when compared to survivors 24. However, it remains far less clear whether apoptosis in the lung during indirect ALI has any pathological mechanistic significance. In this regard, blocking of the Fas-FasL system reduced the sequelae of endotoxin mediated experimental ALI 25 and Fas deficient mice even showed protection in hemorrhage induced septic ALI 12. Fas-FasL deficiency was also associated with a reduction in the degree of ALI seen after infection 26,27. However, there are limitations to the use of knockout or mutant animals and part of the effects seen might be co-dependent on the absence of the Fas-receptor in other organs than the lung. Previous studies have indicated that silencing of specific genes in the lungs under pathological conditions was feasible 11;28;29. Furthermore, naked double stranded siRNA appears to localize primarily in lung epithelial cells without causing a marked inflammatory response 11.
In our setting lung apoptosis, as evidenced by the presence of active caspase-3 and double strand DNA breaks, was detected as early as 12 hours following the insult. Immunohistochemistry of caspase cleaved cytokeratin indicated that lung epithelial cells were the primary cell type to undergo apoptosis here, which is very much in line with recent clinical findings reporting increased caspase-cleaved cytokeratin-18 concentrations in BALF of ARDS patients 22. Lung fibroblasts do not seem to be very susceptible to Fas induced apoptosis 30. The fact that local siRNA delivery into the lung is particularly well-suited to affect epithelial cell is supported by recent literature 31;32. Silencing of caspase-3 markedly reduced lung apoptosis, even when siRNA was instilled after the resuscitation period of hemorrhagic shock. The presence of TUNEL positive cells after HEM+Sepsis – although markedly reduced after caspase-3 siRNA treatment – despite caspase-3 silencing does not preclude that alternative apoptotic pathways might also be activated by the insult. Interestingly, caspase-3 silencing not only abrogated lung apoptosis but also mitigated lung inflammation. In this regard, the fact that apoptotic pathway molecules, particularly of the extrinsic pathway, are involved in the generation of inflammatory mediators has been described previously 33. Faouzi et al. observed that activation of Fas in mice in vivo induced apoptosis as well as inflammation and blockade of caspase-3 markedly diminished activator protein-1 as well as hepatic chemokine production 34. Following ischemia and reperfusion inhibition of caspases not only markedly abrogated renal apoptosis but also inflammation and tissue injury 35. In addition, using a broad spectrum caspase inhibitor in bleomycin induced pneumopathy, a substantial decrease of lung apoptosis as well as pulmonary inflammation was observed 36. The findings of Matute-Bello’s group as well as our own results indicated that it might be the lung epithelial cell, which, upon Fas activation, is responsible for apoptotic lung damage as well as the release of certain inflammatory mediators 12;37. In addition, it appears that by down regulating the local inflammatory response in the lung, this partly translated into a change in the systemic compartment. Whether this might indicate that the lung was able to function as a motor of the systemic inflammatory response following ALI and why this was not observed for all cytokines and chemokines remains to be elucidated. Interestingly, we also observed a reduction of neutrophil sequestration into the lung, evidenced by decreased lung myeloperoxidase activity, in response to caspase-3 silencing. In this regard, it is well known that granulocytes can contribute to ALI, even in our model 15, and inhibition of PMN migration or activation has previously been shown to ameliorate hemorrhage induced ALI 28. Clinically increased recruitment of neutrophils into the bronchoalveolar fluid of ARDS patients correlated significantly with a local rise in chemokines/cytokines as well as soluble Fas 22. Thus, decreased recruitment of activated PMNs into the lungs might have also contributed to the beneficial effects on ALI seen in our experiments.
Most interestingly, the reduction of lung apoptosis and inflammation through caspase-3 silencing and its sequelae had beneficial impact on the degree of lung injury seen in response to HEM+Sepsis as evidenced by reduced capillary leak and improved lung histology. This clearly suggests a patho-mechanistic role of lung apoptosis during hemorrhaged induced septic ALI. Furthermore, the fact that the reduction of lung injury was associated with an improved survival of the animals supports the concept, that the lung is a major player in orchestrating/initiating multiple organ failure during sepsis and/ or trauma. The survival rate of non-siRNA treated animals has been shown to be comparable to that of CTRL siRNA treated mice 12;15.
