Abstract
The cephalochordate amphioxus is now established as an important model system for understanding the evolution of vertebrate novelties from an invertebrate chordate ancestor. It is also emerging as a serious candidate for studies of organ regeneration. We extend here our previous observations on the European amphioxus´ extensive adult regenerative capacity. The expression of Wnt5 and the presence of β-catenin protein in the early bud-stage blastema support a role for Wnt signaling during tail regeneration in amphioxus. We also present data showing that Branchiostoma lanceolatum continues to regenerate well after repeated amputation of the post-anal tail. These results are discussed in relation to vertebrate regeneration and other stem cell systems, and in the context of regeneration decline with aging.
Keywords: amphioxus, Branchiostoma lanceolatum, Wnt, repeated regeneration
Cephalochordates as Emerging Regeneration Models
Cephalochordates (“amphioxus”) are considered to be the closest living relatives of vertebrates and ascidians based on molecular phylogenetic studies.1 As chordates, they share many anatomical, developmental and genomic similarities with vertebrates, but are simpler. Although invertebrates, and possessing their fair share of derived features, they are the perfect extant group for understanding the evolution of vertebrate characters, including regenerative ability (For a review see ref. 2). Nevertheless, evidence for regeneration in cephalochordates, while often cited, is relatively sparse.3,4 Biberhofer in 1906,5 and later Probst in 1930,6 were among the first to document regenerative ability in amphioxus, which they characterized as generally poor. It is only recently that we7 and another group8 have addressed in some depth the nature and molecular basis of the regenerative process in European (Branchiostoma lanceloatum) and Asian (B. belcheri) species. Amphioxus can regenerate oral cirri,7,8 specialized buccal structures involved in food particle capture, in a process that appears to mainly involve tissue remodelling (morphallaxis).8 We on the other hand favor a vertebrate-like epimorphic process, with active proliferation in the blastema, at least during tail regeneration.7 Though we are only beginning to dissect the regeneration process in cephalochordates, these studies highlight the potential for amphioxus as a non-vertebrate chordate regeneration model.
The Usual Suspects?
The same pathways are often implicated during regeneration as during development, even if their mode of action may be different in different phyla, and even in different structures of the same organism. During oral cirri regeneration, several orthologs of vertebrate skeletogenic markers are upregulated in the cartilaginous rods, providing insight into the origins of the vertebrate skeleton.8 We previously showed expression of the BMP signaling target msx, a good marker for undifferentiated cells, in the early bud-stage tail blastema. Moreover, the BMP antagonist Chordin is expressed in the notochord blastema prior to overt differentiation.6 Although we did not dissect the dynamics of BMP signaling during tail regeneration, these data support a large body of evidence demonstrating an important role for BMP during regeneration in invertebrates and vertebrates.9-11
Wnt ligands are also critical for regeneration polarity in various systems, including the basally divergent metazoan Hydra.9-12 Similarly, we find expression of components of the Wnt signaling pathways during amphioxus tail regeneration. Preliminary data suggest that at least one of the signals may include Wnt5 (Fig. 1A), whose expression in the blastema is remarkably similar to that of msx.7 In Xenopus laevis, Wnt5a can specifically induce an ectopic tail at a wound site, suggesting an instructive role during tail regeneration.13 However, a complex interplay appears to exist among Wnts, as exemplified by the opposing effects of non-canonical Wnt5b and canonical Wnt3 during fin regeneration in zebrafish,14,15 and the differential effects of Wnt orthologs in planaria.16 The function of many Wnt ligands during regeneration remains unexplored, but is not necessarily strictly conserved across phyla.
