Abstract
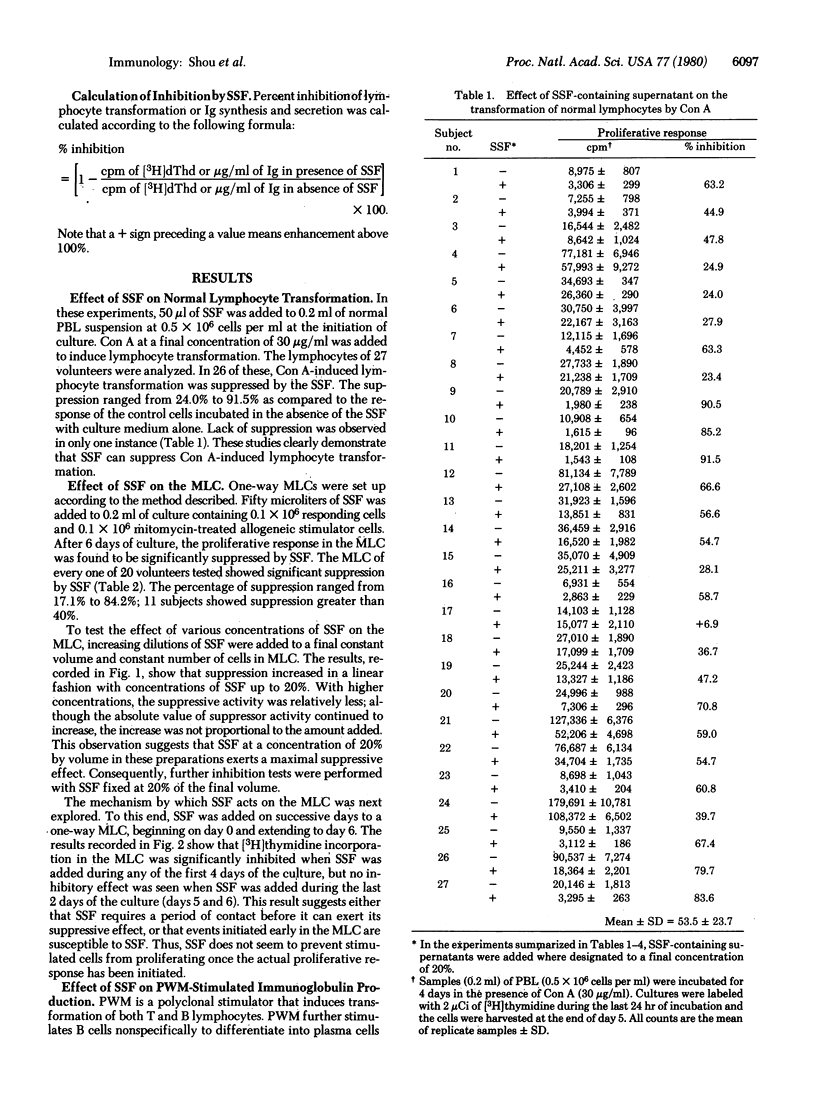
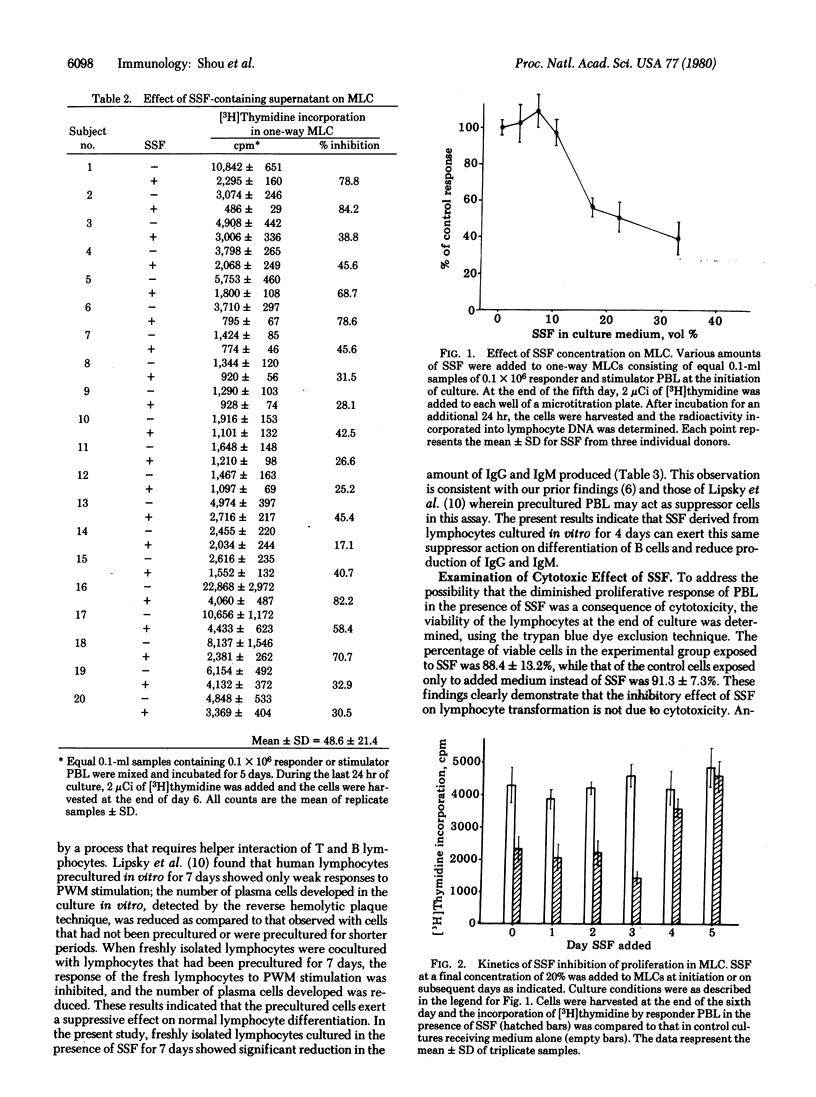
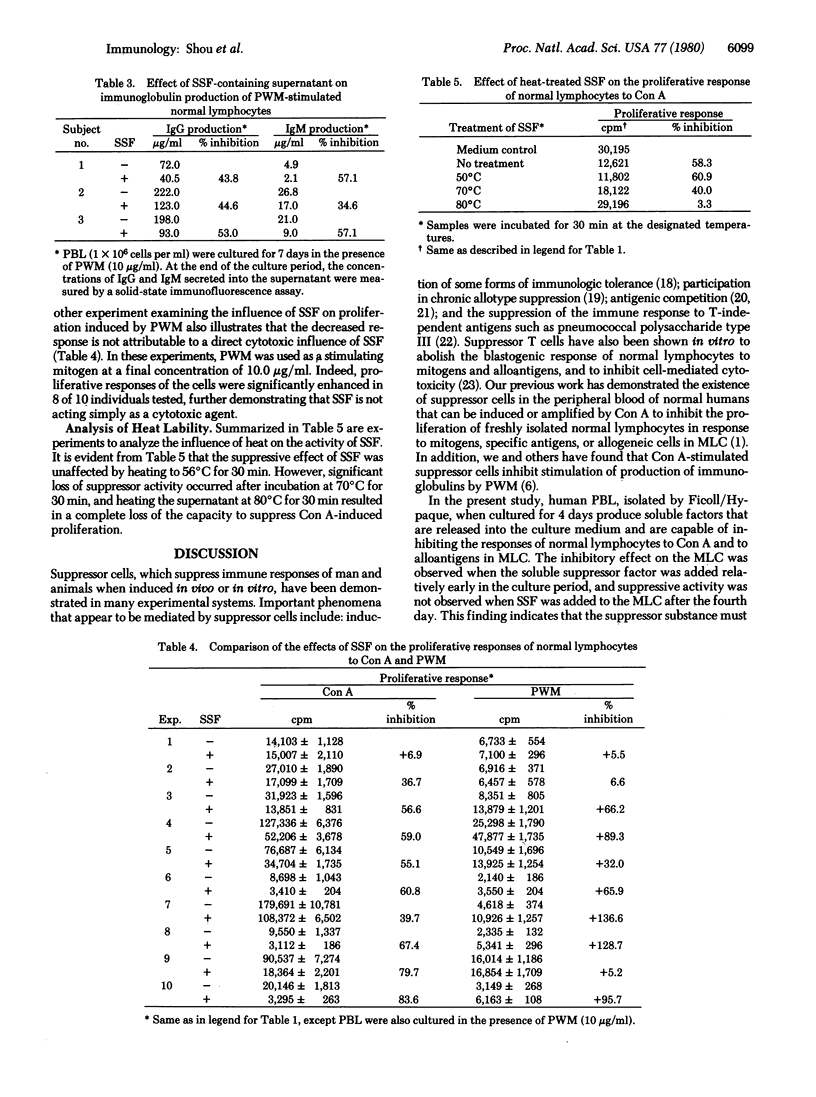
A soluble suppressor factor (SSF) has been demonstrated in the supernatant of normal human peripheral blood lymphocyte cultures that exhibits suppressive activity toward the proliferative response of normal lymphocytes to concanavalin A or alloantigens in mixed lymphocyte culture (MLC) or toward pokeweed mitogen-stimulated immunoglobulin synthesis and secretion in vitro. Suppression of the proliferative response in MLC reached maximal levels when added SSF-containing supernatant approximated 20% by volume of the culture medium. Suppression in the MLC was found to act at the proliferative stage. SSF acts independently of cytotoxicity and is stable at 56 degrees C for 30 min but is inactivated at higher temperatures. Addition of SSF to the MLC as late as day 4 after initiation of the culture results in suppression of transformation. This factor(s) may regulate the magnitude of several immune responses in humans.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker P. J., Stashak P. W., Amsbaugh D. F., Prescott B. Regulation of the antibody response to type 3 pneumococcal polysaccharide. II. Mode of action of thymic-derived suppressor cells. J Immunol. 1974 Jan;112(1):404–409. [PubMed] [Google Scholar]
