Abstract
Septins are a family of GTP-binding, membrane-interacting cytoskeletal proteins, highly conserved and essential in all eukaryotes (with the exception of plants). Septins play important roles in a number of cellular events that involve membrane remodeling and compartmentalization. One such event is cytokinesis, the last stage of cell division. While cytokinesis is ultimately achieved via the mechanical contraction of an actomyosin ring at the septum, determination of the location where cytokinesis will take place, and recruitment of factors involved in signaling events leading to septation requires the activity of septins. We are working towards dissecting the properties of septins from the budding yeast Saccharomyces cerevisiae, where they were first discovered as cell cycle mutants. In our studies we have employed several complementary electron microscopy techniques to describe the organization and structure of septins both in vitro and in situ.
Keywords: septins, cytokinesis, bud neck filaments, electron tomography, cytoskeleton
The four essential mitotic yeast septins form an octameric, rod-shaped, symmetric complex with the order: cdc11-cdc12-cdc3-cdc10-cdc10-cdc3-cdc12-cdc11. Under physiological salt concentration the rods self-assemble into “railroad track” paired filaments via end-one interaction of cdc11 subunits. Importantly, septin polymerization is greatly facilitated by their interaction with PI(4,5)P2-containing membranes, a lipid enriched at the bud neck. This addendum concentrates on our latest studies, involving the characterization of the yeast septin organization in situ. Using electron tomography we have shown that, at the bud neck, septins form an array of highly organized, orthogonal set of filaments connected to the membrane.
Septins were discovered in budding yeast about forty years ago in a screen for cell division cycle mutants.1 Since then, septins have been shown to be conserved from fungi to mammals2 and to constitute a fourth cytoskeletal system in eukaryotic cells.3 Septins, like tubulins and actins, bind nucleotides. However they do not exhibit any apparent nucleotide-dependent dynamics, and while guanine nucleotide binding promotes septin stability, hydrolysis is not needed in vivo.4,5 A unique property of septins is that they contain a lipid-binding motif that allows them to interact directly with membranes. This property is important for their cellular roles, which involve membrane remodeling and compartmentalization processes.6,7 Among such functions is their essential role in cytokinesis. Septins have been shown to be either up- or down-regulated in cancer cells,8,9 and they appear to be involved in a variety of neurological pathologies, such as Alzheimer and Parkinson diseases.10,11
Budding Yeast Septins: Molecular Organization, Self-Assembly and Lipid Interactions
In budding yeast septin’s essential function in cytokinesis involves their role as a scaffold for the recruitment of both structural and signaling proteins in the septation process.12 Septins also form a barrier that prevents the diffusion of proteins between daughter and mother cells.13,14
Depending on the organisms, the number of septin genes varies from 2 for C. elegans to 14 for humans.2 In all cases, a subset of septins assembles in a specific manner into symmetrical rods that serve as structural units for the polymerization of filaments. In budding yeast, four septins are essential during mitosis: Cdc3, Cdc10, Cdc11 and Cdc12. We have shown that they form an octameric complex in the order: Cdc11-Cdc12-Cdc3-Cdc10-Cdc10-Cdc3-Cdc12-Cdc11. In low salt conditions (below 150 mM) those octamers assemble into non polar paired filaments.15 During mitosis, a fifth sub-stoichiometric septin, Shs1, is expressed. Although Shs1 is dispensable for cell survival, it dramatically affects the organization of the septin complex. Shs1 has the ability to replace the end subunit, Cdc11, and then make the septin complex spontaneously assemble into a ring-like structure.16 The phosphorylation state of Shs1 generally disrupts ring assembly, but a specific phophomimetic mutant site gives rise to an orthogonal array of filaments.16
The quaternary organization of septins is not only affected by specific septin composition and post-translational modifications, but by their interaction with specific lipids. In budding yeast, during cytokinesis, septins are localized at the bud neck where they interact directly with the cell membrane.17 Biochemical and cellular assays have shown septins to bind to phosphoinositides.18 To get further insights into the septin-lipid interaction and its effect on septin ultrastructural organization, we have used a lipid monolayer assay. We showed that budding yeast septins interact with PI(4,5)P2, specially through a basic domain in Cdc10. PI(4,5)P2 facilitates septin filament assembly, even under conditions that prevent polymerization in solution. Furthermore, on PI(4,5)P2 –containing lipid monolayers septin filaments are tightly paired, and can further organize into sheets or orthogonal arrays.19