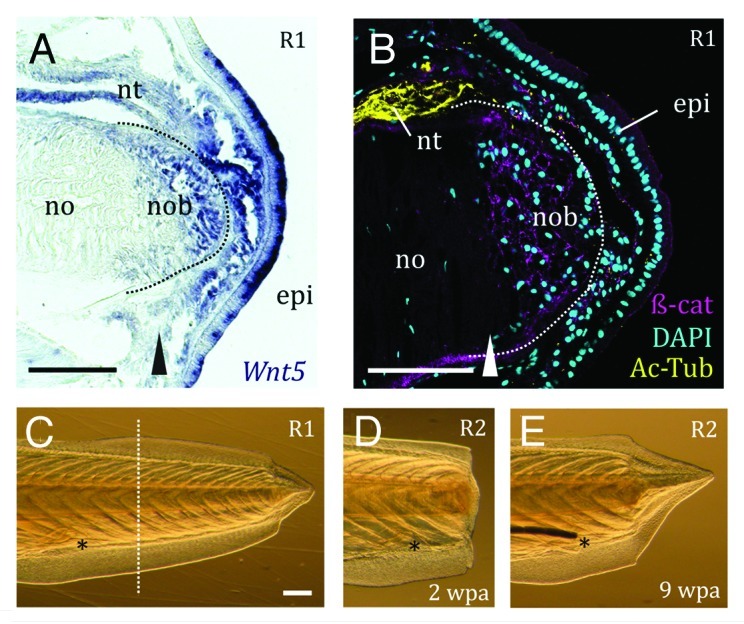
Figure 1. Amphioxus expresses Wnt pathways components in the blastema, and is capable of repeated and robust regeneration of the postanal tail. (A) Wnt5 is expressed in the epidermis (epi) overlying the R1 blastema, the notochord blastema (nob) and the neural tube even anterior to the amputation plane (arrowhead). (B) Immunohistochemistry reveals β-catenin (β-cat, magenta; C2206, Sigma) in the membranes of blastema cells of the notochord (no) in an R1 regenerate (bud stage); acetylated tubulin (Ac-Tub, yellow; T7693 Sigma) labels the axons of the neural tube (nt). The dotted white line demarcates the notochord blastema. (C) Regenerate 1 (R1) pre-amputation. The dotted white line indicates the amputation plane. (D) The second regenerate (R2) two weeks post-amputation (2 wpa), with a clear blastema. (E) The same R2 individual 9 weeks post-amputation (9 wpa). This particular example illustrates a case of the second regenerate exceeding the size of the first. The anus is a reference point, and is indicated by an asterisk. Scale bars, 100μm.
β-catenin is the nuclear effector of canonical Wnt signaling and an important component of adherens junctions, where it links E-Cadherin to the actin cytoskeleton. In planaria, β-catenin acts as the fulcrum of a negative feedback loop regulating head vs. tail regeneration.17 An activated form is also able to induce limb regeneration in vertebrates, most spectacularly in the chick embryo, considered to lack regeneration capability.18 We find that β-catenin protein is specifically stabilized in the cell membranes of the nascent notochord, but is not nuclear (Fig. 1B). Since this β-catenin antibody is predominantly located to the membranes of late amphioxus larvae,19 we cannot exclude a canonical Wnt function during tail regeneration. Interestingly, β-catenin also accumulates in the membranes of regenerating fin epidermis where Wnt3a is expressed, but little in the mesenchyme, and none colocalized with lef1.20 Our results are also intriguing in light of recent work in zebrafish showing that noncanonical Wnt11 and BMP are required to prevent formation of a secondary tail bud. The authors speculate that cohesion of the notochord progenitors is maintained through localization of E-Cadherin.21 Future studies should be geared toward identifying the differential spatial and temporal requirements of BMP and Wnt pathways in the amphioxus tail regenerative program.
Repeated Regeneration
In Hydra and planaria, specialized stem cell populations are constantly turning over, permitting unlimited regeneration and clonal propagation. This, however, does not appear to be the norm in regeneration-competent vertebrates, which are characterized by progenitors with restricted potential (For a review see ref. 22). A corollary to this is that regeneration capacity may be limited to certain organs or to specific life-stages, and may decline with aging. If regeneration relies on stem cells that are in limited supply, the prediction is that repeated injuries would be unable to sustain a comparable regeneration response over the lifetime of the organism. Alternatively, if dedifferentiation programmes can be faithfully re-initiated, then one might expect a constant ability to regenerate, though perhaps not a perfect regeneration response.
