Abstract
The interactions between the plant hormones auxin and cytokinin throughout plant development are complex, and genetic investigations of the interdependency of auxin and cytokinin signaling have been limited. We have characterized the cytokinin sensitivity of the auxin-resistant diageotropica (dgt) mutant of tomato (Lycopersicon esculentum Mill.) in a range of auxin- and cytokinin-regulated responses. Intact, etiolated dgt seedlings showed cross-resistance to cytokinin with respect to root elongation, but cytokinin effects on hypocotyl growth and ethylene synthesis in these seedlings were not impaired by the dgt mutation. Seven-week-old, green wild-type and dgt plants were also equally sensitive to cytokinin with respect to shoot growth and hypocotyl and internode elongation. The effects of cytokinin and the dgt mutation on these processes appeared additive. In tissue culture organ regeneration from dgt hypocotyl explants showed reduced sensitivity to auxin but normal sensitivity to cytokinin, and the effects of cytokinin and the mutation were again additive. However, although callus induction from dgt hypocotyl explants required auxin and cytokinin, dgt calli did not show the typical concentration-dependent stimulation of growth by either auxin or cytokinin observed in wild-type calli. Cross-resistance of the dgt mutant to cytokinin thus was found to be limited to a small subset of auxin- and cytokinin-regulated growth processes affected by the dgt mutation, indicating that auxin and cytokinin regulate plant growth through both shared and separate signaling pathways.
The hormones auxin and cytokinin control plant development through a multitude of complex interactions. The balance between auxins and cytokinins controls the formation of roots, shoots, and callus tissue in vitro (Skoog and Miller, 1957), the outgrowth of shoot axillary buds (Sachs and Thimann, 1967; Cline, 1994, 1996; Tamas, 1995), and the formation of lateral roots (Wightman et al., 1980; Hinchee and Rost, 1986). Cytokinins synergistically enhance auxin-induced ethylene production in pea stem sections (Fuchs and Lieberman, 1968) and in tobacco leaf discs (Aharoni et al., 1979), whereas they inhibit the auxin-induced elongation of sunflower (DeRopp, 1956) and soybean (Vanderhoef and Stahl, 1975) hypocotyl segments. The mode of interaction between auxins and cytokinins can therefore be synergistic, antagonistic, or additive and is dependent on the type of tissue and on the plant species in which the interaction occurs. Although the molecular mechanisms underlying most of these auxin-cytokinin interactions are unknown, they are thought to include mutual control of auxin and cytokinin metabolism, interactions in the control of gene expression, and post-transcriptional interactions (for review, see Coenen and Lomax, 1997).
Mutants that are disrupted in their response to hormones or environmental signals can often be used to demonstrate or exclude interactions between signal transduction pathways. For example, light-insensitive mutants of Arabidopsis have been used to show that the inhibition of hypocotyl elongation by cytokinins does not depend on light effects (Su and Howell, 1995), and that ethylene-insensitive Arabidopsis mutants have helped to establish that cytokinin effects on hypocotyl and root growth in intact seedlings are partially mediated by ethylene (Cary et al., 1995; Su and Howell, 1995).
Research on auxin and cytokinin signal transduction has benefited from an increasing number of hormone-response mutants identified in a number of different plant species (Reid, 1990; Hobbie and Estelle, 1994). Some of these mutants appear to be affected exclusively in either auxin (Blonstein et al., 1991; Hobbie and Estelle, 1995; Simmons et al., 1995) or cytokinin (Deikman and Ulrich, 1995) responses. However, many of the mutants isolated in screens for reduced sensitivity to one class of exogenous hormones are cross-resistant to at least one other class of plant hormones, reflecting the strongly interactive nature of hormone signaling (Hobbie and Estelle, 1994). For example, the auxin-resistant Arabidopsis mutants aux1, axr1, and axr3 show increased resistance to ethylene and cytokinin as well as to auxin (Hobbie and Estelle, 1994; Timpte et al., 1995; Leyser et al., 1996), and the auxin-resistant axr2 mutant was also found to be resistant to ethylene and ABA (Wilson et al., 1990). However, with the exception of the auxin-overresponding axr3 mutant (Leyser et al., 1996), the characterization of auxin-cytokinin cross-resistance in these Arabidopsis mutants has thus far been limited to a single assay, namely, the inhibition of root elongation by exogenously applied hormones. Extending this approach to a broader range of physiological responses may therefore help to unravel the complexities of auxin-cytokinin interactions.
The single-gene, recessive diageotropica (dgt) mutant of tomato (Lycopersicon esculentum Mill.) exhibits pleiotropic phenotypic effects that include reduced apical dominance; stunting of root and shoot growth; dark-green, hyponastic leaves; thin, rigid stems; and primary and adventitious roots that lack lateral root primordia unless the root apex has been severely damaged (Zobel, 1972, 1973). Much of the complex dgt phenotype can be explained by the reduced sensitivity of mutant tissues to auxin, which has been demonstrated in dgt hypocotyl segments and roots (Kelly and Bradford, 1986; Muday et al., 1995). The mutation is thought to affect the auxin response mechanism rather than auxin metabolism or transport because dgt hypocotyls are also insensitive to 2,4-D (Kelly and Bradford, 1986), which is less efficiently metabolized and transported than IAA. Furthermore, dgt shoot tips contain normal auxin levels (Fujino et al., 1988) and the velocity of auxin transport in dgt shoots and roots is normal (Daniel et al., 1989; Muday et al., 1995). Increased auxin transport capacity found in both roots and shoots of dgt seedlings (Daniel et al., 1989; Muday et al., 1995) may be related to the altered vascular differentiation in the mutant (Zobel, 1974). The reduced auxin responsiveness of rapidly auxin-inducible genes, such as tomato homologs of the Small Auxin Up-Regulated RNA, ACC synthase, and IAA gene families, in dgt hypocotyl segments (Mito and Bennett, 1995; C. Coenen, A. Nebenführ, and T.L. Lomax, unpublished data) indicates that the Dgt gene product may function in an early event of auxin signaling. Reduced labeling of an auxin-binding protein in dgt hypocotyls (Hicks et al., 1989) was later found not to be correlated with the dgt phenotype (Lomax et al., 1993); therefore, the nature of the signaling event affected by the dgt mutation remains unknown. The characterization of cross-resistance of the dgt mutant to other classes of plant hormones has been limited to the demonstration that shoots of the dgt mutant show normal responses to GA (Zobel, 1974; Scott, 1988) and ethylene (Zobel, 1973).
