Abstract
A method of liposome modification of cell surfaces to render unsuitable target cells susceptible to lysis by anti-viral cytotoxic T lymphocytes (CTLs) is described. Liposomes containing the hemagglutinin-neuraminidase (HN) and fusion (F) glycoproteins of Sendai virus as well as purified H-2Kk cells and rendering those cells susceptible to lysis by B10. A anti-Sendai virus or anti-H-2Kk CTLs. The absence from the modifying liposomes of the HN or F proteins or H-2Kk antigens eliminated the ability of the target cells to be recognized and lysed by either effector cell population. Vesicles containing HN, H-2Kk molecules, and inactive fusion protein (Fo) were not capable of increasing the susceptibility of h-2-negative target cells to lysis. Liposomes containing inactive fusion protein were similarly unable to render H-2-positive target cells susceptible to lysis by anti-Sendai virus CTLs, suggesting that fusion of the liposomes to the cell surface is a prerequisite to lysis. It did not appear that attachement of liposomes to the cell surface was sufficient for generation of susceptible targets, however, because attachment to the cell surface was observed, as long as the HN glycoprotein was present in the liposomes. These results indicate that purified H-2Kk glycoproteins are target antigens for anti-H-2k CTLs and that B10 . A anti-Sendai virus CTLs recognize in an H-2-restricted manner the HN, F, or both glycoproteins of Sendai virus in the context of the purified H-2Kk glycoproteins. This technique of liposome modification of cell surfaces has potential applications in the examination of CTL antigen recognition and immunotherapy of many viral and neoplastic diseases.
Full text
PDF
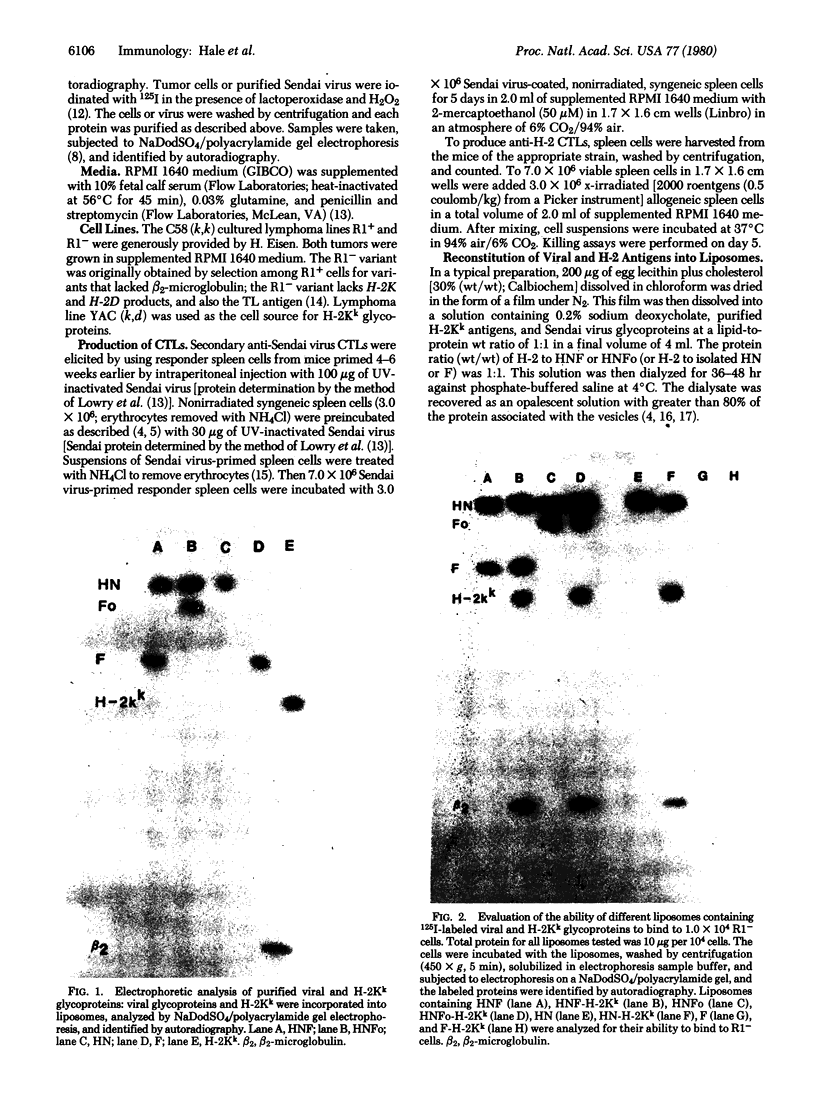
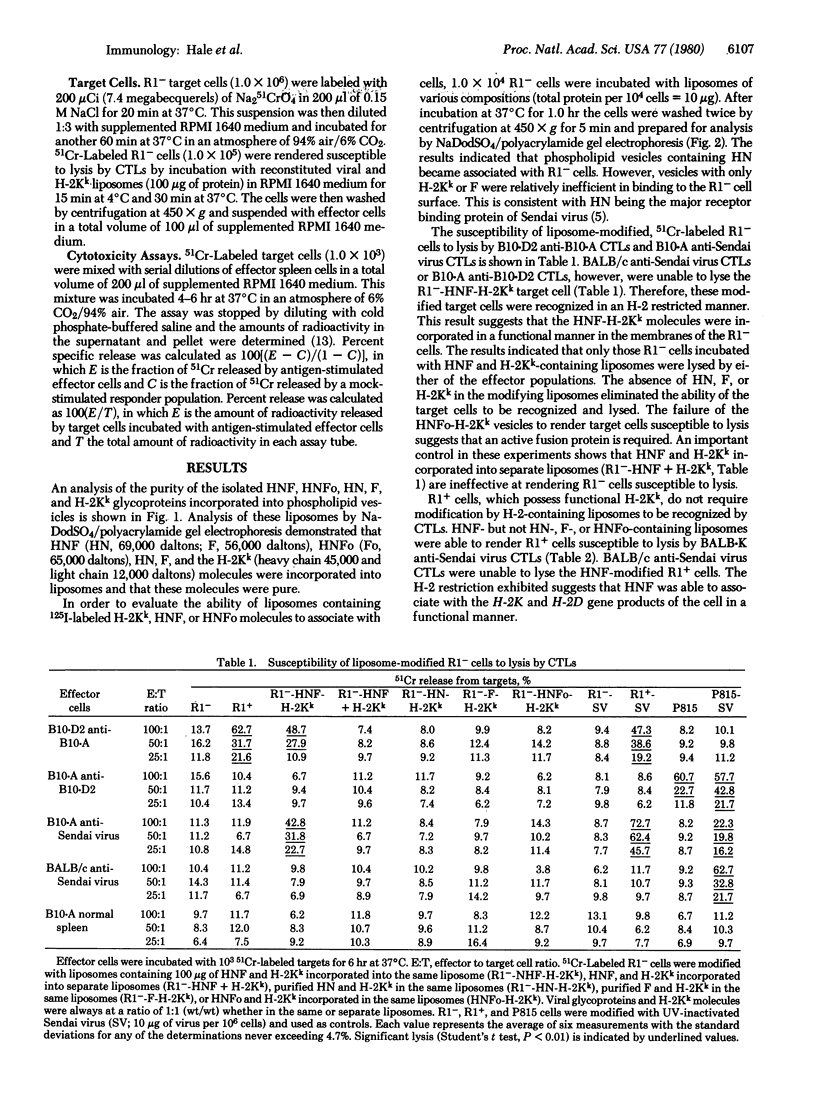