Budding Yeast Septins: In Situ Ultrastructure
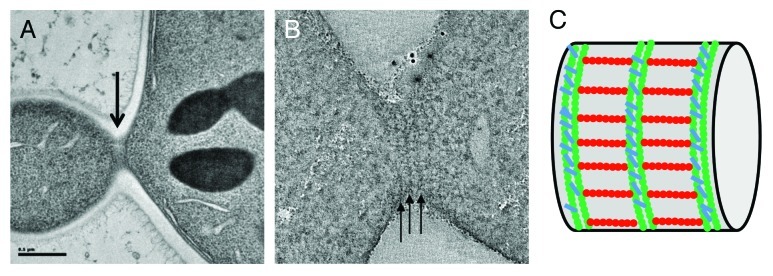
Our in vitro studies have demonstrated that septin organization is highly variable and plastic, from short rods to paired straight filaments, ring-like structures or orthogonal arrays of filaments. Which of these arrangements, if any, are present in the cell? To answer this question, we decided to investigate the organization of septins in their cellular environment using electron tomography(Fig. 1).
Figure 1. Electron microscopy characterization of bud neck septin filaments in S. cerevisiae. (A) Projection view of a 50 nm section from a dividing budding yeast. Grazing filaments are seen close to the membrane (arrow). (B) Single slice from a tomographic reconstruction of a grazing section of budding yeast. Circumferential filaments are indicated with arrows. (C) Schematic representation of septin filaments at the bud neck based on tomographic analysis, with circumferential filaments shown in green and axial filaments drawn in red.
To this end, two sample preparation methods were used. First of all, we carried out high pressure freezing followed by freeze substitution and embedding into epon resin. This method is more preservative than chemical standard fixation techniques and provides high contrast for the cellular features under study. In addition, we performed cryo-sectioning of the high-pressure frozen samples followed by cryo-electron tomography (TOVIS). This method is the most preservative, since it prevents any dehydration or the requirement to use a fixative or a stain, but it suffers from greater technical difficulty and reproducibility, and lower contrast. Using either technique, we clearly visualized an array of highly organized and perpendicular filaments at the bud neck of budding yeast. Circumferential filaments running below the cell membrane and around the bud neck can be seen. These filaments are periodically repeated and the distance from a filament to another equals the length of a septin octamer.20 From cryo-EM of cryo-sections, it was clearer that the circumferential filaments are actually made up of two individual filaments, tightly paired, and resembling those seen for septin assembly on PIP2-containing lipid monolayers. Importantly, a second set of filaments is present, running along the mother-bud axis, and thus creating a perpendicular array of bud neck filaments. Based on previous experiments using inmunolabeling,21 our in our own in vitro observations of perpendicular arrays of septin filaments, and, most importantly, on the distance between circumferential filaments, we strongly believe that these sets of filaments correspond to septins. In support of this interpretation, the analysis of a mutant lacking cdc10, one of the septin subunits, showed a similar, although more disorganize array of filaments, but with a shorter repeat corresponding to a hexameric distance (~24 nm) between circumferential filaments. This size is exactly what would be expected from filaments where the repeating rod is missing two cdc10 subunits.
This orthogonal organization of two sets of septin filaments may likely serve several physiological purposes. First, such an arrangement of filamentous material at the membrane would physically generate a diffusion barrier stopping membrane proteins from moving from the mother cell to the bud. Second, this interconnected network of filaments is likely to have a mechanical effect on the stability of the membrane.22 Importantly, the overall septin at the bud neck should facilitate a quick reorganization of the septin cytoskeleton and thus give rise to alternative morphologies as cell division proceeds. Specifically, one could envision the targeted disassembly of the longitudinal filaments concomitant with the appearance of the acto-myosin contractile ring and the split of the septin collar into the two rings visualized by fluorescence microscopy.23 Some recent studies can be interpreted in support of such model. Using fluorescence polarization spectroscopy, two studies have shown a global rotation of 90° of the septin filaments. The targeting of a set of filaments with respect to the other for disassembly that we propose would likely build on a differential composition that could involve different titrations of the cdc11 and Shs1 subunits.