- Burns F. D., Marrack P. C., Kappler J. W., Janeway C. A., Jr Functional heterogeneity among the T-derived lymphocytes of the mouse. IV. Nature of spontaneously induced suppressor cells. J Immunol. 1975 Apr;114(4):1345–1347. [PubMed] [Google Scholar]
- Gershon R. K., Kondo K. Antigenic competition between heterologous erythrocytes. I. Thymic dependency. J Immunol. 1971 Jun;106(6):1524–1531. [PubMed] [Google Scholar]
- Green S. S., Wistar R., Jr Characterization of lymphocyte inhibition by supernatants of crowded lymphocytoblasts. J Immunol. 1976 Nov;117(5 Pt 1):1429–1433. [PubMed] [Google Scholar]
- Green S. S., Wistar R., Jr, Sell K. W. Mitogen-stimulated lymphocyte inhibition by supernatants of crowded lymphocytoblasts. Transplantation. 1974 Dec;18(6):496–502. doi: 10.1097/00007890-197412000-00004. [DOI] [PubMed] [Google Scholar]
- Hallgren H. M., Yunis E. J. Suppressor lymphocytes in young and aged humans. J Immunol. 1977 Jun;118(6):2004–2008. [PubMed] [Google Scholar]
- Han T., Pauly J. L., Minowada J. Disparity in the production of lymphoblastogenesis inhibition factor by cultured human B and T lymphoid cell lines. Clin Exp Immunol. 1975 Apr;20(1):73–81. [PMC free article] [PubMed] [Google Scholar]
- Haynes B. F., Fauci A. S. Activation of human B lymphocytes. III. Concanavalin A-induced generation of suppressor cells of the plaque-forming cell response of normal human B lymphocytes. J Immunol. 1977 Jun;118(6):2281–2287. [PubMed] [Google Scholar]
