Abstract
Background
Tissue analysis of bone regenerate has suggested an intense vascular response after mandibular distraction osteogenesis (DO). Quantifying and 3-dimensionally imaging this vascular response could be of immense clinical import in efforts to advance the utility of bone regeneration and repair. Conventional quantification of vascular responses has heretofore focused on inexact, cumbersome measurements of blood flow and histological vessel counting. Utilizing Micro-Computed Tomography after vessel perfusion we posit that quantitative vascular metrics will be significantly higher in mandibular DO compared to those observed in fracture repair (FxR) after bony union.
Methods
Sprague-Dawley rats underwent mandibular osteotomy and external fixator placement. A DO group (n=9) underwent a 5.1 mm distraction, while a FxR group (n=12) had a 2.1mm fixed gap set. 40 days after surgery Microfil was perfused into the vasculature and imaging ensued. Vascular radiomorphometrics were calculated for the regions of interest. Independent samples-t test were performed for comparisons with statistical significance at p≤0.05.
Results
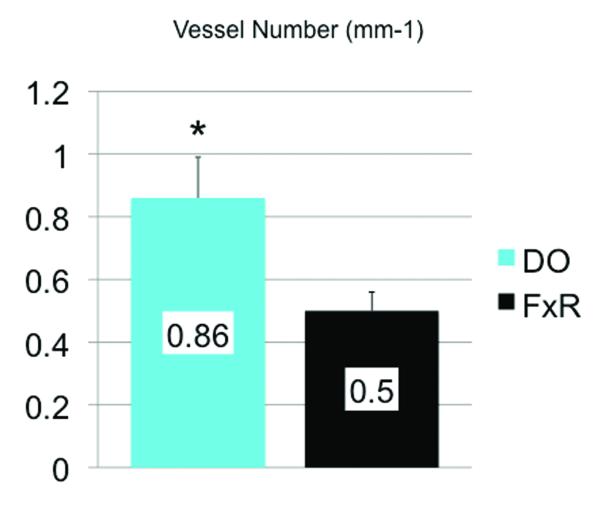
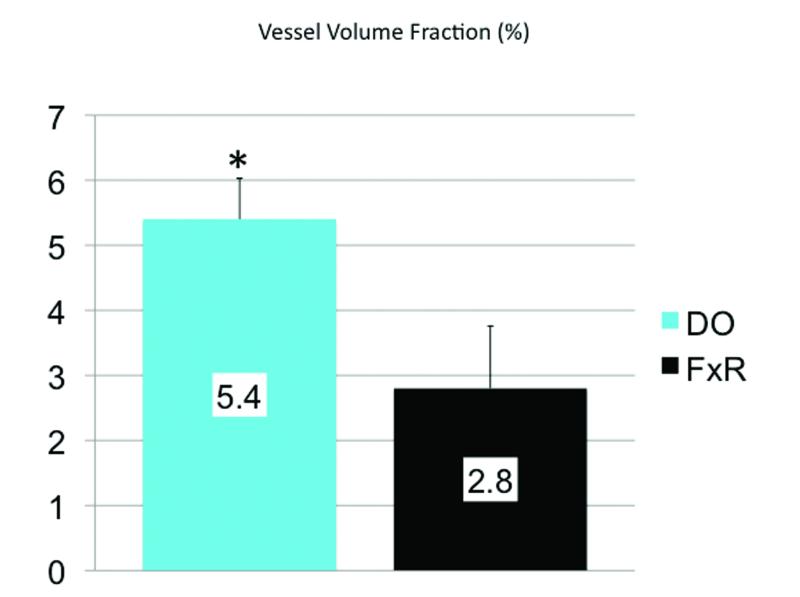
Stereological analysis demonstrated statistically significant increases in the distracted vasculature compared to fracture repair: Vessel volume fraction (5.4% vs. 2.8%, p = 0.030), and vessel number (0.86 mm−1 vs. 0.50 mm−1, p = 0.014).
Conclusion
We report robust and quantifiable increases in vascular density in DO compared to FxR. Our findings support a significant distinction between the regenerative processes of mandibular DO from the reparative mechanisms controlling fracture healing. A better understanding of the differences between the two types of bone formation may enable clinicians to selectively optimize therapeutic outcomes in the future.
Introduction
Although the process of bone healing inherent in fracture repair (FxR) and the mechanisms of bone regeneration that occur during distraction osteogenesis (DO) are thought to be closely related, understanding the unique differences between the two could be pivotal in efforts to optimize therapeutic interventions.1,2,3,4 Flow studies and histological analyses have demonstrated evidence of robust recruitment and sustenance of vascularity at the site of distraction existing long after the active distraction and consolidation phases have ended.5,6,7,8 These findings highlight an important distinction regarding the body’s ability to respond to the hyper-metabolic demands placed on it as it is challenged with the mechanical forces necessary for the engineering of new bone generated by DO.