The fact that a single intratracheal instillation of anti-apoptotic siRNA during the onset of ALI was sufficient to beneficially impact the pathologic consequences of hemorrhage induced septic ALI is also worth pointing out. Phase I intranasal trials with an siRNA targeting the viral nucleocapsid gene of the Respiratory Syncytial Virus, have already been performed 38;39. Based on this phase II clinical studies designed to evaluate the safety, tolerability, and antiviral activity of this siRNA have now been initiated 40 and the potential for local delivery of siRNAs to the lung for the treatment of diverse respiratory diseases including asthma, chronic obstructive pulmonary disease (COPD), and viral infections is now being considered 41.
In summary, we provide evidence here, that apoptosis of lung epithelial cells is a patho-physiologically significant event in a clinically relevant double-hit model of indirect ALI. Furthermore, via an approach involving in vivo gene silencing by the deposition of small interfering RNA against caspase-3 into the lungs, not only were several hallmarks of ALI, i.e. lung apoptosis, pulmonary inflammation and neutrophil recruitment markedly suppressed, but an increase in survival rate of the animals was observed. Together, these data suggest that local siRNA delivery to the lungs holds considerable promise as an anti-apoptotic/anti-inflammatory therapeutic approach in the clinical setting of acute lung injury in man.
Acknowledgements
We thank Virginia Hovanesian and Paul Monfils of Core Research Laboratories at Lifespan, Rhode Island Hospital for their excellent technical assistance.
Support Grants from the National Institutes of Health, HL73525, GM53209 (to A.A.) and from Rhode Island Hospital.
Footnotes
The authors have no potential conflicts of interest to disclose.
Reference List
- 1.Rubenfeld GD, Caldwell E, Peabody E, et al. Incidence and outcomes of acute lung injury. N Engl J Med. 2005;353:1685–1693. doi: 10.1056/NEJMoa050333. [DOI] [PubMed] [Google Scholar]
- 2.Wheeler AP, Bernard GR. Acute lung injury and the acute respiratory distress syndrome: a clinical review. Lancet. 2007;369:1553–1564. doi: 10.1016/S0140-6736(07)60604-7. [DOI] [PubMed] [Google Scholar]
- 3.Herridge MS, Angus DC. Acute lung injury--affecting many lives. N Engl J Med. 2005;353:1736–1738. doi: 10.1056/NEJMe058205. [DOI] [PubMed] [Google Scholar]
- 4.Brun-Buisson C, Minelli C, Bertolini G, et al. Epidemiology and outcome of acute lung injury in European intensive care units. Results from the ALIVE study. Intensive Care Med. 2004;30:51–61. doi: 10.1007/s00134-003-2022-6. [DOI] [PubMed] [Google Scholar]
- 5.Goss CH, Brower RG, Hudson LD, et al. Incidence of acute lung injury in the United States. Crit Care Med. 2003;31:1607–1611. doi: 10.1097/01.CCM.0000063475.65751.1D. [DOI] [PubMed] [Google Scholar]
- 6.Shah CV, Localio AR, Lanken PN, et al. The impact of development of acute lung injury on hospital mortality in critically ill trauma patients. Crit Care Med. 2008;36:2309–2315. doi: 10.1097/CCM.0b013e318180dc74. [DOI] [PubMed] [Google Scholar]
- 7.Vincent JL, Zambon M. Why do patients who have acute lung injury/acute respiratory distress syndrome die from multiple organ dysfunction syndrome? Implications for management. Clin Chest Med. 2006;27:725–731. doi: 10.1016/j.ccm.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 8.Monchi M, Bellenfant F, Cariou A, et al. Early predictive factors of survival in the acute respiratory distress syndrome. A multivariate analysis. Am J Respir Crit Care Med. 1998;158:1076–1081. doi: 10.1164/ajrccm.158.4.9802009. [DOI] [PubMed] [Google Scholar]
- 9.ARDS Network: Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The Acute Respiratory Distress Syndrome Network. N Engl J Med. 2000;342:1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 10.Perl M, Chung CS, Ayala A. Apoptosis. Crit Care Med. 2005;33:S526–S529. doi: 10.1097/01.ccm.0000185499.28006.4c. [DOI] [PubMed] [Google Scholar]