We have characterized the effects of the dgt mutation on the cytokinin responsiveness of etiolated seedlings and green plants and on auxin and cytokinin responses of tissues cultured in vitro. The results demonstrate that the occurrence of auxin-cytokinin cross-resistance is highly dependent on the type of tissue and on the specific hormone response studied.
MATERIALS AND METHODS
For experiments testing the effect of long-term cytokinin application on wild-type and dgt tomato (Lycopersicon esculentum Mill.) development in the light, we used dgt and the isogenic parent VFN8, which were originally a gift from Dr. K. Bradford (University of California, Davis). The dgt mutant extensively back-crossed into the more fertile Ailsa Craig (AC) background (originally obtained from Dr. C.M. Rick, University of California, Davis) was used for studies of etiolated seedlings because of the large amounts of mutant seed required in these experiments. The dgt line used in tissue-culture experiments was an ethyl methanesulfonate-induced allele of the dgt mutant in the background line VF36, which was originally obtained from Dr. R. Zobel (Cornell University, Ithaca, NY). The morphological traits of dgt are the same in the AC, VFN8, and VF36 backgrounds (data not shown). All seeds used in this study came from field plants propagated by selfing at the Oregon State University botany farm.
Seedling Growth Measurements
For experiments on cytokinin-induced growth inhibition in intact seedlings, seeds were treated with 20% (v/v) household bleach for 10 min, rinsed in tap water, sown in plastic boxes (32 × 26 × 10 cm) onto two layers of Whatman No. 3 filter paper moistened with distilled water, and incubated for 2 d at 28°C in the dark. Germinated seeds with radicles of 3 to 5 mm in length were placed on agar plates (15 cm in diameter) containing 100 mL of 0.8% (w/v) Bacto-Agar (Difco, Detroit, MI), Murashige-Skoog salts (pH adjusted to 6.5 with KOH), and the indicated concentrations of the cytokinin BA. Sixteen seedlings were aligned on each plate with radicles pointing down, and the plates were incubated vertically at 28°C in the dark. After 3 d the root and hypocotyl lengths of each seedling were measured to the nearest millimeter with a ruler.
Ethylene Production
For experiments on cytokinin-induced ethylene biosynthesis in intact seedlings, 10 surface-sterilized seeds were sown in a 10-mL vial containing 1 mL of autoclaved agar medium prepared as described for growth measurements. The open vials were incubated for 5 d in Magenta boxes (7.5 × 7.5 × 10 cm, six vials to a box; Sigma) at 28°C in the dark. For the last 8 h of the 5th d, each vial was sealed with a serum stopper. One milliliter of the gas phase of each sample was analyzed on a gas chromatograph (model GC-8A, Shimadzu, Kyoto, Japan) equipped with a 4-foot Poropak Q-column (Waters) and a flame-ionization detector.
Long-Term Cytokinin Treatment
Seeds were surface sterilized, rinsed in tap water, and sown in Magenta boxes (7.5 × 7.5 × 10 cm) on absorbent paper (Kimtowels, Kimberly-Clark, Roswell, GA) wetted with aqueous solutions of 0, 3, 10, and 30 μm BA. In preliminary experiments it was determined that long-term treatments with 1 μm or less BA had little or no effect on the morphological characteristics of mature light-grown plants, whereas long-term treatment with 100 μm BA was lethal (data not shown). After 2 d in the dark at 28°C, the boxes were transferred to an incubator equipped with wide-spectrum fluorescent lights (General Electric). Seedlings were grown for 7 d at 28°C under a cycle of 16 h of light (50 μE PAR m−2 s−1) and 8 h of dark. Nine-day-old seedlings were transplanted into 5- × 6- × 6-cm plastic pots containing a soil-free potting mixture wetted with the appropriate BA solutions. The mixture consisted of 3 L of vermiculite:1 L of expanded clay:50 g of Osmocote (14:14:14, N:P:K):3 g of Micromax Micronutrients (Osmocote and micronutrients were obtained from Grace-Sierra Horticultural Products Co., Milpitas, CA). After 2 d more in the incubator, transplanted plants were grown in a greenhouse under natural light conditions at 24°C (day) and at 18°C (night). During this period the plants were watered with the appropriate BA solutions and fertilized as needed with Osmocote and micronutrients. Seven weeks after sowing, between 12 and 27 plants from each treatment were harvested for determination of total shoot fresh weight and hypocotyl and internode lengths.
Tissue Culture
Seeds (0.7 g per experiment) were surface sterilized in 80 mL of 50% (v/v) household bleach containing two drops of Tween 20. The bleach solution was removed by rinsing the seeds five times for 2 min each in 200 mL of sterile distilled water. Seeds (0.15 g) were then spread onto four layers of Whatman No. 1 filter paper wetted with 9 mL of sterile distilled water in a sterile plastic Petri dish (9 cm in diameter, 1.5 cm deep). The dishes were sealed with Parafilm and incubated at 28°C in a light incubator equipped with wide-spectrum fluorescent lights (General Electric). Seedlings were grown for 9 d under a cycle of 16 h of light (50 μE PAR m−2 s−1) and 8 h of dark.