An ability to quantify and 3 dimensionally image this augmented vascular response could be of immense clinical import in efforts to optimize and expand the utility of DO, or improve the current regenerative capacities of the procedure.9,10 Further, finding ways to trigger, augment and maintain vascularity during bone turn-over may provide avenues for the optimization of fracture healing in complicated, metabolically demanding clinical scenarios such as in cases of osteoradionecrosis, osteomyelitis, and non-unions.11,12
Although enhancing the vascular response to DO has been suggested, quantifying the changes in vascular anatomy that parallel this unique characteristic has proved challenging.13 Illizarov postulated that the process of DO could be used to heal chronic osteomyelitis.14-16 Aronson posited that this assertion could be in part attributed to an enhanced vascular response in comparison with normal fracture healing leading to clinically evident increases in blood flow.5 Subsequent examination of this observation is rooted in the scintigraphic evaluation of blood flow in long bone canine models. In long bone FxR a 5-7-fold peak in blood flow occurring between 7-14 days has been observed, followed by a prolonged remodeling period before a return to near pre-fracture flow rates.17,18 Aronson demonstrated vascular flow outcomes in DO. He reported a peak in blood flow 8-10 times normal, occurring one week after osteotomy followed by a plateau elevation at four weeks of 4 times normal. Finally, he demonstrated increased blood flow was maintained at approximately 2 times normal as far out as five months post-operatively.5,6 This observation was subsequently corroborated in the rat mandible by serial histological blood vessel counting.7 Such an endeavor is commendable as it represents a clear attempt to quantify the anatomic changes in vascular response; yet, intrinsic drawbacks limit histological examination from adequately representing complex vascular anatomy. Inherent technicalities oblige the observer to make inferences about 3-dimensional structures from 2-dimensional serial slides.13 Further, it is protracted and time-consuming leading to studies with small numbers and low statistical power. Comparatively, we have previously utilized μCT after vessel perfusion to provide 3-dimensional quantitative analysis of mandibular vascular networks to generate control data germane to this line of inquiry.9,19,20 This is an accurate, non-destructive, rapid, stereological assessment of vascular network microarchitecture dependant on automated computer algorithms capable of accurately quantifying and assessing differences in vessel volume and projection.13
Here we make use of this powerful tool for the quantification and 3-dimensional analysis of the vascular responses after mandibular DO and FxR. We aim to offer computational anatomic metrics to gain insight into the understanding of how mechanical force impacts vascular responses by providing volumetric data highlighting unique differences between these two distinct processes.
We hypothesize that the metrics of vascular density will be significantly higher in DO compared to those same measures observed in fracture healing after bony union in the murine mandible. Furthermore, we posit that μCT after vessel perfusion will demonstrate 3-dimensional imaging substantiating the enhanced vascularity of the regenerative processes of DO in comparison to the reparative processes of fracture healing.
Materials and Methods
Adult male Sprague-Dawley rats weighing approximately 400 g were paired in cages and maintained in a pathogen-free environment on a 12-hour light/dark schedule. Rats were fed standard hard chow and water ad libitum during a seven-day acclimation period prior to surgery. All animal procedures were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and approved by the University of Michigan Committee on the Use and Care of Animals.
Surgical Procedure and Device Placement
Preoperative subcutaneous injections of Gentamycin (5 mg/kg), Buprenorphine (0.03 mg/kg) and Lactated Ringer’s solution (25cc/kg) were administered prior to surgery. Anesthesia was achieved with the inhalation of an oxygen/isoflurane mixture. The animals were prepared for surgery, and underwent placement of custom titanium external mandibular fixator devices followed by surgical osteotomy as previously described.21
The critical size defect utilized in our DO model is predicated on our previous experimental experience demonstrating that gradual distraction to 5.1 mm with a 28 day consolidation period consistently resulted in bony healing across the entire regenerate. Comparatively, defects created using acute separation to 5.1 mm or greater consistently formed fibrous unions. Previous data also demonstrated that a 2.1 mm partially reduced fracture created by acute separation consistently healed by secondary bone healing. This is consistent with fracture repair without the aid of DO after an analogous 28-day consolidation period, thus defining our FxR model.2
Postoperative Procedures
The FxR group (n=12) had a fixed 2.1 mm fracture gap set four hours after surgery. This was accomplished by turning the distraction screw clockwise a total of seven times.