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bevan M. J. The major histocompatibility complex determines susceptibility to cytotoxic T cells directed against minor histocompatibility antigens. J Exp Med. 1975 Dec 1;142(6):1349–1364. doi: 10.1084/jem.142.6.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durda P. J., Shapiro C., Gottlieb P. D. Partial molecular characterization of the Ly-1 alloantigen on mouse thymocytes. J Immunol. 1978 Jan;120(1):53–57. [PubMed] [Google Scholar]
- Engelhard V. H., Strominger J. L., Mescher M., Burakoff S. Induction of secondary cytotoxic T lymphocytes by purified HLA-A and HLA-B antigens reconstituted into phospholipid vesicles. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5688–5691. doi: 10.1073/pnas.75.11.5688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gething M., Koszinowski U., Waterfield M. Fusion of Sendai virus with the target cell membrane is required for T cell cytotoxicity. Nature. 1978 Aug 17;274(5672):689–691. doi: 10.1038/274689a0. [DOI] [PubMed] [Google Scholar]
- Hale A. H., Lyles D. S., Fan D. P. Elicitation of anti-Sendai virus cytotoxic T lymphocytes by viral and H-2 antigens incorporated into the same lipid bilayer by membrane fusion and by reconstitution into liposomes. J Immunol. 1980 Feb;124(2):724–731. [PubMed] [Google Scholar]
- Hale A. H., Paulus L. K. Loss of reactivity of a myeloma tumor with H-2 compatible thymus-derived lymphocytes. Cell Immunol. 1979 Sep 1;46(2):337–347. doi: 10.1016/0008-8749(79)90421-0. [DOI] [PubMed] [Google Scholar]
- Herrmann S. H., Mescher M. F. Purification of the H-2Kk molecule of the murine major histocompatibility complex. J Biol Chem. 1979 Sep 25;254(18):8713–8716. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Littman D. R., Cullen S. E., Schwartz B. D. Insertion of Ia and H-2 alloantigens into model membranes. Proc Natl Acad Sci U S A. 1979 Feb;76(2):902–906. doi: 10.1073/pnas.76.2.902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowinski R. C., Klein P. A. Anomalous reactions of mouse alloantisera with cultured tumor cells. II. Cytotoxicity is caused by antibodies to leukemia viruses. J Immunol. 1975 Nov;115(5):1261–1268. [PubMed] [Google Scholar]