Our previous work suggested a regeneration process involving activation of local progenitors, but a reduced ability to regenerate in older animals.7 We therefore wanted to determine whether or not amphioxus has an inherent capacity to regenerate after repeated amputation. We performed a second series of experiments on a subset of the juveniles (n = 29) amputated during the initial study. This removed the potentially confounding factors of age and size, but also of systemic environment. We found that, with the exception of two animals that were discarded from further analysis due to infection, amphioxus produced healthy blastemas (Fig. 1C and D), even after 3 rounds of amputation (not shown). We saw no evidence for a significant decline in regenerative capacity; on the contrary, after the second amputation, amphioxus regenerates tended to be larger (Wilcoxon Matched pairs test, p = 0,039, Figure 1E), a difference that became more prominent with regeneration time. Further, there were no overt differences in quality of the regenerates (see ref. 7) when using the first regenerate size as baseline (Wilcoxon Matched pairs test, p = 0,57). Nevertheless, several factors may affect the size of the second regenerate: namely, the size of tail removed during the second amputation, and secondarily, how well the animal regenerated after the first amputation at the equivalent post-regenerative time-point (Table 1). Taken together, these results suggest that regeneration is not dependent upon an exhaustible local supply of stem cells.
Amphioxus´ repeated tail regeneration capacity falls within the range of different salamander systems. In Notophthalmus viridescens, limb regeneration is near perfect after the first amputation. However, after the second, almost 30% of animals show abnormalities, the most severe concerning patterning of the distal digit tips, defects that exceeded 80% after 5 amputations.23 Since the amphioxus tail is considerably less complex than a vertebrate limb, our gross observations may fail to detect cryptic patterning defects. Our second amphioxus tail regenerates compare favorably with those of Triturus carnifex in size and quality achieved relative to the originals, but detailed histology and expression data may reveal similar increases in variability in number and distribution of ependymal glia.24 It remains to be seen whether or not the amphioxus tail possesses the incredible capacity of the newt Cynops pyrrhogaster lens, which regenerated faithfully after 18 lentectomies over 15 y.25
Differences across different organs or lifestages are not uncommon even in the same species. For instance, unlike tadpoles, which regenerate well, adult Xenopus only regenerate spikes upon limb amputation, an ontogenetic change that is accompanied by shifts in the importance of Wnt/β-catenin.26 In contrast, digit tips can regenerate with high fidelity throughout the life of the frog, even with repeated amputations.27 Similarly, the zebrafish caudal fin regenerates faithfully after 27 amputations and repeated cycles of Wnt signaling inhibition, although bone structure anterior to the amputation plane is affected.28 In contrast, the maxillary barbel regenerate is hypomorphic, and its regenerative capacity is compromised further with repeated amputation.29 Comparison of repeated regeneration capacity in different amphioxus organs may reveal similar variation.
Perspectives
“Immortal” organisms, like Hydra and planaria, are ideal systems for unravelling the basis of pluripotency and proportion control. However, they are likely to be less useful for understanding the factors that promote regenerative decline, particularly during aging or after repeated injury, or to explain why organ systems may have variable regenerative potential in a single species. Such “mosaic” regenerates, of which amphioxus is one, may answer other questions: If long-range inputs, like chemical or bioelectric signals, transmit positional information,30 then is it a failure of the regenerate to respond, or poor signal transmission that explains regeneration decline? Why does repeated amputation of one organ succeed in recapitulating the original, when another doesn’t? How do the new and old tissues integrate different sources of information to control growth and final regenerate size? And finally, what can we learn about the evolution of regenerative mechanisms? Though many traditional model systems are beginning to make clear progress toward deciphering the complexity of regeneration, the humble amphioxus may yet find its niche in stem cell and regeneration research.
Table 1. Multiple regression on Reg R2*.
| Best Model§ F(2,21) = 39,58 SEest = 36,97 Adj R2: 0,77 p ≤ 0,0000001 |
|
n = 24 |
||||||
|---|---|---|---|---|---|---|---|---|
| β | SE of β | B | SE of B | t(21) | p-level | |||
| Intercept |
|
|
5,81 |
49,28 |
0,12 |
0,91 |
||
|
Reg R1* |
0,25 |
0,10 |
0,44 |
0,18 |
2,44 |
0,02 |
||
|
Rem Tail* |
0,79 |
0,10 |
0,31 |
0,04 |
7,65 |
≤ 0,0000001 |
||
Notes: *Reg R1 and R2 = length of Regenerates R1 and R2, respectively; Rem Tail = length of Removed Tail §Image quantification and statistical analyses were performed using NIH ImageJ and Statistica (Statsoft) software, respectively.