The DO group (n=9) underwent distraction after 4 days of latency. One 180-degree clockwise turn of the distraction screw corresponded to a 0.3-mm separation of the osteotomy fronts. Active distraction began on the evening of post-operative day 4 and went through the evening of post-operative day 12. A total of 17 half-turns were performed on an every-12-hour interval, resulting in a maximum 5.1-mm distraction gap. No analgesic or sedation was required during the distraction.
Postoperative Animal Care
Animals were housed one per cage and fed moist chow with Hill’s high-calorie diet (Columbus Serum, Columbus, Ohio) and water ad libitum. Two postoperative doses of Gentamycin (5 mg/kg subcutaneously every 12 hours) were given as well as continuation of Buprenorphine (0.03 mg/kg) and Lactated Ringer’s solution (10cc) subcutaneously every 12 hours through postoperative day 4, and as needed thereafter. Weights were monitored daily and diets adjusted as needed. Pin care was performed with Silvadene (Monarch Pharmaceuticals, Inc., Bristol, Tenn.) every other day. Maxillary incisors were clipped weekly due to overgrowth from cross bite and staples were removed by postoperative day 10. Animals completed a 40-day recovery period prior to perfusion and sacrifice.
Perfusion Protocol
All rats were anesthetized prior to thoracotomy and underwent left cardio-ventricular catheterization. Perfusion with heparinized normal saline followed by pressure fixation with normal buffered formalin solution ensured euthanasia. After fixation, the vasculature was injected with Microfil (MV-122, Flow Tech, Carver, MA). Mandibles were harvested and demineralized using Cal-Ex II (Fisher Scientifics; Fairlawn, NJ), a formic acid solution. Leeching of mineral was confirmed with serial radiographs to ensure adequate demineralization prior to scanning.
Micro-Computed Tomography
Specimens were scanned at 18μm voxel size with μCT. We have previously utilized this voxel size and have found it optimal to adequately resolve small murine mandibular vessel networks. The region of interest was defined as a distance measuring 2.1 mm after the third molar for the FxR group and 5.1 mm for the DO group, corresponding to the surgical site of the osteotomy and respective gap distance. It should be noted that this difference in geometry is accounted for in our calculations. We only report radiomorphometrics as calculated percentages of the region of interest or comparisons of vascular parameters per millimeter of region of interest. For example, one metric that is not reported is Vessel Volume (VV) or the actual volume of vessels in the entire region of interest, instead we report Vessel Volume Fraction (VVF), which is the percentage of vessel density in the region of interest and is thus comparable regardless of differences in dimensions of analyzed regions. The other metrics are given per cubic millimeter of region of interest therefore; again, these would be comparable despite differences in the entire volume. Analysis of the left hemimandible region of interest (ROI) was then performed with MicroView 2.2 software (GE Healthcare, Milwaukee, WI.). Contours were defined highlighting the regions of interest using the spline function. Analysis of vascularity in this region is accomplished by setting a global grayscale threshold of 1000 to differentiate vessels from surrounding tissue as previously described.19 Using this information, MicroView allocates only the number of voxels above this threshold and uses stereologic algorithms to assign relative densities to the voxels based on the contrast content within the vessels. The ROI was analyzed for vessel volume fraction, and vessel number. While vessel volume fraction depicts the fraction of bone regenerate occupied by the volume of vessels within the ROI tissue, vessel number is a reflection of the actual number of vessels per mm within the ROI. Vessel volume fraction is calculated based on the voxel size and the number of segmented voxels in the 3-D image after application of the binarization threshold . To calculate vessel number, a segmented volume is skeletonized, leaving just the voxels above our binarization threshold at the midaxes of the vascular structures. The vessel number is defined as the inverse of the mean spacing between the midaxes of the structures in the segmented volume. Vessel thickness is measured by calculating an average of the local voxel thicknesses within the vessel. Local voxel thickness is defined as the diameter of the largest sphere that both contains the point regardless of position within the sphere, and is completely within the structure of interest. This distinguishes the blood vessel from the background space surrounding the object.13 It is worth noting that vessel thickness, in this case, defines the diameter of the vessel and not the thickness of the vessel wall. In addition, for qualitative comparison, 3-dimensional visualization of vascular anatomy was accomplished utilizing maximal intensity projections (MIP’s). This enables instant volume rendering of a volumetric data set yielding a 3-dimensional representation of the scanned specimen.
Stastical Analysis
The independent samples t-test was used to analyze differences between group means (SPSS version 16.0; SPSS, Inc., Chicago, IL.). Vascular metrics were compared between groups and reported as their respective means with significance indicated at p < .05.
Results
All animals tolerated the osteotomy procedure without incident. Gross union of the left hemi-mandibles was verified upon harvest prior to μCT scanning. Two animals were excluded from the study due to poor perfusion in the FxR group.