Tissue-culture media contained 0.8% (w/v) agar, Murashige-Skoog salts and B vitamins, and the indicated concentrations of hormones. Hormone stocks were prepared as aqueous solutions by heating until dissolved and added before the media were autoclaved. Media (50 mL) were poured into sterile, plastic Petri dishes (10 cm in diameter, 1.5 cm deep) and left to cool and dry at room temperature overnight. Four hypocotyl explants of approximately 1 cm in length, cut from immediately below the cotyledons of 9-d-old light-grown seedlings, were placed in each culture dish. For experiments on organ formation, dishes were incubated under the same conditions as light-grown seedlings (see above). Photographs were taken after 1 month. For callus induction, dishes were incubated for 1 month at 28°C in the dark. To characterize growth of transferred callus, explants were placed onto plates containing 3 μm 2,4-D and 3 μm BA and incubated in the dark at 28°C for 1 month. Callus was then cut away from the original explants and callus pieces (0.5 × 0.5 × 0.5 cm) were transferred to fresh plates containing the same hormone concentrations (four pieces per plate). After 1 month the callus was cut into pieces as before and transferred to fresh plates containing hormone combinations as indicated, incubated for 1 month, and then harvested and weighed.
RESULTS
Cytokinin Responses in Etiolated Seedlings
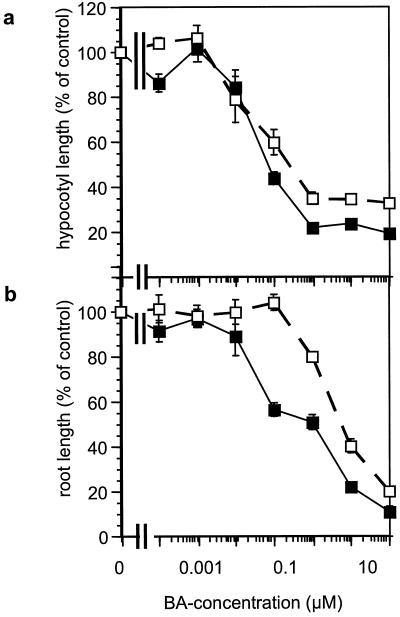
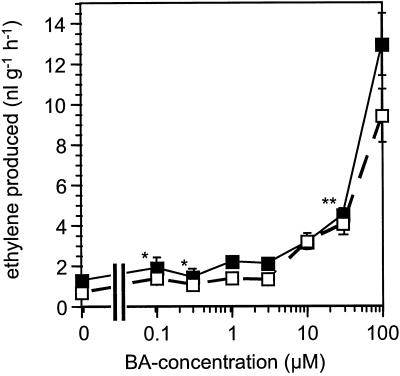
The dgt mutation affects auxin-induced root growth inhibition (Muday et al., 1995) and ethylene formation (Zobel, 1974) in intact seedlings. To assess the cytokinin sensitivity of these responses in the dgt mutant, we examined growth and ethylene synthesis in 5-d-old etiolated dgt and wild-type seedlings that had been exposed to the cytokinin BA for 3 d. Hypocotyls and roots of untreated dgt seedlings were shorter than those of wild-type seedlings (29 versus 41 mm for hypocotyls, and 28 versus 36 mm for roots). Whereas the sensitivity of dgt and wild-type hypocotyls to increasing concentrations of BA appeared similar (Fig. 1a), dgt roots were markedly less inhibited by 0.1 μm BA than were wild-type roots (Fig. 1b), indicating reduced cytokinin sensitivity in the mutant. As a second response of intact, young seedlings to cytokinin, we compared BA-induced ethylene synthesis and found that wild-type and dgt seedlings responded in a similar manner to relatively high (10–100 μm) concentrations of BA (Fig. 2).
Figure 1.
BA effect on length of hypocotyls (a) and roots (b) of 5-d-old etiolated seedlings. Wild-type (▪) and dgt (□) seeds were germinated for 2 d and then grown on BA media for 3 d. Error bars represent the ses from three independent experiments.
Figure 2.
BA-induced ethylene biosynthesis by etiolated wild-type (▪) and dgt (□) seedlings. Error bars represent the ses from three independent experiments (*, n = 3; **, n = 8; all others, n = 11).
Effects of Cytokinin on Shoot Growth in Green Plants
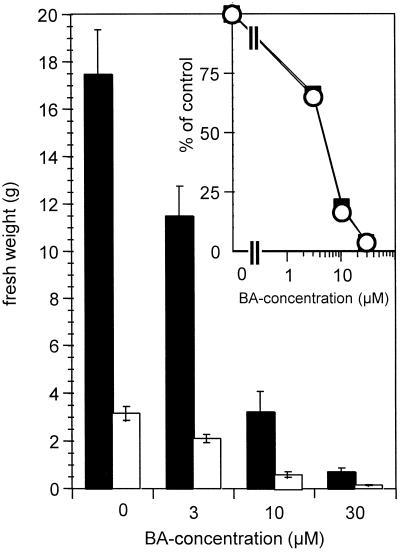
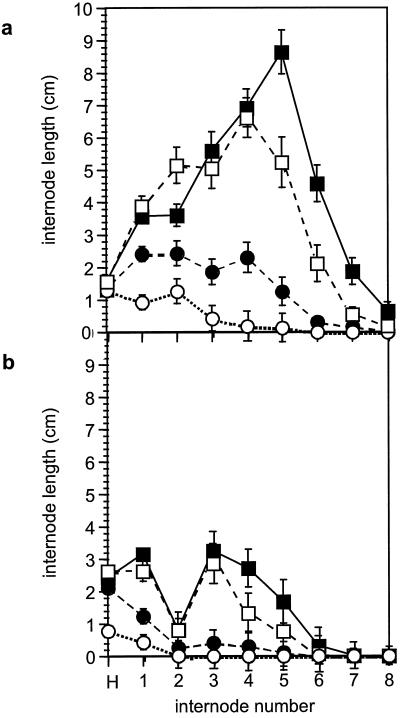
Long-term effects of cytokinin treatment on the development of wild-type and dgt shoots were compared in light-grown plants continuously watered with BA solutions. Untreated 7-week-old dgt plants had dramatically reduced shoot biomass as compared with wild-type plants (Fig. 3), but the sensitivity of fresh weight accumulation to BA in wild-type and dgt shoots was exactly the same (Fig. 3, inset). To specifically analyze the effects of BA and the dgt mutation on shoot elongation, we compared hypocotyl and internode lengths in wild-type and dgt plants grown at various BA concentrations. In contrast to the reduced hypocotyl lengths observed in etiolated dgt seedlings (see above), hypocotyls of untreated 7-week-old dgt plants were longer than wild-type hypocotyls (Fig. 4). Surprisingly, the cytokinin treatment did not affect hypocotyl lengths of mature dgt and wild-type plants (Fig. 4), indicating that the effects of cytokinin on hypocotyl elongation (Fig. 1a) are either transient or limited to etiolated plants. The only exception was the 30 μm BA treatment in dgt plants (Fig. 4), in which the shorter hypocotyls were probably due to severe overall growth retardation caused by additive effects of cytokinin and the dgt mutation. Internodes of untreated dgt plants were much shorter than wild-type internodes, and specifically the second internodes of dgt shoots were severely shortened compared with the other internodes (Fig. 4). The relative inhibition of internode elongation by BA was similar in wild-type and dgt plants (Fig. 4).