- Hersh E. M., McCredie K. B., Freireich E. J. Mechanism of production of inhibitor of lymphocyte blastogenic response to mitogens by cultured lymphoblastoid cell line cells. Transplantation. 1974 Feb;17(2):221–227. doi: 10.1097/00007890-197402000-00011. [DOI] [PubMed] [Google Scholar]
- Herzenberg L. A., Chan E. L., Ravitch M. M., Riblet R. J., Herzenberg L. A. Active suppression of immunoglobulin allotype synthesis. 3. Identification of T cells as responsible for suppression by cells from spleen, thymus, lymph node, and bone marrow. J Exp Med. 1973 Jun 1;137(6):1311–1324. doi: 10.1084/jem.137.6.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipsky P. E., Ginsburg W. W., Finkelman F. D., Ziff M. Control of human B lymphocyte responsiveness: enhanced suppressor T cell activity after in vitro incubation. J Immunol. 1978 Mar;120(3):902–910. [PubMed] [Google Scholar]
- Nadler L. M., Hodes R. J. Regulatory mechanisms in cell-mediated immune responses. II. Comparison of culture-induced and alloantigen-induced suppressor cells in MLR and CML. J Immunol. 1977 May;118(5):1886–1895. [PubMed] [Google Scholar]
- Parks D. E., Weigle W. O. Regulation of B cell unresponsiveness by suppressor cells. Immunol Rev. 1979;43:217–240. doi: 10.1111/j.1600-065x.1979.tb00423.x. [DOI] [PubMed] [Google Scholar]
- Reinherz E. L., Schlossman S. F. Con A-inducible suppression of MLC: evidence for mediation by the TH2 + T cell subset in man. J Immunol. 1979 Apr;122(4):1335–1341. [PubMed] [Google Scholar]
- Rice L., Laughter A. H., Twomey J. J. Three suppressor systems in human blood that modulate lymphoproliferation. J Immunol. 1979 Mar;122(3):991–996. [PubMed] [Google Scholar]