Qualitative Assessments
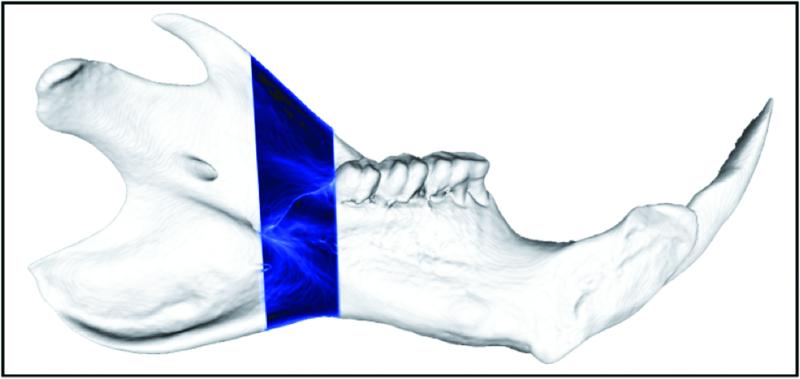
Representative MIP’s are shown demonstrating 3-dimensional microangiograms of the vascular networks. For orientation purposes, a schematic is shown outlining the figure of the normal left hemi-mandible with the region of interest highlighted for visual comparison (Figure 1).
Figure 1.
Schematic outlining the figure of the normal left hemi-mandible with the region of interest highlighted for visual comparison.
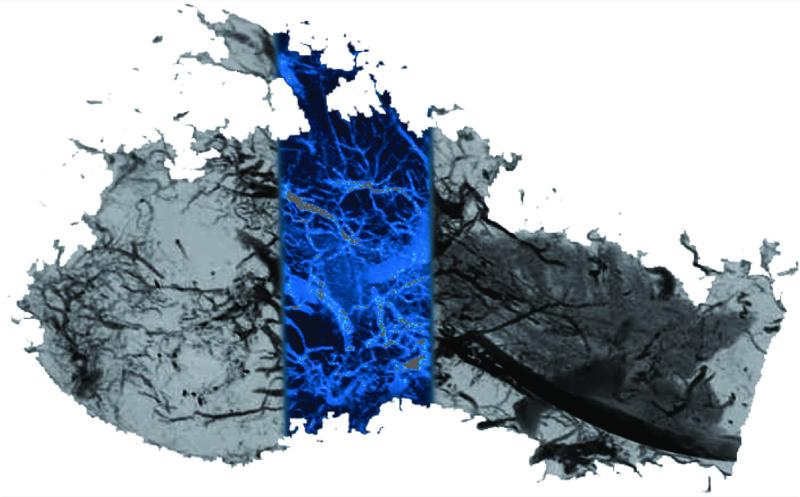
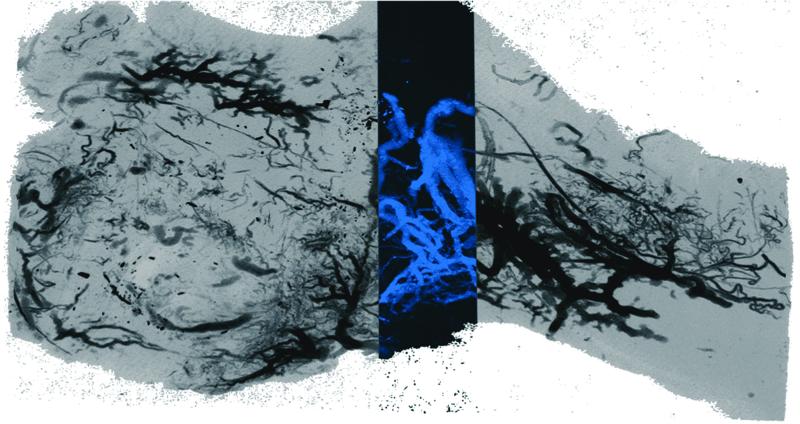
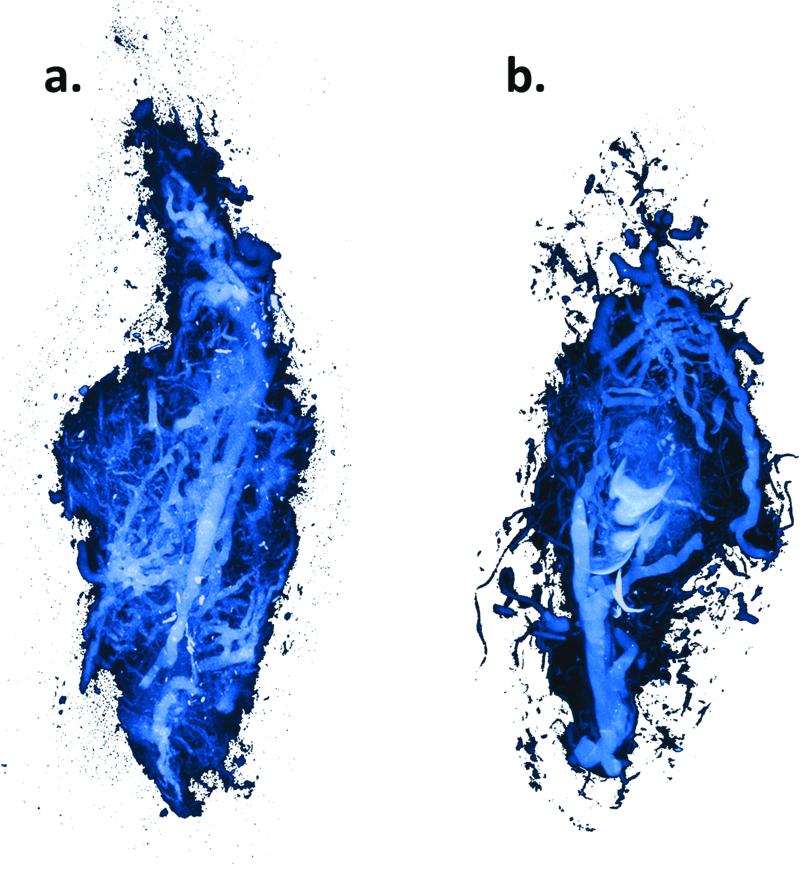
Appraisal of mandibular vascular networks in the DO group characteristically revealed enhanced vascularity filling the regenerate area from condyle to mandibular notch, with clearly visualized vessels of all sizes seen throughout the distracted regenerate. Both contributions from the central medullary vessels, and dense contributions of small vessels from surrounding periosteal networks appear to fill the regenerate area in a uniformly distributed pattern (Figure 2). Comparatively, in the FxR group, besides less vascular density, there is also a more centralized, convergent pattern at the fracture repair site (Figure 3). These findings are clearly visualized in the coronal sections. (Figure 4) Similar sources of vascularity are observed in both groups. The dominant medullary vessels receive contributions from the surrounding coronoid, condylar and angular vessel networks, though these appear much less dense in the FxR group.22
Figure 2.
DO saggital Maximal Intensity Projection image after vessel perfusion demonstrating a robust vascular density.
Figure 3.
FxR saggital Maximal Intensity Projection image after vessel perfusion.
Figure 4.
Coronal Maximal Intensity Projections. a) DO, b) FxR.