Figure 3.
Inhibition of shoot fresh weight accumulation by BA in 7-week-old light-grown wild-type (black bars) and dgt (white bars) plants. Error bars represent the ses from all harvested plants (n >12). Inset contains the same data expressed as percentage inhibition relative to untreated controls for both wild type (squares) and dgt (circles). Similar effects were observed in three independent experiments.
Figure 4.
Reduction of internode length by BA in 7-week-old light-grown wild-type (a) and dgt (b) plants watered with 30 (○), 10 (•), 3 (□), and 0 (▪) μm BA. Error bars represent ses (n >12). Effects were similar in three independent experiments.
Organ Regeneration from Hypocotyl Explants
To investigate auxin-cytokinin interactions in a variety of differentiation processes, we compared the regeneration of organs and callus production by wild-type and dgt hypocotyl explants in culture. Cultured wild-type hypocotyl explants regenerated vigorous and prolific roots and shoots in the absence of hormones (Fig. 5a, left). Hypocotyl explants of dgt were also able to regenerate both leaves and roots. However, these organs were much less prolific and vigorous than those formed by wild-type explants (Fig. 5a, right). Similar to intact dgt plants, roots regenerated by dgt hypocotyl explants did not form lateral roots. Like wild-type explants, dgt hypocotyl explants developed leaves at the apical end and roots at the basal end, indicating that the polarity of the hypocotyl segments was retained.
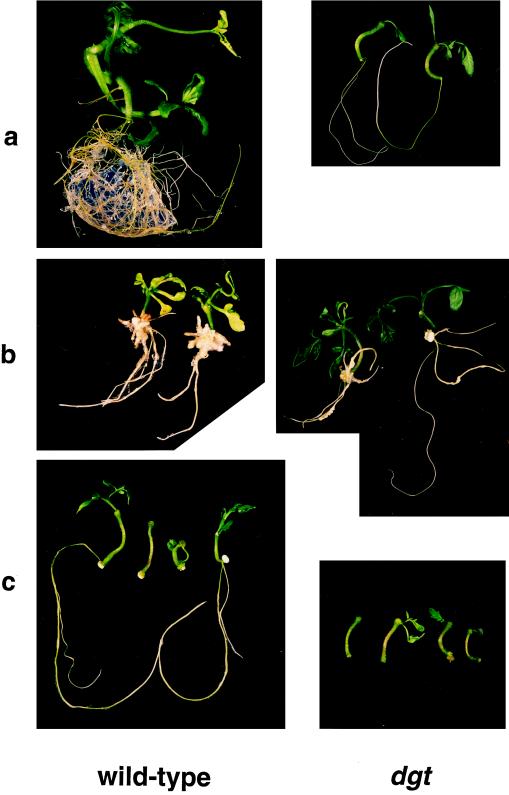
Figure 5.
Low levels of auxin or cytokinin differentially modulate organ regeneration in wild-type and dgt hypocotyl explants. Hypocotyl segments from 9-d-old light-grown seedlings were incubated on Murashige-Skoog media in the light for 4 weeks. a, Regeneration of shoots and roots from untreated wild-type and mutant hypocotyl segments; b, organ formation in the presence of 0.3 μm 2,4-D; and c, inhibition of organ formation by 0.3 μm BA.
In the presence of 0.3 μm BA, wild-type explants produced much weaker and smaller roots and leaves than the control explants, and root branching was nearly absent (Fig. 5c), resulting in a phenocopy of dgt regeneration (Fig. 5, compare a, right, with c, left). Organ formation by the already stunted dgt explants was further reduced by cytokinin treatment; therefore, roots were completely absent and the leaves produced were smaller than those of the controls (Fig. 5, compare c, right, with a, right).
In contrast to the cytokinin effects, the effects of auxin on morphogenesis in dgt and wild-type explants differed. Wild-type leaves produced in the presence of 0.3 μm 2,4-D were smaller and much more pale than those produced by untreated wild-type explants, and roots were severely thickened and shortened (Fig. 5, compare explants of a, left, and b, left), indicating that this auxin concentration was too high for proper organ regeneration in wild-type hypocotyl segments. In contrast, dgt leaves and roots regenerated in the presence of 0.3 μm 2,4-D were more vigorous and numerous than in untreated dgt explants (Fig. 5, compare explants of a, right, and b, right). Auxin treatment thus partially rescued the dgt phenotype with respect to leaf number and vigor, as well as root formation, although it did not restore root branching in dgt. Reduced auxin sensitivity of root formation in dgt explants was also apparent at higher concentrations of 2,4-D (Fig. 6). At 3 μm 2,4-D, mutant calli formed roots, whereas this concentration of 2,4-D completely suppressed root formation in wild-type explants.