- Roder J. C., Bell D. A., Singhal S. K. Regulation of the immune response in autoimmune NZB/NZW F1 mice. II. Age dependent release of suppressive factors from spleen cells. Immunology. 1978 Jun;34(6):1017–1026. [PMC free article] [PubMed] [Google Scholar]
- Sakane T., Green I. Human suppressor T cells induced by concanavalin A: suppressor T cells belong to distinctive T cell subclasses. J Immunol. 1977 Sep;119(3):1169–1178. [PubMed] [Google Scholar]
- Schwartz S. A. Heavy chain-specific suppression of immunoglobulin synthesis and secretion by lymphocytes from patients with selective IgA deficiency. J Immunol. 1980 Apr;124(4):2034–2041. [PubMed] [Google Scholar]
- Schwartz S. A., Shou L., Good R. A., Choi Y. S. Suppression of immunoglobulin synthesis and secretion by peripheral blood lymphocytes from normal donors. Proc Natl Acad Sci U S A. 1977 May;74(5):2099–2103. doi: 10.1073/pnas.74.5.2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shou L., Schwartz S. A., Good R. A. Suppressor cell activity after concanavalin A treatment of lymphocytes from normal donors. J Exp Med. 1976 May 1;143(5):1100–1110. doi: 10.1084/jem.143.5.1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagart V. B., Thomas W. R., Asherson G. L. Suppressor T cells which block the induction of cytotoxic T cells in vivo. Immunology. 1978 Jun;34(6):1109–1116. [PMC free article] [PubMed] [Google Scholar]


