Abstract
Intrabodies can be powerful reagents to effect modulation of aberrant intracellular proteins that underlie a range of diseases. However, their cytoplasmic solubility can be limiting. We previously reported that overall charge and hydrophilicity can be combined to provide initial estimates of intracellular solubility, and that charge engineering via fusion can alter solubility properties experimentally. Additional studies showed that fusion of a proteasome-targeting PEST motif to the anti-huntingtin intrabody scFv-C4 can degrade mutant huntingtin proteins by directing them to the proteasome, while also increasing the negative charge. We now validate the generality of this approach with intrabodies against α-synuclein (α-syn), an important target in Parkinson disease. In this study, fusion of the PEST sequence to a set of four diverse, poorly soluble anti-α-syn intrabodies (D5E, 10H, D10 scFv, VH14 nanobody) significantly increased steady-state soluble intrabody protein levels in all cases, despite fusion with the PEST proteasomal-targeting signal. Furthermore, adding this PEST motif to the least soluble construct, VH14, significantly enhanced degradation of the target protein, α-syn~GFP. The intrabody-PEST fusion approach thus has dual advantages of potentially solubilizing intrabodies and enhancing their functionality in parallel. Empirical testing of intrabody-PEST fusions is recommended for enhancement of intrabody solubility from diverse sources.
Keywords: Parkinson disease, intrabodies, intrabody-PEST fusions, proteasome, α-synuclein
Introduction
Many neurodegenerative and other diseases are thought to be triggered by the abnormal aggregation of intracellular proteins, which then interferes with critical functioning of the cell, eventually leading to degeneration. Existing symptom-based treatments do not address this underlying process, and will necessarily be short-term solutions. Intrabodies, which can be selected, engineered, and delivered as genes, offer a novel protective therapeutic approach.1,2 Consisting of the antigen binding domains, these single-chain Fv (scFv) or single-domain (VH or VL) fragments display the specificity and affinity of antibodies.3 However, many promising intrabodies suffer from reduced cytoplasmic solubility,4 and are aggregation-prone due to the intracellular redox potential and macromolecular crowding.5-7 Such aggregation-prone intrabodies may in fact exacerbate the proteostatic burden in proteinopathies such as Parkinson disease (PD). Currently, only a small fraction of intrabodies is intrinsically soluble in cytoplasm. Intrabody solubility is difficult to predict accurately, but overall negative charge at cytoplasmic pH plays an important role, with a contribution from reduced hydrophilicity. Both of these can be altered by acidification using highly charged peptides, as shown with proof-of-concept fusion of 3xFLAG tag to an aggregation-prone scFv.5
Functionality of soluble intrabodies against toxic proteins can be improved by modifications that increase the probability of degradation of the pathogenic protein target. Proteins containing regions enriched in prolyl (P), glutamyl (E), aspartyl (D), seryl (S) and threonyl (T) residues (PEST regions) are targeted for accelerated proteasomal degradation, and typically have a short half life.8 The PEST motifs contain negatively charged residues,9 and PEST fusions to small heterologous proteins may exert a strong effect on the net protein charge. Recently our lab showed that fusion of the C-terminal PEST motif of mouse ornithine decarboxylase (mODC; amino acids 422–461) to the anti-huntingtin C4 scFv greatly reduced the level of mutant htt exon 1 protein fragments compared with the parent intrabody.10 In this case, the scFv intrabody was already highly soluble, and the enhanced function appeared to be due to retargeting, although improved folding and enhanced solubility could also be contributing factors.
In the current study, we have broadened this bifunctional antibody engineering approach, examining the effects of mODC PEST fusion on four poorly soluble intrabodies selected against α-syn as potential PD therapeutics. We show that the increased negative charge on a series of structurally diverse anti-syn scFv and VH intrabodies can increase their soluble expression, greatly improving functionality. This novel intrabody-PEST fusion technology may therefore be generally applicable, increasing intracellular solubility due to increased net negative charge with enhanced degradation of antigen-intrabody-PEST complexes by proteasomal targeting in numerous diseases.