Acknowledgments
We apologize to all those whose work we were unable to cite due to space restrictions. This work was funded by a Marie Curie IEF postdoctoral fellowship to IMLS (FP7 People Programme). HE´s laboratory is supported by ANR-2010-BLAN-1716 01 and ANR-2010-BLAN-1234 02 grants from the Agence Nationale de la Recherche. JGF is funded by the ICREA Academia Prize (Generalitat de Catalunya) and by grant BFU2011–23921 (Ministerio de Economia y Competitividad, Spain).
Footnotes
Previously published online: www.landesbioscience.com/journals/cib/article/21075
References
- 1.Delsuc F, Brinkmann H, Chourrout D, Philippe H. Tunicates and not cephalochordates are the closest living relatives of vertebrates. Nature. 2006;439:965–8. doi: 10.1038/nature04336. [DOI] [PubMed] [Google Scholar]
- 2.Bertrand S, Escrivà H. Evolutionary crossroads in developmental biology: amphioxus. Development. 2011;138:4819–30. doi: 10.1242/dev.066720. [DOI] [PubMed] [Google Scholar]
- 3.Bely AE, Nyberg KG. Evolution of animal regeneration: re-emergence of a field. Trends Ecol Evol. 2010;25:161–70. doi: 10.1016/j.tree.2009.08.005. [DOI] [PubMed] [Google Scholar]
- 4.Sánchez Alvarado A, Tsonis PA. Bridging the regeneration gap: genetic insights from diverse animal models. Nat Rev Genet. 2006;7:873–84. doi: 10.1038/nrg1923. [DOI] [PubMed] [Google Scholar]
- 5.Biberhofer R. Über Regeneration bei Amphioxus lanceolatus. Arch EntwMech Org. 1906;22:15–7. [Google Scholar]
- 6.Probst G. Regenerationsstudien an Anneliden und Branchiostoma lanceolatum (Pallas) Rev Suisse Zool. 1930;37:343–52. [Google Scholar]
- 7.Somorjai IML, Somorjai RL, Garcia-Fernàndez J, Escrivà H. Vertebrate-like regeneration in the invertebrate chordate amphioxus. Proc Natl Acad Sci USA. 2012;109:517–22. doi: 10.1073/pnas.1100045109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaneto S, Wada H. Regeneration of amphioxus oral cirri and its skeletal rods: implications for the origin of the vertebrate skeleton. J Exp Zoolog B Mol Dev Evol. 2011;316:409–17. doi: 10.1002/jez.b.21411. [DOI] [PubMed] [Google Scholar]
- 9.Beck CW, Christen B, Slack JM. Molecular pathways needed for regeneration of spinal cord and muscle in a vertebrate. Dev Cell. 2003;5:429–39. doi: 10.1016/S1534-5807(03)00233-8. [DOI] [PubMed] [Google Scholar]
- 10.Reddien PW. Constitutive gene expression and the specification of tissue identity in adult planarian biology. Trends Genet. 2011;27:277–85. doi: 10.1016/j.tig.2011.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stoick-Cooper CL, Moon RT, Weidinger G. Advances in signaling in vertebrate regeneration as a prelude to regenerative medicine. Genes Dev. 2007;21:1292–315. doi: 10.1101/gad.1540507. [DOI] [PubMed] [Google Scholar]
- 12.Lengfeld T, Watanabe H, Simakov O, Lindgens D, Gee L, Law L, et al. Multiple Wnts are involved in Hydra organizer formation and regeneration. Dev Biol. 2009;330:186–99. doi: 10.1016/j.ydbio.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 13.Sugiura T, Tazaki A, Ueno N, Watanabe K, Mochii M. Xenopus Wnt-5a induces an ectopic larval tail at injured site, suggesting a crucial role for noncanonical Wnt signal in tail regeneration. Mech Dev. 2009;126:56–67. doi: 10.1016/j.mod.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 14.Lee Y, Hami D, De Val S, Kagermeier-Schenk B, Wills AA, Black BL, et al. Maintenance of blastemal proliferation by functionally diverse epidermis in regenerating zebrafish fins. Dev Biol. 2009;331:270–80. doi: 10.1016/j.ydbio.2009.05.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stoick-Cooper CL, Weidinger G, Riehle KJ, Hubbert C, Major MB, Fausto N, et al. Distinct Wnt signaling pathways have opposing roles in appendage regeneration. Development. 2007;134:479–89. doi: 10.1242/dev.001123. [DOI] [PubMed] [Google Scholar]