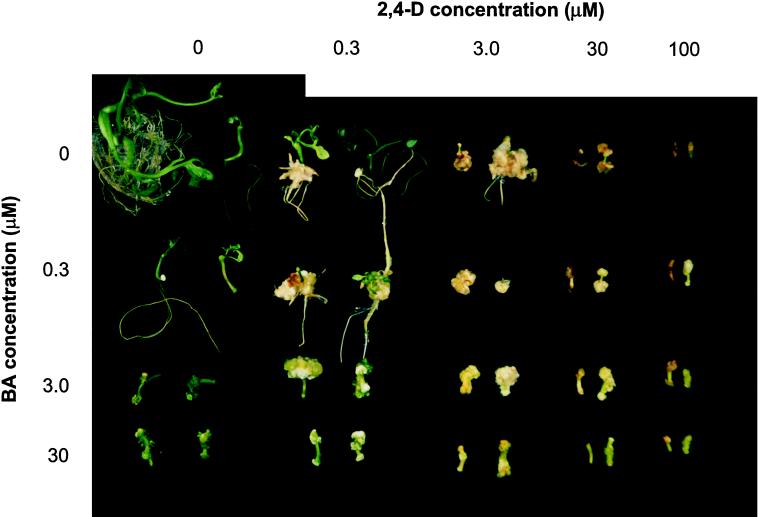
Figure 6.
Auxin (2,4-D) and cytokinin (BA) effects on callus production and root formation by wild-type and dgt hypocotyl segments. Hypocotyl segments from 9-d-old, light-grown seedlings were incubated for 1 month in the light on Murashige-Skoog media containing the indicated concentrations of 2,4-D and BA. For each treatment, a representative wild-type explant is shown to the left of the corresponding dgt explant.
Although differences in organ regeneration between wild-type and dgt explants were apparent at low hormone concentrations, the overall response pattern to higher concentrations of BA and 2,4-D in mutant and wild-type explants was similar (Fig. 6). At the higher cytokinin concentrations (3 and 30 μm), dgt and wild-type explants looked very similar, irrespective of the auxin concentration used. When high auxin concentrations were used in combination with high cytokinin concentrations, mutant explants produced slightly more callus and remained greener. Another subtle difference was that calli formed by wild-type explants tended to be browner than dgt calli when high auxin concentrations (30 or 100 μm) were applied.
Induction and Growth of Callus
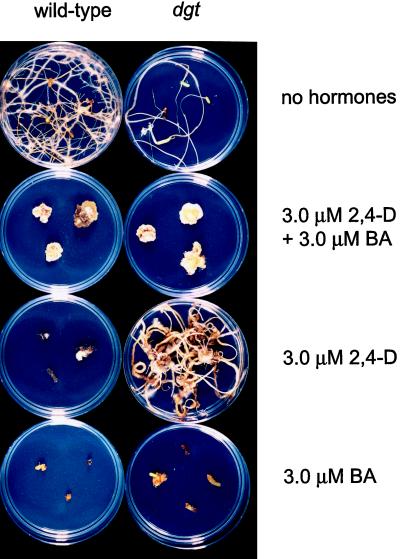
To specifically characterize the effects of the dgt mutation on callus growth, shoot formation was suppressed by incubation of explants in the dark. The formation of large calli in both genotypes was dependent on the presence of both auxin and cytokinin (Fig. 7). With no growth regulators added, both wild-type and dgt hypocotyl explants produced roots and small amounts of callus. Roots formed from untreated explants were more prolific in wild-type explants than in dgt explants. Addition of 3 μm 2,4-D to the medium suppressed root formation in wild-type explants, whereas it stimulated root formation in the mutant (Fig. 7), similar to the shifted auxin sensitivity of dgt root formation previously observed in the light (Figs. 5 and 6). BA suppressed root formation in both genotypes in the light (Figs. 5 and 6) as well as in the dark (Fig. 7), suggesting that cytokinin suppresses adventitious root formation through a Dgt-independent mechanism.
Figure 7.
Callus and root formation in wild-type and dgt hypocotyl explants from 9-d-old light-grown seedlings incubated for 1 month in the dark on Murashige-Skoog media containing the indicated hormones.
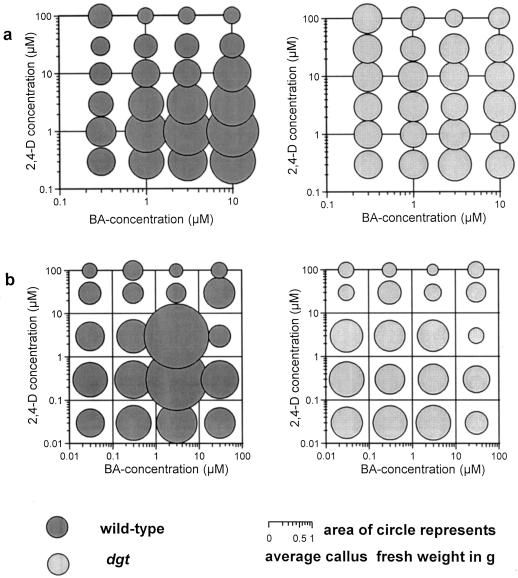
For a quantitative analysis of auxin and cytokinin effects on callus growth in the absence of regenerated organs, the formation of adventitious roots was suppressed by concomitantly treating explants with 0.3 μm or more of both 2,4-D and BA. When production of callus by hypocotyl segments was studied on a hormone matrix (Fig. 8a), wild-type calli were largest in the presence of 10 μm BA and 1 μm 2,4-D, and callus production showed clearly defined responses to varying doses of both auxin and cytokinin. In contrast, mutant hypocotyl explants produced approximately equal amounts of callus tissue in response to all growth regulator combinations tested in this experiment, with no increase in response to changes in cytokinin or auxin concentration (Fig. 8a). To test whether the lack of hormone responsiveness in dgt calli was dependent on the presence of the hypocotyl explant, we investigated the hormone responsiveness of subcultured callus. As in newly initiated calli (Fig. 8a), the growth response of passaged wild-type calli showed clear concentration optima for both 2,4-D and BA, whereas there were no such optima for dgt callus growth (Fig. 8b).
Figure 8.
Responses of callus induction and growth to 2,4-D and BA. a, Callus induction from hypocotyl tissue; b, growth of callus subcultured in the dark for 2 additional months. The average fresh weight of four callus pieces is represented by the area of each circle. The experimental setup was the same as for Figure 7.