Results
Sources and sequence comparisons of α-syn intrabodies
We characterized four intrabodies for their soluble expression in the cytoplasm. Three of these were human scFv intrabodies (D5E, 10H and D10 scFv); and one was a human single-domain nanobody (VH14). Basic characteristics and sources of the intrabodies are shown in Table 1. These intrabodies target the protein α-syn, which aggregates to form Lewy bodies that are major markers for PD pathology. These intrabodies were selected against monomeric or oligomeric forms of α-syn from non-immune libraries.11-14 VH14 binds strongly to the non-amyloid component (NAC) of α-syn, shown previously to be critical for aggregation and toxicity.15,16 Human D10 scFv is pan-specific and can bind both monomeric and higher molecular weight forms of human α-syn. Human D5E and 10H scFv recognize oligomeric forms of α-syn.
Table 1. Sources and targets of α-syn intrabodies.
| Intrabody | Type | Target | Reference | Source |
|---|---|---|---|---|
|
D5E |
Human scFv |
Oligomeric α-syn |
Emadi et al. 2007 |
Tomlinson I and J antibody libraries |
|
10H |
Human scFv |
Oligomeric α-syn |
Emadi et al. 2009 |
Tomlinson I and J antibody libraries |
|
D10 |
Human scFv |
Pan-specific |
Zhou et al., 2004 |
Human scFv phage Griffin I library |
| VH14 (NAC14) | Human Nanobody | Hydrophobic non-amyloid component (NAC) of α-synuclein, amino acids 53–95 | Lynch et al. 2008 | Human non-immune yeast surface display library |
Calculated physio-chemical properties of parent and fusion intrabodies
Isoelectric point, net charge and GRAVY were estimated from the amino-acid sequence of each intrabody fused to an HA epitope tag (YPYDVPDYA) or HA-PEST tag (YPYDVPDYA-SHGFPPEVEEQDDGTLPMSCAQESGMDRHPAACASARINV), as outlined in Materials and Methods (Table 2).5 The PEST fusion clearly increases the negative charge, and enhances the theoretical solubility of all four intrabodies. The algorithm PEST-FIND can be used to identify PEST motifs within a sequence. PEST-FIND scores range from -50 to +50. Plus scores are considered possible PEST regions; scores higher than +5 predict effective targeting functionality.8 The PEST sequence used in this investigation gives a PEST-FIND score of +5.16 and thus is indicative of a moderately strong PEST sequence. Positively charged residues are excluded from functional PEST regions. As a scrambled control, we repositioned the PEST motif in such a way that positively charged residues were within the scrambled PEST region.10 The scrambled PEST (SCRPEST) therefore maintained the same net charge, but decreased the proteasomal targeting propensity of its fusion intrabody (PEST-FIND calculated score of -2.41). The PEST-FIND scores, along with the net negative charge, predicted that the solubility of the PEST and scrambled PEST intrabody proteins should be similar. Turnover of the intrabody or the intrabody-antigen complex, however, may be different, depending on the ability of PEST sequence in context to direct each antigen to the proteasome.
Table 2. Calculated physio-chemical properties of parent and fusion intrabodies.
| scFv | Antigen | Fusion | PI (Putnam/Scripps) | Net Charge at pH 7.4 | Gravy Score |
|---|---|---|---|---|---|
|
D5E |
Amyloid oligomers |
HA |
8.38 |
2.7 |
-0.37 |
|
D5E |
|
HA-PEST |
6.53 |
-2.2 |
-0.403 |
|
D5E |
|
HA-SCR-PEST |
6.53 |
-2.2 |
-0.403 |
|
D10 |
α-Syn monomers and oligomers |
HA |
8.95 |
5.6 |
-0.263 |
|
D10 |
|
HA-PEST |
7.66 |
0.6 |
-0.312 |
|
D10 |
|
HA-SCR-PEST |
7.66 |
0.6 |
-0.312 |
|
10H |
α-Syn oligomers |
HA |
5.69 |
-1.5 |
-0.275 |
|
10H |
|
HA-PEST |
5.15 |
-6.5 |
-0.319 |
|
10H |
|
HA-SCR-PEST |
5.15 |
-6.5 |
-0.319 |
|
VH14 |
α-Syn monomer |
HA |
9.32 |
5.7 |
-0.399 |
|
VH14 |
|
HA-PEST |
7.8 |
0.8 |
-0.442 |
| VH14 | HA-SCR-PEST | 7.8 | 0.8 | -0.442 |
Data in Table 2 shows that fusion of PEST to intrabodies takes the net charge from strongly positive to much less positive for intrabodies VH14 and D10 scFv; from positive to negative for D5E scFv; and from negative to more negative for 10H scFv. These predictions are in approximate agreement with the in situ experimental data below.