- 16.Gurley KA, Elliott SA, Simakov O, Schmidt HA, Holstein TW, Sánchez Alvarado A. Expression of secreted Wnt pathway components reveals unexpected complexity of the planarian amputation response. Dev Biol. 2010;347:24–39. doi: 10.1016/j.ydbio.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Petersen CP, Reddien PW. Polarized notum activation at wounds inhibits Wnt function to promote planarian head regeneration. Science. 2011;332:852–5. doi: 10.1126/science.1202143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kawakami Y, Rodriguez Esteban C, Raya M, Kawakami H, Martí M, Dubova I, et al. Wnt/beta-catenin signaling regulates vertebrate limb regeneration. Genes Dev. 2006;20:3232–7. doi: 10.1101/gad.1475106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oda H, Akiyama-Oda Y, Zhang S. Two classic cadherin-related molecules with no cadherin extracellular repeats in the cephalochordate amphioxus: distinct adhesive specificities and possible involvement in the development of multicell-layered structures. J Cell Sci. 2004;117:2757–67. doi: 10.1242/jcs.01045. [DOI] [PubMed] [Google Scholar]
- 20.Poss KD, Shen J, Keating MT. Induction of lef1 during zebrafish fin regeneration. Dev Dyn. 2000;219:282–6. doi: 10.1002/1097-0177(2000)9999:9999<::AID-DVDY1045>3.3.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 21.Yang Y, Thorpe C. BMP and non-canonical Wnt signaling are required for inhibition of secondary tail formation in zebrafish. Development. 2011;138:2601–11. doi: 10.1242/dev.058404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tanaka EM, Reddien PW. The cellular basis for animal regeneration. Dev Cell. 2011;21:172–85. doi: 10.1016/j.devcel.2011.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dearlove GE, Dresden MH. Regenerative abnormalities in Notophthalmus viridescens induced by repeated amputations. J Exp Zool. 1976;196:251–62. doi: 10.1002/jez.1401960212. [DOI] [PubMed] [Google Scholar]
- 24.Margotta V, Filoni S, Merante A, Chimenti C. Analysis of morphogenetic potential of caudal spinal cord in Triturus carnifex adults (Urodele Amphibians) subjected to repeated tail amputations. It J Anat Embryol 2002; 127-44. [PubMed] [Google Scholar]
- 25.Eguchi G, Eguchi Y, Nakamura K, Yadav MC, Millán JL, Tsonis PA. Regenerative capacity in newts is not altered by repeated regeneration and ageing. Nat Commun. 2011;2:384. doi: 10.1038/ncomms1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yokoyama H, Maruoka T, Ochi H, Aruga A, Ohgo S, Ogino H, et al. Different requirement for Wnt/β-catenin signaling in limb regeneration of larval and adult Xenopus. PLoS ONE. 2011;6:e21721. doi: 10.1371/journal.pone.0021721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Russell AP, Maddin HC, Chrbet T. Restorative regeneration of digital tips in the African clawed frog (Xenopus laevis daudin) Anat Rec (Hoboken) 2011;294:253–62. doi: 10.1002/ar.21313. [DOI] [PubMed] [Google Scholar]
- 28.Azevedo AS, Grotek B, Jacinto A, Weidinger G, Saúde L. The regenerative capacity of the zebrafish caudal fin is not affected by repeated amputations. PLoS ONE. 2011;6:e22820. doi: 10.1371/journal.pone.0022820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.LeClair EE, Topczewski J. Development and regeneration of the zebrafish maxillary barbel: a novel study system for vertebrate tissue growth and repair. PLoS ONE. 2010;5:e8737. doi: 10.1371/journal.pone.0008737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mondia JP, Levin M, Omenetto FG, Orendorff RD, Branch MR, Adams DS. Long-distance signals are required for morphogenesis of the regenerating Xenopus tadpole tail, as shown by femtosecond-laser ablation. PLoS ONE. 2011;6:e24953. doi: 10.1371/journal.pone.0024953. [DOI] [PMC free article] [PubMed] [Google Scholar]