- 11.Perl M, Chung CS, Lomas-Neira J, et al. Silencing of fas but not caspase-8, in lung epithelial cells ameliorates pulmonary apoptosis, inflammation, and neutrophil influx after hemorrhagic shock and sepsis. Am J Pathol. 2005;167:1545–1559. doi: 10.1016/S0002-9440(10)61240-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Perl M, Chung CS, Perl U, et al. Fas-induced pulmonary apoptosis and inflammation during indirect acute lung injury. Am J Respir Crit Care Med. 2007;176:591–601. doi: 10.1164/rccm.200611-1743OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Perl M, Lomas-Neira J, Chung CS, et al. Epithelial cell apoptosis and neutrophil recruitment in acute lung injury-a unifying hypothesis? What we have learned from small interfering RNAs. Mol Med. 2008;14:465–475. doi: 10.2119/2008-00011.Perl. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Novina CD, Murray MF, Dykxhoorn DM, et al. siRNA-directed inhibition of HIV-1 infection. Nat Med. 2002;8:681–686. doi: 10.1038/nm725. [DOI] [PubMed] [Google Scholar]
- 15.Ayala A, Chung CS, Lomas JL, et al. Shock-induced neutrophil mediated priming for acute lung injury in mice: divergent effects of TLR-4 and TLR-4/FasL deficiency. Am J Pathol. 2002;161:2283–2294. doi: 10.1016/S0002-9440(10)64504-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leers MP, Kolgen W, Bjorklund V, et al. Immunocytochemical detection and mapping of a cytokeratin 18 neo-epitope exposed during early apoptosis. J Pathol. 1999;187:567–572. doi: 10.1002/(SICI)1096-9896(199904)187:5<567::AID-PATH288>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 17.Bachofen M, Weibel ER. Structural alterations of lung parenchyma in the adult respiratory distress syndrome. Clin Chest Med. 1982;3:35–56. [PubMed] [Google Scholar]
- 18.Bardales RH, Xie SS, Schaefer RF, et al. Apoptosis is a major pathway responsible for the resolution of type II pneumocytes in acute lung injury. Am J Pathol. 1996;149:845–852. [PMC free article] [PubMed] [Google Scholar]
- 19.Bem RA, Bos AP, Matute-Bello G, et al. Lung epithelial cell apoptosis during acute lung injury in infancy. Pediatr Crit Care Med. 2007;8:132–137. doi: 10.1097/01.PCC.0000257207.02408.67. [DOI] [PubMed] [Google Scholar]
- 20.Guinee D, Jr, Brambilla E, Fleming M, et al. The potential role of BAX and BCL-2 expression in diffuse alveolar damage. Am J Pathol. 1997;151:999–1007. [PMC free article] [PubMed] [Google Scholar]
- 21.Albertine KH, Soulier MF, Wang Z, et al. Fas and fas ligand are up-regulated in pulmonary edema fluid and lung tissue of patients with acute lung injury and the acute respiratory distress syndrome. Am J Pathol. 2002;161:1783–1796. doi: 10.1016/S0002-9440(10)64455-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee KS, Choi YH, Kim YS, et al. Evaluation of bronchoalveolar lavage fluid from ARDS patients with regard to apoptosis. Respir Med. 2008;102:464–469. doi: 10.1016/j.rmed.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 23.Hashimoto S, Kobayashi A, Kooguchi K, et al. Upregulation of two death pathways of perforin/granzyme and FasL/Fas in septic acute respiratory distress syndrome. Am J Respir Crit Care Med. 2000;161:237–243. doi: 10.1164/ajrccm.161.1.9810007. [DOI] [PubMed] [Google Scholar]
- 24.Matute-Bello G, Liles WC, Steinberg KP, et al. Soluble Fas ligand induces epithelial cell apoptosis in humans with acute lung injury (ARDS) J Immunol. 1999;163:2217–2225. [PubMed] [Google Scholar]
- 25.Kitamura Y, Hashimoto S, Mizuta N, et al. Fas/FasL-dependent apoptosis of alveolar cells after lipopolysaccharide-induced lung injury in mice. Am J Respir Crit Care Med. 2001;163:762–769. doi: 10.1164/ajrccm.163.3.2003065. [DOI] [PubMed] [Google Scholar]