Fusion with PEST improves soluble expression of α−syn intrabodies
In situ testing of steady-state levels of anti-α-syn-intrabody-PEST fusion constructs transiently transfected into the neuronal cell line ST14A was performed using western blots. Controls included the scrambled PEST sequence that does not change the overall charge characteristics of the proteins. pcDNA3.1- (empty vector) served as a negative control. Soluble expression of the intrabodies was assayed in the presence and absence of the specific proteasome inhibitor epoxomicin using HA staining, quantitated by densitometry normalized to actin.
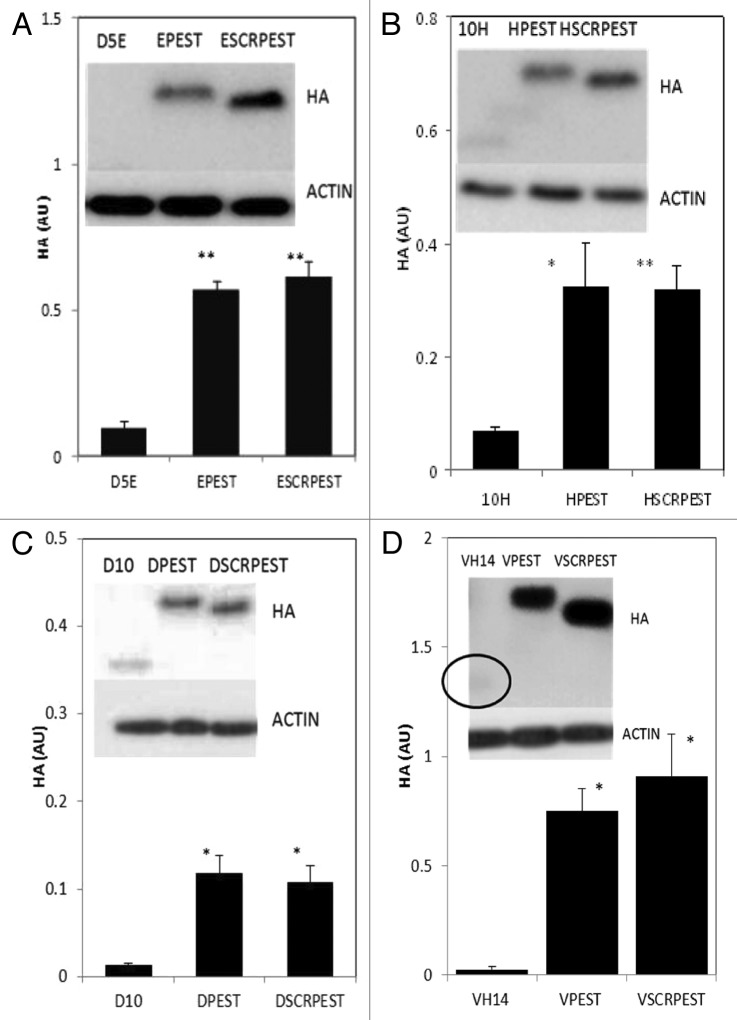
In our previous publication, the anti-oligomeric D5 scFv was tested for proof of concept, using fusion to 3xFLAG and NLS, which changed the calculated charge, and also improved solubility when tested in cells.5 The current version of this intrabody, D5E scFv, is a single substitution variant (E58Q, at the end of HCDR2) that is still significantly unstable. We therefore started this series of experiments testing soluble expression of parent and fusion D5E scFv intrabodies (Fig. 1A). Fusions with PEST or SCRPEST significantly increased the levels of soluble protein.