In addition to callus growth, the dgt mutation also affected callus morphology. In all experiments mutant calli remained hard and white, irrespective of the hormone treatment, whereas wild-type calli grown at near-optimal hormone concentrations were soft and translucent. After two passages on medium containing 3 μm 2,4-D and 3 μm BA, wild-type calli were virtually free of vascular tissue, whereas dgt calli contained large numbers of tracheary elements. Counts of vascular elements in both tissues consistently confirmed this difference (data not shown).
DISCUSSION
Auxin-Cytokinin Cross-Resistance Is Tissue and Response Specific
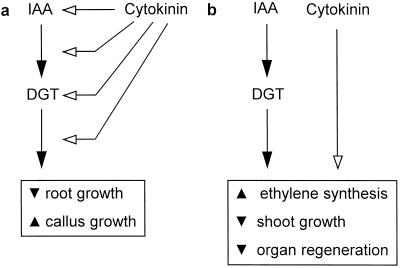
Similar to the auxin-resistant aux1 (Pickett et al., 1990) and axr1 (Lincoln et al., 1990) mutants of Arabidopsis, the roots of young dgt seedlings are resistant to both auxin (Muday et al., 1995) and cytokinins (Fig. 1b). Cytokinin-induced increases in free auxin have been demonstrated in a number of tissues including roots (Coenen and Lomax, 1997). Cross-resistance of mutants such as aux1, axr1, and dgt to cytokinins could thus indicate that cytokinins inhibit root growth by increasing active auxin pools to inhibitory levels. Alternatively, these mutations might affect a signaling pathway that transduces both auxin and cytokinin signals (Fig. 9a).
Figure 9.
Models depicting possible auxin-cytokinin interactions in specific tissues or responses. a, Auxin-cytokinin cross-resistance of dgt tissues could indicate either cytokinin-induced changes in active auxin pools or interactions between cytokinin- and auxin-signaling pathways; b, lack of auxin-cytokinin cross-resistance suggests separate mechanisms of auxin and cytokinin action. ▾ indicates inhibition and ▴ indicates stimulation of the processes or responses specified.
Although the initial characterization of hormone-response mutants routinely involves testing their sensitivity to several hormone classes, tissue or response specificity of cross-resistance has received little attention. Auxin resistance of the dgt mutant is not limited to root growth but is also apparent in ethylene synthesis and hypocotyl and internode elongation (Zobel, 1974; Kelly and Bradford, 1986; M. Rice and T.L. Lomax, unpublished data). However, these latter reactions did not show cytokinin resistance in dgt plants (Figs. 1–4). The limitation of auxin-cytokinin cross-resistance to certain dgt tissues and responses extends to tissue culture, where the regeneration of roots and shoots from dgt hypocotyl explants showed reduced sensitivity to auxin but not to cytokinin (Figs. 5–7). The effects of cytokinin on these responses therefore appear to be mediated by a separate, Dgt-independent mechanism (Fig. 9b) and cannot be ascribed to cytokinin effects on auxin metabolism.
Auxin and Cytokinin Stimulation of Callus Growth Both Require Dgt
Whereas the induction of calli in both dgt and wild-type explants was dependent on the presence of auxin and cytokinin (Fig. 7), differences in the hormonal regulation of callus growth became apparent when a matrix of 2,4-D and BA concentrations was applied (Fig. 8). The absence of clear concentration optima for either auxin or cytokinin in dgt tissues demonstrates that both auxin and cytokinin stimulate callus growth via a Dgt-dependent process (Fig. 9a). It also suggests that the regulatory mechanisms for the induction (Fig. 7) and the prolonged growth (Fig. 8) of callus may differ, with induction being DGT independent and growth regulation being DGT dependent. The signaling pathways for the stimulation of callus growth by auxin and cytokinin therefore share the requirement for a functional DGT gene product. However, our data do not permit conclusions about the point at which the auxin- and cytokinin-signaling pathways feed into the control mechanisms for either root growth or the mitotic cycle (Fig. 9a). Shared signal transduction elements for auxin- and cytokinin-stimulated cell division are also supported by the phenotype of several dominant mutants of tobacco. The axi1 and cyi1 mutants were isolated by activation tagging and confer both auxin- and cytokinin-independent division of mesophyll protoplasts (Hayashi et al., 1992; Miklashevichs et al., 1997). Unlike dgt plants, axi1 plants have no measurable phenotypes besides the hormone-independent protoplast growth, and mapping of the axi1 homolog in tomato indicates that axi1 is not allelic to dgt (M.J. Ellard-Ivey and T.L. Lomax, unpublished data). The dgt effect on cell division is further distinguished from these mutants because dgt plants do not initiate calli in the complete absence of exogenous hormones (Fig. 7).
DGT-Independent Auxin Responses
Traditional physiological experiments (Skoog and Miller, 1957; Fosket and Torrey, 1969) and studies with auxin-auxotrophic cell lines (Blonstein et al., 1988; Oetiker et al., 1990; Fracheboud and King, 1991) demonstrate that auxin is essential for the regeneration of shoots and roots and for the induction of callus. Although hypocotyl segments of the dgt mutant were previously shown to be auxin insensitive (Kelly and Bradford, 1986), they do form leaves and roots (Fig. 5) and callus (Fig. 7). The dgt mutation thus does not eliminate these auxin-dependent developmental programs. One possible explanation for these observations is that the dgt mutant is leaky. However, the complete lack of auxin responsiveness in dgt hypocotyl segments (Kelly and Bradford, 1986) argues against this interpretation. Furthermore, the resistance of dgt roots to auxin (Muday et al., 1995) is comparable to the level of resistance observed in the strongest alleles of auxin-insensitive Arabidopsis mutants (Timpte et al., 1995).