Quantitative Assessments
Stereological analysis demonstrated statistically significant increases in the DO vascular density metrics when compared to FxR (Figure 5,6). Our quantitative results indicate that vascular density as reflected by these metrics is enhanced beyond that of normal FxR in DO after complete bony union at our 40 day mark. These results demonstrate a remarkable quantifiable distinguishing feature between these two processes.
Figure 5.
Vessel Volume Fraction graph demonstrating a statisticaly significant increase in distracted vascularity compared to FxR. Error bars denote the standard error of the means (SEM) for DO vs FxR (SEM=%0.63, %0.96), and p = 0.030.
Figure 6.
Vessel Number graph demonstrating a statisticaly significant increase in distracted vascularity compared to FxR. Error bars denote the SEM for DO vs FxR (SEM=0.86 mm−1, 0.06 mm−1) and p = 0.014.
Discussion
Ilizarov considered DO to be a regenerative process that was to be distinguished from the reparative process of fracture repair. In fact, he used the augmented vascular response associated with DO as a therapeutic means by which to address issues of both infection and non-union.14-16 Reportedly, in post-WWII Siberia, before the ubiquitous use of antibiotics, he had success in treating active cases of osteomyelitis by using DO proximal to an infected site. Evidently, he attributed his success in part to the reaction of the vascular system— “washing the infection from relatively hypovascular bone”.5 Since these initial observations, inherent challenges with visualizing and quantifying changes in the vascular responses to both DO and fracture repair have limited our full understanding of the role of vascularity in regeneration and repair of bone.