Figure 1. Intracytoplasmic soluble expression of intrabody-PEST and intrabody-SCRPEST constructs is increased significantly with respect to intrabody alone. (A) D5E scFv, designated E; (B) 10H scFv, designated H; (C) D10 scFv, designated D; (D) VH14, designated V. ST14A cells were transiently transfected with Intrabody-hemagluttinin (HA), Intrabody-HA-PEST or Intrabody-HA-SCRPEST constructs. 48 h post-transfection, cells were harvested, cell lysates prepared, soluble protein separated and transferred on western blots. Proteins were identified using anti-HA monoclonal antibodies, with actin as a loading control. Proteins were quantified densitometrically and normalized to actin. Negative control lanes, empty vector only (pcDNA3.1-), were always blank. At least 3 independent experiments were performed and representative gels are illustrated. One-way ANOVA with Minitab statistical software was used to perform statistical significance. (*p < 0.05, **p < 0.01, compared with intrabody-HA)
To determine how uniform the solubilizing effect might be, two additional scFv intrabodies were tested in the same formats. Figure 1B shows soluble expression of a second anti-oligomeric intrabody, 10H scFv, and its fusion variants. As was the case with D5E, soluble expression of 10H-PEST and 10H-scrambled-PEST increased about 5× with respect to that of intrabody alone. Figure 1C shows soluble expression of the more broadly interacting pan-specific intrabody scFv D10. The soluble level of D10 increased about 9 and 8 fold with PEST and scrambled-PEST fusions respectively
In a yeast surface display library selection, the strongest binder to the critical hydrophobic interaction region (non-amyloid component; NAC) of α-syn was determined to be a single domain nanobody, VH14.17 As an unprotected heavy chain only intrabody, VH14 is extremely insoluble, and is prone to intracellular aggregation.18 The PEST fusion was also able to rescue intracellular solubility for this construct (Fig. 1D).
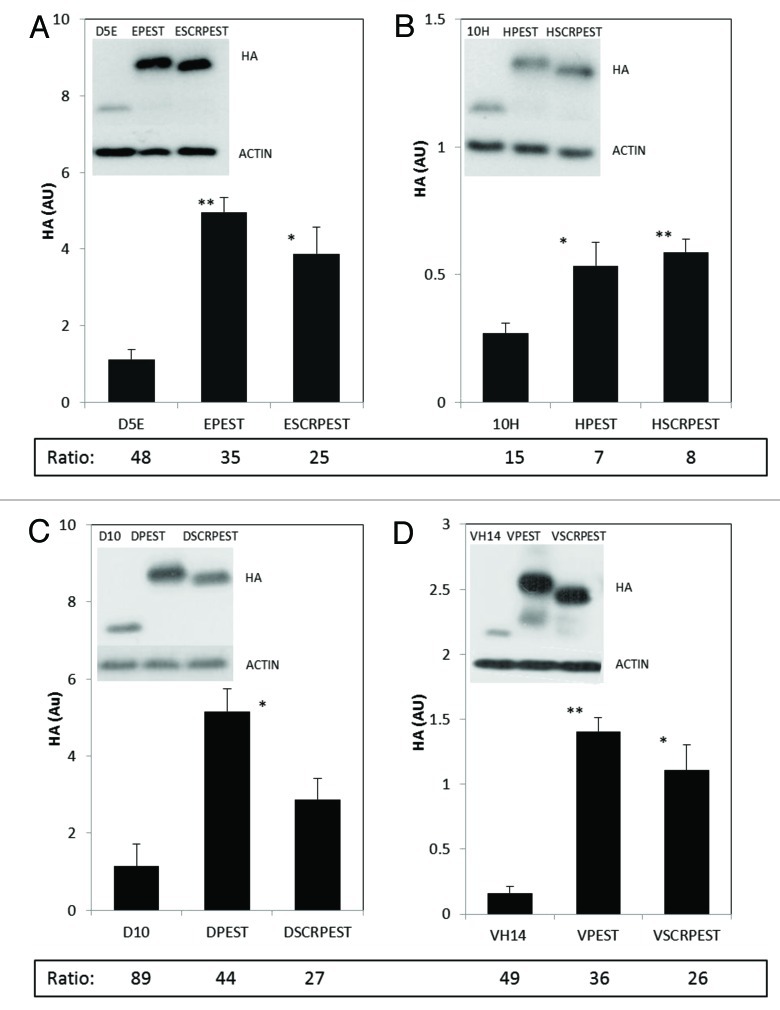
Proteasome inhibition has similar effects on all α-syn intrabody-PEST fusion proteins
Epoxomicin was used to examine the extent to which turnover of the different constructs was proteasome-mediated. Levels of all proteins increased with this inhibitor; individual ratios of inhibitor:uninhibited values are tabulated below each bar (Fig. 2). Proteasome inhibitied:uninhibited ratios for D5E, D5E-PEST and D5E-SCRPEST ranged from 25–48 (Fig. 2A), with a similar pattern for D10 scFv and VH14 nanobody (Fig. 2C and D).The substantial increases in ratios in the presence of specific proteasome inhibitor epoxomicin indicated that all three variants of these intrabodies, including some fraction of the least soluble, can be degraded by proteasome-mediated proteolysis under physiological conditions. For the initially more soluble 10H scFv constructs, protein levels also increased with