The samples we have used for in vivo ultrastructure determination were collected during log phase in order to maximize the number of dividing cells . Importantly, because the split septin ring structure is more transient than the septin collar, most of the cells that we analyzed have not reached the onset of cytokinesis and thus, the septin ultrastructure that we have described corresponds to the collar stage of the septin cytoskeleton that precedes cytokinesis. In an attempt to trap the cells at the septin split ring stage, we used a strain that stops cell division at the onset of cytokinesis24 (BYY116, a gift from David Drubin [UC Berkeley]). Organized septin structure was no longer obvious in this mutant. To discard the possibility that either the character of the mutant or the nature of the split rings is too fragile to withstand the freeze substitution and dehydration steps used in our studies of these mutant cells, such samples should by analyzed by TOVIS, which should provide better preservation of delicate structures. Alternatively to the use of mutant yeast strains, it should be possible to synchronize the cells and to try to trap a population where a significant percentage of the cells are undergoing cytokinesis. Correlative methods could facilitate the identification of cells in the right stage, so that targeted tomography can concentrate the EM efforts on the cells of interest.
Acknowledgments
The authors were funded by a Jane Coffin Childs Postdoctoral Research Fellowship (fellowship 61-1357 to A.B.) and by the Howard Hughes Medical Institute (E.N.).
Footnotes
Previously published online: www.landesbioscience.com/journals/cib/article/21125
References
- 1.Hartwell LH. Genetic control of the cell division cycle in yeast. II. Genes controlling DNA replication and its initiation. J Mol Biol. 1971;59:183–94. doi: 10.1016/0022-2836(71)90420-7. [DOI] [PubMed] [Google Scholar]
- 2.Pan F, Malmberg RL, Momany M. Analysis of septins across kingdoms reveals orthology and new motifs. BMC Evol Biol. 2007;7:103. doi: 10.1186/1471-2148-7-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mostowy S, Cossart P. Septins: the fourth component of the cytoskeleton. Nat Rev Mol Cell Biol. 2012;13:183–94. doi: 10.1038/nrm3284. [DOI] [PubMed] [Google Scholar]
- 4.McMurray MA, Thorner J. Biochemical properties and supramolecular architecture of septin hetero-oligomers and septin filaments. In: Hall PA, Russell SEH, Pringle JR, eds. The Septins. Chicester, West Sussex, UK, John Wiley & Sons, Ltd., 2008; In press. [Google Scholar]
- 5.Versele M, Thorner J. Septin collar formation in budding yeast requires GTP binding and direct phosphorylation by the PAK, Cla4. J Cell Biol. 2004;164:701–15. doi: 10.1083/jcb.200312070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Caudron F, Barral Y. Septins and the lateral compartmentalization of eukaryotic membranes. Dev Cell. 2009;16:493–506. doi: 10.1016/j.devcel.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 7.McMurray MA, Thornerr J. Septins: molecular partitioning and the generation of cellular asymmetry. Cell Div. 2009;4:1–40. doi: 10.1186/1747-1028-4-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gonzalez ME, Peterson EA, Privette LM, Loffreda-Wren JL, Kalikin LM, Petty EM. High SEPT9_v1 expression in human breast cancer cells is associated with oncogenic phenotypes. Cancer Res. 2007;67:8554–64. doi: 10.1158/0008-5472.CAN-07-1474. [DOI] [PubMed] [Google Scholar]
- 9.Russell SE, McIlhatton MA, Burrows JF, Donaghy PG, Chanduloy S, Petty EM, et al. Isolation and mapping of a human septin gene to a region on chromosome 17q, commonly deleted in sporadic epithelial ovarian tumors. Cancer Res. 2000;60:4729–34. [PubMed] [Google Scholar]