Aronson examined the validity of Ilizarov’s observations of increased blood flow to the DO lengthened bone by scintigraphic examination in distracted canine long bone models. He confirmed an increase in initial amplitude of blood flow to the lengthened tibia; however, he could not show statistically significant differences regarding temporal and spatial patterns of blood flow when compared to fracture repair. His inability to demonstrate these differences can be plausibly attributed to the limitations of Tc Scintigraphy analyses and the small groups used in the experiments.5
Our experience with μCT after vessel perfusion has enabled us to study vascular responses from a different perspective and generate valuable data substantiating the initial observations of Ilizarov. While vascular analysis has largely relied on flow metrics to ascertain quantitative data about vascular responses, our focus was redirected towards examination of changes in vascular network density during vascular responses. This perspective affords us a way to observe vascular phenomenon at a given point in time and generate calculated data in a manner amenable to a small animal model thus overcoming some of the cumbersome shortcomings of flow analyses and allowing for an adequately powered quantitative study. Specifically, in this case, our results demonstrate a 1.93 fold increase in vessel volume fraction and a 1.73 fold increase in vessel number within our regenerate area existing at 40 days post osteotomy in the DO group compared to the FxR group. These findings clarify and substantiate Aronson’s long bone flow observations and those posited initially by Ilizarov, specifically, at our measured time point. These findings also, for the first time, allow for the 3-dimensional comparison of the form (vascular microarchitecture) that follows the reported function (enhanced vascular flow) in a murine model comparing DO and FxR.
It is also worth noting that the basic science literature has been slow in reflecting that the utility of DO has clinically shifted from a largely orthopaedic procedure to what we observe today as a procedure utilized frequently in the craniofacial skeleton. This poses an interesting consideration in that the simple extrapolation of findings in the long bone with the assumption of analogous events in the mandible may be erroneous. Certain distinctions are worth clarifying in these regards. The mandible is a flat membranous bone that is predominantly cortical and highly dependent on a haversion based vascular network. These characteristics are in contradistinction to the tubular, predominantly trabecular structure of the long bone which principally gets its blood supply from a medullary system. These findings exemplify the unique structural and functional complexity of the mandible and lend support for establishing a foundation of evidence specific to the biological phenomenon occurring in the mandible. Here we have succeeded in establishing baseline quantitative analyses regarding the mandibular vascular responses to both FxR and DO. Further, our comparison between these two processes mirrors findings in long bones, at least at our indicated time period, thus also establishing a basis of comparison between long bone and the mandible.
Here we provide 3-dimensional, visual and stereological analysis of the vascular responses to mandibular DO and FxR, and highlight—perhaps the most distinguishable vascular difference between these two processes; a quantifiable enhancement in vascular density that may reasonably be attributable to mechanical force. We have successfully illustrated and calculated an enhancement in vascular network density evidenced by an increased vessel volume fraction and vessel number observed 40 days after osteotomy in mandibular DO compared to successful FxR. Our results demonstrate increases in vessel volume fraction (5.4% vs. 2.8%, p = 0.030), and vessel number (0.86 mm−1 vs. 0.50 mm−1, p = 0.014) within our regenerate area in the DO group compared to the FxR group.
Our qualitative analysis also depicts unique differences between DO and FxR. An organized array of vascularity existing throughout the regenerate area is observed in the DO group as opposed to a more centralized pattern observed in the FxR specimens. Both groups exhibited an array of vessels of all sizes, and quantitative comparison of vessel diameter as measured by vessel thickness did not reveal significant differences (150 μm vs. 52 μm, p = 0.234). This taken together with the overall increase in vessel density would not be surprising as we would expect that the generation of an environment under a uniform vector of mechanical force would yield a uniform enhanced regenerative vascular response. This uniform response was indeed demonstrated by vessels of all sizes throughout the ROI. This finding may also be correlated to the predominance of intramembranous ossification found in DO as opposed to the largely endochondral reparative response existing in FxR. Intramembranous ossification is thought to be a more uniform mechanism of bone development.14,15 This is in contradistinction to FxR, where healing is more dependant on resorption of a cartilaginous callous, vascularization, and secondary bone formation; a more random process lacking the uniformity noted in intramembranous ossification.1,23
In conclusion, we have succeeded in demonstrating, quantifying, and comparing the regenerative vascular response as a consequence of DO, from the reparative vascular response resulting from FxR. Our findings both substantiated the theoretical assertions in long bone studies as well as provided evidence for an important distinction. DO demonstrated a more robust and significantly greater vascular response than that of FxR as measured by vessel number and vessel volume fraction. Furthermore, we observed a more uniform arrangement of vascular networks in DO in comparison to FxR, conceivably, due to the mechanical exertion of a vector of force. Finally, we have succeeded in clarifying distinctions between the mandible and long bone vascular response and report quantitative vascular metrics specific to mandibular analysis for both DO and FxR after successful consolidation. These findings set the foundation for investigation of the impact of vascular responses in mandibular bone regeneration and repair. Having the pre-requisite knowledge of a calculated vascular density needed to supply the metabolic demands required to heal bone in these two scenarios, we hope to establish means to meet and exceed that demand with therapeutic interventions in future studies. We hope to use these calculated insights to selectively optimize vascular responses in translatable, clinically relevant scenarios, such as cases of radiation damage, ballistic injury, and osteomyelitis, where FxR and DO currently have limited reconstructive capabilities.