Alternatively, Dgt may modulate the sensitivity of organ and callus formation to changing auxin concentrations without being required for elicitation of these developmental programs by the hormone. This interpretation would explain why dgt hypocotyl explants regenerate organs (Fig. 5) and also produce a certain amount of callus in response to auxin and cytokinin (Fig. 7) but do not show the typical auxin stimulation of callus growth (Fig. 8) seen in wild-type tissues. The possibility that a Dgt-independent auxin-response pathway is involved in the induction of cell division is supported by studies demonstrating auxin-induced increases in transcript levels for two auxin-regulated genes in dgt tissues (Young et al., 1994; Mito and Bennett, 1995). One of these genes, LePAR, has high sequence similarity to the tobacco gene parA (Mito and Bennett, 1995), which is expressed in tobacco mesophyll protoplasts during the transition from the G0 to S phase, and has been proposed to play a role in cell division (Takahashi et al., 1989). The RSI-1 gene is also auxin inducible in dgt tissues (Young et al., 1994) and is thought to play a role in the cell divisions initiating lateral root formation (Taylor and Scheuring, 1994). These two genes may therefore be involved in an auxin-induced, Dgt-independent pathway leading to cell division.
Conclusions
The dgt mutant exhibits altered auxin responses in a number of different tissues and reactions, suggesting that the Dgt gene product could be part of a signaling pathway or regulate one of its components. By surveying a range of responses in which auxin and cytokinin interact, we found that auxin-cytokinin cross-resistance of the dgt mutant was apparent in only two of the responses studied. This suggests that auxin and cytokinin can act on a common set of responses through separate signaling pathways, as well as through a common, Dgt-dependent series of events.
ACKNOWLEDGMENTS
We thank Karen Cardozo for the photograph presented in Figure 5, Dr. Donald Armstrong for advice concerning tissue culture techniques and evaluation, the Oregon State University Laboratory for Nitrogen Fixation for the use of their gas chromatograph, Dr. Elke Duwenig for critical reading of the manuscript, and Dr. Rainer Hertel for helpful discussions.
Footnotes
This work was supported by the National Science Foundation (grants no. BIR-9314619 and IBN-9423651).
LITERATURE CITED
- Aharoni N, Anderson JD, Lieberman M. Production and action of ethylene in senescing leaf discs. Effects of indoleacetic acid, kinetin, silver ion, and carbon dioxide. Plant Physiol. 1979;64:805–809. doi: 10.1104/pp.64.5.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blonstein AD, Stirnberg P, King PJ. Mutants of Nicotiana plumbaginifolia with specific resistance to auxin. Mol Gen Genet. 1991;228:361–371. doi: 10.1007/BF00260628. [DOI] [PubMed] [Google Scholar]
- Blonstein AD, Vahala T, Koorneef M, King PJ. Plants regenerated from auxin-auxotrophic variants are inviable. Mol Gen Genet. 1988;215:58–64. [Google Scholar]
- Cary AJ, Liu W, Howell SS. Cytokinin action is coupled to ethylene in its effects on the inhibition of root and hypocotyl elongation in Arabidopsis thaliana seedlings. Plant Physiol. 1995;107:1075–1082. doi: 10.1104/pp.107.4.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cline MG. The role of hormones in apical dominance. New approaches to an old problem in plant development. Physiol Plant. 1994;90:230–237. [Google Scholar]
- Cline MG. Exogenous auxin effects on lateral bud outgrowth in decapitated shoots. Ann Bot. 1996;78:255–266. [Google Scholar]
- Coenen C, Lomax TL. Auxin-cytokinin interactions in higher plants: old problems and new tools. Trends Plant Sci. 1997;2:351–356. doi: 10.1016/S1360-1385(97)84623-7. [DOI] [PubMed] [Google Scholar]
- Daniel SG, Rayle DL, Cleland E. Auxin physiology of the tomato mutant diageotropica. Plant Physiol. 1989;91:804–807. doi: 10.1104/pp.91.3.804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deikman J, Ulrich M. A novel cytokinin-resistant mutant of Arabidopsis with abbreviated shoot development. Planta. 1995;195:440–449. doi: 10.1007/BF00202603. [DOI] [PubMed] [Google Scholar]
- DeRopp R. Kinetin and auxin activity. Plant Physiol. 1956;31:253–254. doi: 10.1104/pp.31.3.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fosket DE, Torrey JG. Hormonal control of cell proliferation and xylem differentiation in cultured tissues of Glycine max var. Biloxi. Plant Physiol. 1969;44:871–880. doi: 10.1104/pp.44.6.871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fracheboud Y, King PJ. An auxin-auxotrophic mutant of Nicotiana plumbaginifolia. Mol Gen Genet. 1991;227:397–400. doi: 10.1007/BF00273929. [DOI] [PubMed] [Google Scholar]
- Fuchs Y, Lieberman M. Effect of kinetin, IAA, and gibberellin on ethylene production, and their interactions in growth of seedlings. Plant Physiol. 1968;43:2029–2036. doi: 10.1104/pp.43.12.2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujino DW, Nissen SJ, Jones AD, Burger DW, Bradford KJ. Quantification of the indole-3-acetic acid in dark-grown seedlings of the diageotropica and epinastic mutants of tomato (Lycopersicon esculentum Mill) Plant Physiol. 1988;88:780–784. doi: 10.1104/pp.88.3.780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi H, Czaja I, Lubenow H, Schell J, Walden R. Activation of a plant gene by T-DNA tagging: auxin-independent growth in vitro. Science. 1992;258:1350–1353. doi: 10.1126/science.1455228. [DOI] [PubMed] [Google Scholar]
- Hicks GR, Rayle DL, Lomax TL. The diageotropica mutant of tomato lacks high specific activity auxin binding sites. Science. 1989;254:52–54. doi: 10.1126/science.245.4913.52. [DOI] [PubMed] [Google Scholar]
- Hinchee MA, Rost TL. The control of lateral root development in cultured pea seedlings. I. The role of seedling organs and plant growth regulators. Bot Gaz. 1986;147:137–147. [Google Scholar]