- Oi V. T., Jones P. P., Goding J. W., Herzenberg L. A., Herzenberg L. A. Properties of monoclonal antibodies to mouse Ig allotypes, H-2, and Ia antigens. Curr Top Microbiol Immunol. 1978;81:115–120. doi: 10.1007/978-3-642-67448-8_18. [DOI] [PubMed] [Google Scholar]
- Russell J. H., Hale A. H., Ginns L. C., Eisen H. N. Periodic loss of reactivity of a myeloma tumor with cytotoxic thymus-derived lymphocytes. Proc Natl Acad Sci U S A. 1978 Jan;75(1):441–445. doi: 10.1073/pnas.75.1.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheid A., Choppin P. W. Identification of biological activities of paramyxovirus glycoproteins. Activation of cell fusion, hemolysis, and infectivity of proteolytic cleavage of an inactive precursor protein of Sendai virus. Virology. 1974 Feb;57(2):475–490. doi: 10.1016/0042-6822(74)90187-1. [DOI] [PubMed] [Google Scholar]
- Schrader J. W., Edelman G. M. Joint recognition by cytotoxic T cells of inactivated Sendai virus and products of the major histocompatibility complex. J Exp Med. 1977 Mar 1;145(3):523–539. doi: 10.1084/jem.145.3.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shearer G. M. Cell-mediated cytotoxicity to trinitrophenyl-modified syngeneic lymphocytes. Eur J Immunol. 1974 Aug;4(8):527–533. doi: 10.1002/eji.1830040802. [DOI] [PubMed] [Google Scholar]
- Volsky D. J., Cabantchik Z. I., Beigel M., Loyter A. Implantation of the isolated human erythrocyte anion channel into plasma membranes of Friend erythroleukemic cells by use of Sendai virus envelopes. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5440–5444. doi: 10.1073/pnas.76.11.5440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinkernagel R. M., Doherty P. C. Major transplantation antigens, viruses, and specificity of surveillance T cells. Contemp Top Immunobiol. 1977;7:179–220. doi: 10.1007/978-1-4684-3054-7_5. [DOI] [PubMed] [Google Scholar]