- 10.Choi P, Snyder H, Petrucelli L, Theisler C, Chong M, Zhang Y, et al. SEPT5_v2 is a parkin-binding protein. Brain Res Mol Brain Res. 2003;117:179–89. doi: 10.1016/S0169-328X(03)00318-8. [DOI] [PubMed] [Google Scholar]
- 11.Takehashi M, Alioto T, Stedeford T, Persad AS, Banasik M, Masliah E, et al. Septin 3 gene polymorphism in Alzheimer’s disease. Gene Expr. 2004;11:263–70. doi: 10.3727/000000003783992243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gladfelter AS, Pringle JR, Lew DJ. The septin cortex at the yeast mother-bud neck. Curr Opin Microbiol. 2001;4:681–9. doi: 10.1016/S1369-5274(01)00269-7. [DOI] [PubMed] [Google Scholar]
- 13.Dobbelaere J, Barral Y. Spatial coordination of cytokinetic events by compartmentalization of the cell cortex. Science. 2004;305:393–6. doi: 10.1126/science.1099892. [DOI] [PubMed] [Google Scholar]
- 14.Takizawa PA, DeRisi JL, Wilhelm JE, Vale RD. Plasma membrane compartmentalization in yeast by messenger RNA transport and a septin diffusion barrier. Science. 2000;290:341–4. doi: 10.1126/science.290.5490.341. [DOI] [PubMed] [Google Scholar]
- 15.Bertin A, McMurray MA, Grob P, Park SS, Garcia G, 3rd, Patanwala I, et al. Saccharomyces cerevisiae septins: supramolecular organization of heterooligomers and the mechanism of filament assembly. Proc Natl Acad Sci USA. 2008;105:8274–9. doi: 10.1073/pnas.0803330105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garcia G, 3rd, Bertin A, Li Z, Song Y, McMurray MA, Thorner J, et al. Subunit-dependent modulation of septin assembly: budding yeast septin Shs1 promotes ring and gauze formation. J Cell Biol. 2011;195:993–1004. doi: 10.1083/jcb.201107123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Byers B, Goetsch L. A highly ordered ring of membrane-associated filaments in budding yeast. J Cell Biol. 1976;69:717–21. doi: 10.1083/jcb.69.3.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tanaka-Takiguchi Y, Kinoshita M, Takiguchi K. Septin-mediated uniform bracing of phospholipid membranes. Curr Biol. 2009;19:140–5. doi: 10.1016/j.cub.2008.12.030. [DOI] [PubMed] [Google Scholar]
- 19.Bertin A, McMurray MA, Thai L, Garcia G, 3rd, Votin V, Grob P, et al. Phosphatidylinositol-4,5-bisphosphate promotes budding yeast septin filament assembly and organization. J Mol Biol. 2010;404:711–31. doi: 10.1016/j.jmb.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bertin A, McMurray MA, Pierson J, Thai L, McDonald KL, Zehr EA, et al. Three-dimensional ultrastructure of the septin filament network in Saccharomyces cerevisiae. Mol Biol Cell. 2012;23:423–32. doi: 10.1091/mbc.E11-10-0850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rodal AA, Kozubowski L, Goode BL, Drubin DG, Hartwig JH. Actin and septin ultrastructures at the budding yeast cell cortex. Mol Biol Cell. 2005;16:372–84. doi: 10.1091/mbc.E04-08-0734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gilden J, Krummel MF. Control of cortical rigidity by the cytoskeleton: emerging roles for septins. Cytoskeleton (Hoboken) 2010;67:477–86. doi: 10.1002/cm.20461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Faty M, Fink M, Barral Y. Septins: a ring to part mother and daughter. Curr Genet. 2002;41:123–31. doi: 10.1007/s00294-002-0304-0. [DOI] [PubMed] [Google Scholar]
- 24.Young BA, Buser C, Drubin DG. Isolation and partial purification of the Saccharomyces cerevisiae cytokinetic apparatus. Cytoskeleton (Hoboken) 2010;67:13–22. doi: 10.1002/cm.20412. [DOI] [PMC free article] [PubMed] [Google Scholar]