- Hobbie L, Estelle M. Genetic approaches to auxin action. Plant Cell Environ. 1994;17:525–540. doi: 10.1111/j.1365-3040.1994.tb00147.x. [DOI] [PubMed] [Google Scholar]
- Hobbie L, Estelle M. The axr4 auxin-resistant mutants of Arabidopsis thaliana define a gene important for root gravitropism and lateral root initiation. Plant J. 1995;7:211–220. doi: 10.1046/j.1365-313x.1995.7020211.x. [DOI] [PubMed] [Google Scholar]
- Kelly MO, Bradford KJ. Insensitivity of the diageotropica tomato mutant to auxin. Plant Physiol. 1986;82:713–717. doi: 10.1104/pp.82.3.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leyser HMO, Pickett BF, Dharmasiri S, Estelle M. Mutations in the AXR3 gene of Arabidopsis result in altered auxin response including ectopic expression from the SAUR-AC1 promoter. Plant J. 1996;10:403–413. doi: 10.1046/j.1365-313x.1996.10030403.x. [DOI] [PubMed] [Google Scholar]
- Lincoln C, Britton J, Estelle M. Growth and development of the axr1 mutant of Arabidopsis. Plant Cell. 1990;2:1071–1080. doi: 10.1105/tpc.2.11.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomax TL, Coenen C, Gaiser JC, Hopkins R, Rayle DL, Rice MS (1993) Auxin perception and the regulation of tomato growth and development. In J Yoder, ed, Molecular Biology of Tomato: Fundamental Advances and Crop Improvement. Technomic Publishing Co., Lancaster, PA, pp 129–138
- Miklashevichs E, Czaja I, Cordeiro A, Prinsen E, Schell J, Walden R. T-DNA tagging reveals a novel cDNA triggering cytokinin- and auxin-independent protoplast division. Plant J. 1997;12:489–498. doi: 10.1046/j.1365-313x.1997.00489.x. [DOI] [PubMed] [Google Scholar]
- Mito N, Bennett A. The diageotropica mutation and synthetic auxin differentially affect the expression of auxin-regulated genes in tomato. Plant Physiol. 1995;109:293–297. doi: 10.1104/pp.109.1.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muday GK, Lomax TL, Rayle DL. Characterization of the growth and auxin physiology of roots of the tomato mutant, diageotropica. Planta. 1995;195:548–553. doi: 10.1007/BF00195714. [DOI] [PubMed] [Google Scholar]
- Oetiker J, Gebhardt C, King PJ. A temperature-sensitive auxin auxotroph not deficient in indole-3-acetic acid. Planta. 1990;180:220–228. doi: 10.1007/BF00194000. [DOI] [PubMed] [Google Scholar]
- Pickett FB, Wilson AK, Estelle M. The aux1 mutation of Arabidopsis confers both auxin and ethylene resistance. Plant Physiol. 1990;94:1462–1466. doi: 10.1104/pp.94.3.1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid JB. Phytohormone mutants in plant research. J Plant Growth Regul. 1990;9:97–111. [Google Scholar]
- Sachs T, Thimann KV. The role of auxins and cytokinins in the release of buds from dominance. Am J Bot. 1967;54:136–144. [Google Scholar]
- Scott IM. Effects of gibberellin on shoot development in the dgt mutant of tomato. Ann Bot. 1988;61:389–392. [Google Scholar]
- Simmons C, Migliaccio F, Masson P, Caspar T, Söll D. A novel root gravitropism mutant of Arabidopsis thaliana exhibiting altered auxin physiology. Physiol Plant. 1995;93:790–798. [PubMed] [Google Scholar]
- Skoog F, Miller CO. Chemical regulation of growth and organ formation in plant tissues cultured in vitro. Soc Exp Biol Symp. 1957;11:118–131. [PubMed] [Google Scholar]
- Su W, Howell SH. The effects of cytokinin and light on hypocotyl elongation in Arabidopsis seedlings are independent and additive. Plant Physiol. 1995;108:1423–1430. doi: 10.1104/pp.108.4.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi Y, Kuroda H, Tanaka T, Machida Y, Takebe I, Nagata T. Isolation of an auxin-regulated gene cDNA expressed during the transition from Go to S phase in tobacco mesophyll protoplasts. Proc Natl Acad Sci USA. 1989;86:9279–9282. doi: 10.1073/pnas.86.23.9279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamas IA. Hormonal regulation of apical dominance. In: Davies PJ, editor. Plant Hormones: Physiology, Biochemistry and Molecular Biology. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1995. pp. 572–597. [Google Scholar]
- Taylor BH, Scheuring CF. A molecular marker for lateral root initiation: The RSI-1 gene of tomato (Lycopersicon esculentum Mill) is activated in early lateral root primordia. Mol Gen Genet. 1994;243:148–157. doi: 10.1007/BF00280311. [DOI] [PubMed] [Google Scholar]
- Timpte C, Lincoln C, Pickett FB, Turner J, Estelle M. The AXR1 and AUX1 genes of Arabidopsis function in separate auxin-response pathways. Plant J. 1995;8:561–569. doi: 10.1046/j.1365-313x.1995.8040561.x. [DOI] [PubMed] [Google Scholar]
- Vanderhoef LN, Stahl C. Separation of two responses to auxin by means of cytokinin inhibition. Proc Natl Acad Sci USA. 1975;72:1822–1825. doi: 10.1073/pnas.72.5.1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wightman F, Schneider EA, Thimann KV. Hormonal factors controlling the initiation and development of lateral roots. II. Effects of exogenous growth factors on lateral root formation in pea roots. Physiol Plant. 1980;49:304–314. [Google Scholar]
- Wilson AK, Pickett FB, Turner JC, Estelle M. A dominant mutation in Arabidopsis confers resistance to auxin, ethylene and abscisic acid. Mol Gen Genet. 1990;222:377–383. doi: 10.1007/BF00633843. [DOI] [PubMed] [Google Scholar]
- Young R, Scheurig CF, Lee GH, Taylor BH. Genes regulated by auxin in tomato seedling roots (abstract no. 24) Plant Physiol. 1994;105:S-16. [Google Scholar]
- Zobel RW. Genetics of the diageotropica mutant in the tomato. J Hered. 1972;63:91–97. [Google Scholar]
- Zobel RW. Some physiological characteristics of the ethylene-requiring tomato mutant diageotropica. Plant Physiol. 1973;52:385–389. doi: 10.1104/pp.52.4.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zobel RW. Control of morphogenesis in the ethylene-requiring tomato mutant, diageotropica. Can J Bot. 1974;52:735. [Google Scholar]