- 26.Matute-Bello G, Frevert CW, Liles WC, et al. Fas/Fas ligand system mediates epithelial injury, but not pulmonary host defenses, in response to inhaled bacteria. Infect Immun. 2001;69:5768–5776. doi: 10.1128/IAI.69.9.5768-5776.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tateda K, Deng JC, Moore TA, et al. Hyperoxia mediates acute lung injury and increased lethality in murine Legionella pneumonia: the role of apoptosis. J Immunol. 2003;170:4209–4216. doi: 10.4049/jimmunol.170.8.4209. [DOI] [PubMed] [Google Scholar]
- 28.Lomas-Neira JL, Chung CS, Wesche DE, et al. In vivo gene silencing (with siRNA) of pulmonary expression of MIP-2 versus KC results in divergent effects on hemorrhage-induced, neutrophil-mediated septic acute lung injury. J Leukoc Biol. 2005;77:846–853. doi: 10.1189/jlb.1004617. [DOI] [PubMed] [Google Scholar]
- 29.Zhang X, Shan P, Jiang D, et al. Small interfering RNA targeting heme oxygenase-1 enhances ischemia-reperfusion-induced lung apoptosis. J Biol Chem. 2004;279:10677–10684. doi: 10.1074/jbc.M312941200. [DOI] [PubMed] [Google Scholar]
- 30.Tanaka T, Yoshimi M, Maeyama T, et al. Resistance to Fas-mediated apoptosis in human lung fibroblast. Eur Respir J. 2002;20:359–368. doi: 10.1183/09031936.02.00252602. [DOI] [PubMed] [Google Scholar]
- 31.Sun L, Guo RF, Gao H, et al. Attenuation of IgG immune complex-induced acute lung injury by silencing C5aR in lung epithelial cells. FASEB J. 2009 doi: 10.1096/fj.09-133694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Whitehead KA, Langer R, Anderson DG. Knocking down barriers: advances in siRNA delivery. Nat Rev Drug Discov. 2009;8:129–138. doi: 10.1038/nrd2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kataoka T, Budd RC, Holler N, et al. The caspase-8 inhibitor FLIP promotes activation of NF-kappaB and Erk signaling pathways. Curr Biol. 2000;10:640–648. doi: 10.1016/s0960-9822(00)00512-1. [DOI] [PubMed] [Google Scholar]
- 34.Faouzi S, Burckhardt BE, Hanson JC, et al. Anti-Fas induces hepatic chemokines and promotes inflammation by an NF-kappa B-independent, caspase-3-dependent pathway. J Biol Chem. 2001;276:49077–49082. doi: 10.1074/jbc.M109791200. [DOI] [PubMed] [Google Scholar]
- 35.Daemen MA, van 't Veer C, Denecker G, et al. Inhibition of apoptosis induced by ischemia-reperfusion prevents inflammation. J Clin Invest. 1999;104:541–549. doi: 10.1172/JCI6974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kuwano K, Kunitake R, Maeyama T, et al. Attenuation of bleomycin-induced pneumopathy in mice by a caspase inhibitor. Am J Physiol Lung Cell Mol Physiol. 2001;280:L316–L325. doi: 10.1152/ajplung.2001.280.2.L316. [DOI] [PubMed] [Google Scholar]
- 37.Bem RA, Farnand AW, Wong V, et al. Depletion of resident alveolar macrophages does not prevent Fas-mediated lung injury in mice. Am J Physiol Lung Cell Mol Physiol. 2008;295:L314–L325. doi: 10.1152/ajplung.00210.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.de FA, Vornlocher HP, Maraganore J, et al. Interfering with disease: a progress report on siRNA-based therapeutics. Nat Rev Drug Discov. 2007;6:443–453. doi: 10.1038/nrd2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thomas M, Lu JJ, Ge Q, et al. Full deacylation of polyethylenimine dramatically boosts its gene delivery efficiency and specificity to mouse lung. Proc Natl Acad Sci U S A. 2005;102:5679–5684. doi: 10.1073/pnas.0502067102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Akhtar S, Benter IF. Nonviral delivery of synthetic siRNAs in vivo. J Clin Invest. 2007;117:3623–3632. doi: 10.1172/JCI33494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thomas M, Lu JJ, Chen J, et al. Non-viral siRNA delivery to the lung. Adv Drug Deliv Rev. 2007;59:124–133. doi: 10.1016/j.addr.2007.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]