Acknowledgements
Funding was provided by National Institutes of Health grant RO1 CA 12587-01 to Steven R. Buchman, M.D. The authors thank Elizabeth R. Razdolsky and Melody Merati for technical assistance during surgery, animal care, and Micro–Computed Tomographic analysis.
Funding supported by: NIH RO1 #CA12587-01 “Optimization of Bone Regeneration in the Irradiated Mandible” to PI: Steven R. Buchman, MD.
Footnotes
No commercial association or financial interests exist by any author.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Alexis Donneys, University of Michigan Department of Plastic Surgery, Ann Arbor, MI.
Catherine N. Tchanque-Fossuo, University of Michigan Department of Plastic Surgery, Ann Arbor, MI.
Aaron S. Farberg, University of Michigan Medical School, Ann Arbor, MI.
Sagar S. Deshpande, University of Michigan, Ann Arbor, MI
Steven R. Buchman, University of Michigan Department of Plastic Surgery, Ann Arbor, MI.
References
- 1.AI-Aql ZS, Alagl AS, Graves DT, Gerstenfeld LC, Einhorn TA. Molecular Mechanisms Controlling Bone Formation during Fracture Healing and Distraction Osteogenesis. Journal of Dental Research. 2008;87(2):107–118. doi: 10.1177/154405910808700215. Available at: http://jdr.sagepub.com/cgi/doi/10.1177/154405910808700215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tong L, Buchman SR, Ignelzi MA, Rhee S, Goldstein SA. Focal adhesion kinase expression during mandibular distraction osteogenesis: evidence for mechanotransduction. Plastic and reconstructive surgery. 2003;111(1):211–22. doi: 10.1097/01.PRS.0000033180.01581.9A. discussion 223-4. Available at: http://www.ncbi.nlm.nih.gov/pubmed/12496582. [DOI] [PubMed] [Google Scholar]
- 3.Fang TD, Salim A, Xia W, et al. Angiogenesis is required for successful bone induction during distraction osteogenesis. Journal of bone and mineral research: the official journal of the American Society for Bone and Mineral Research. 2005;20(7):1114–24. doi: 10.1359/JBMR.050301. Available at: http://www.ncbi.nlm.nih.gov/pubmed/15940364. [DOI] [PubMed] [Google Scholar]
- 4.McCarthy JG, Stelnicki EJ, Mehrara BJ, Longaker MT. Special Topic Distraction Osteogenesis of the Craniofacial Skeleton. New York: 2000. pp. 1812–1827. [DOI] [PubMed] [Google Scholar]
- 5.Aronson J. Temporal and spatial increases in blood flow during distraction osteogenesis. Clinical orthopaedics and related research. 1994;(301):124–31. Available at: http://www.ncbi.nlm.nih.gov/pubmed/8156663. [PubMed]
- 6.Aronson J. Experimental and clinical experience with distraction osteogenesis. The Cleft Palate-Craniofacial Journal. 1994 doi: 10.1597/1545-1569_1994_031_0473_eacewd_2.3.co_2. Available at: http://www.cpcjournal.org/doi/abs/10.1597/1545-1569(1994)031<0473:EACEWD>2.3.CO;2. [DOI] [PubMed]
- 7.Rowe NM, Mehrara BJ, Luchs JS, Dudziak ME, Steinbrech DS, Illei PB, Fernandez GJ, Gittes GK, Longaker MT. Angiogenesis During Distraction Osteogenesis. Annals of Plastic Surgery. 1999;42(5):470–475. doi: 10.1097/00000637-199905000-00002. Available at: http://journals.lww.com/annalsplasticsurgery/abstract/1999/05000/angiogenesis_during_mandibu lar_distraction.2.aspx. [DOI] [PubMed] [Google Scholar]
- 8.Pacicca D. Expression of angiogenic factors during distraction osteogenesis. Bone. 2003;33(6):889–898. doi: 10.1016/j.bone.2003.06.002. Available at: http://linkinghub.elsevier.com/retrieve/pii/S8756328203002382. [DOI] [PubMed] [Google Scholar]
- 9.Donneys A, Farberg A, Tchanque-Fossuo C, Buchman SR, et al. Angiogenic Effect of Deferoxamine in Mandibular Distraction Osteogenesis. Plastic and reconstructive surgery. 2010;125(6S):130. doi: 10.1097/PRS.0b013e31824422f2. Available at: http://www.ncbi.nlm.nih.gov/pubmed/20736650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wan C, Gilbert SR, Wang Y, et al. Activation of the hypoxia-inducible factor-1alpha pathway accelerates bone regeneration. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(2):686–91. doi: 10.1073/pnas.0708474105. Available at: http://www.ncbi.nlm.nih.gov/pubmed/18184809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shen X, Wan C, Ramaswamy G, et al. Prolyl hydroxylase inhibitors increase neoangiogenesis and callus formation following femur fracture in mice. Journal of orthopaedic research : official publication of the Orthopaedic Research Society. 2009;27(10):1298–305. doi: 10.1002/jor.20886. Available at: http://www.ncbi.nlm.nih.gov/pubmed/19338032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Street J, Bao M, DeGuzman L, et al. Vascular endothelial growth factor stimulates bone repair by promoting angiogenesis and bone turnover. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(15):9656–61. doi: 10.1073/pnas.152324099. Available at: http://www.ncbi.nlm.nih.gov/pubmed/12118119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Duvall CL, Taylor WR, Weiss D, Guldberg RE. Quantitative microcomputed tomography analysis of collateral vessel development after ischemic injury. American journal of physiology. Heart and circulatory physiology. 2004;287(1):H302–10. doi: 10.1152/ajpheart.00928.2003. Available at: http://www.ncbi.nlm.nih.gov/pubmed/15016633. [DOI] [PubMed] [Google Scholar]
- 14.Ilizarov GA. The tension-stress effect on the genesis and growth of tissues: Part I. The influence of stability of fixation and soft tissue preservation. Clinical orthopaedics and related research. 1989;(238):249–281. Available at: http://www.ncbi.nlm.nih.gov/pubmed/2912628. [PubMed]
- 15.Ilizarov GA. The tension-stress effect on the genesis and growth of tissues: Part II. The influence of the rate and frequency of distraction. Clinical orthopaedics and related research. 1989;(239):263–85. Available at: http://www.ncbi.nlm.nih.gov/pubmed/2912628. [PubMed]
- 16.Ilizarov GA, Ledayev VI. The replacement of long tubular bone defects by lengthening distraction osteotomy of one of the fragments. Clinical orthopaedics and related research. 1992;280:7–10. [PubMed] [Google Scholar]
- 17.Williams E, Rand J, An K, Chao E, Kelly P. The early healing of tibial osteotomies stabilized by one-plane or two-plane external fixation. J. Bone Joint Surg. Am. 1987;69(3):355–365. Available at: http://www.ejbjs.org/cgi/content/abstract/69/3/355. [PubMed] [Google Scholar]
- 18.Wu J, Shyr H, Chao E, Kelly P. Comparison of osteotomy healing under external fixation devices with different stiffness characteristics. J. Bone Joint Surg. Am. 1984;66(8):1258–1264. Available at: http://www.ejbjs.org/cgi/content/abstract/66/8/1258. [PubMed] [Google Scholar]
- 19.Jing X, Farberg A, Monson L, Buchman SR. Radiomorphometric Quantitative Analysis of Vasculature in Rat Mandible Utilizing Microcomputed Tomography. Plastic and Reconstructive Surgery. 2009;124(4S):26–27. Available at: http://journals.lww.com/plasreconsurg/Citation/2009/10002/Radiomorphometric_Quantitative_Analysis_of.32.aspx. [Google Scholar]
- 20.Donneys A, Tchanque-Fossuo CN, Farberg AS, Buchman SR, et al. Quantitative Analysis of Vascular Response after Mandibular Fracture Repair Utilizing Micro-Computed Tomography with Vessel Perfusion. Plastic and Reconstructive Surgery. 2010;126(4S):5–6. doi: 10.1097/PRS.0b013e318208f3c1. Available at: http://journals.lww.com/plasreconsurg/Abstract/2010/10002/Quantitative_Analysis_of_Vascular_Response_after.7.aspx. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Buchman SR, Ignelzi MA, Wilensky J, et al. Unique Rodent Model of Distraction Osteogenesis of the Mandible. Annals of Plastic Surgery. 2002;49(5):511–519. doi: 10.1097/00000637-200211000-00012. [DOI] [PubMed] [Google Scholar]
- 22.Huelke DF, Castelli W a. The blood supply of the rat mandible. The Anatomical Record. 1965;153(4):335–341. doi: 10.1002/ar.1091530402. Available at: http://doi.wiley.com/10.1002/ar.1091530402. [DOI] [PubMed] [Google Scholar]
- 23.Glowacki J. Angiogenesis in fracture repair. Clinical orthopaedics and related research. 1998;355:S82. doi: 10.1097/00003086-199810001-00010. Available at: http://journals.lww.com/corr/Abstract/1998/10001/Angiogenesis_in_Fracture_Repair.10.aspx. [DOI] [PubMed] [Google Scholar]