inhibitor, but to a lesser extent relative to rest of the intrabodies tested (15 to 8 fold; Figure 2B). Proteasome inhibitor had a stronger effect on the intact PEST variants than on scrambled PEST variants of D5E, D10 and VH14, while these values were similar for10H scFv. All intrabody proteins were increased multifold by epoxomicin, indicating that even without a physiologically functional PEST motif these intrabodies may be normally degraded by the proteasome to some extent.
Figure 2. Intracytoplasmic soluble expression of intrabody-PEST and intrabody-scrambled PEST constructs is increased significantly with respect to intrabody alone when treated with epoxomicin. (A) D5E scFv, designated E; (B) 10H scFv, designated H; (C) D10 scFv, designated D; (D) VH14, designated V. For all intrabodies, the level of soluble protein is significantly higher in epoxomicin treated cells compared with that of vehicle (DMSO) treated cells in Figure 1; Ratio of Figure 2 (+inhibitor) to Figure 1 (no inhibitor) values are shown at the bottom of each graph in Figure 2. Multiple fold increase is not apparent by just looking at the y axis scales of the Figure 1 and 2 because multiple exposures were taken for each treatment. Actin was used as a control to evaluate protein levels across various exposures. ST14A cells were transiently transfected with intrabody, intrabody-PEST or intrabody-scrambled-PEST constructs. Twelve hours prior to harvest cells were treated with 9 μM epoxomicin and 48 h after the transfection cells were harvested, cell lysates prepared, soluble protein separated and run on western blots. Proteins were identified using anti-HA, with actin as a loading control. Proteins were quantified densitometrically and normalized to actin. Inhibitor and non-inhibitor lanes actin bands were used separately from different exposures to calculate ratios, using only non-saturated exposures. At least 3 independent experiments were performed and representative gels are illustrated. One-way ANOVA with Minitab statistical software was used to perform statistical significance. (*p < 0.05, **p < 0.01, compared with intrabody-HA)
PEST fusion can increase antigen clearance
Given that the PEST fusion did not lead to greatly enhanced turnover of the scFv and VH intrabody proteins, it was unclear whether the fusion could increase the turnover of the antigen targets. VH14 is the least soluble of our four constructs, and binds to the hydrophobic region thought to be critical to α-syn misfolding and aggregation. To determine if PEST-fused intrabody can enhance the clearance of its target, and that the enhanced clearance is not due only to the increased solubility, we transiently co-transfected α-syn~GFP plasmid with the three VH14 variants. We then quantified the levels of synuclein~GFP monomer using anti-GFP antibody. Figure 3 shows that VH14-PEST reduced the synuclein~GFP significantly relative to control.
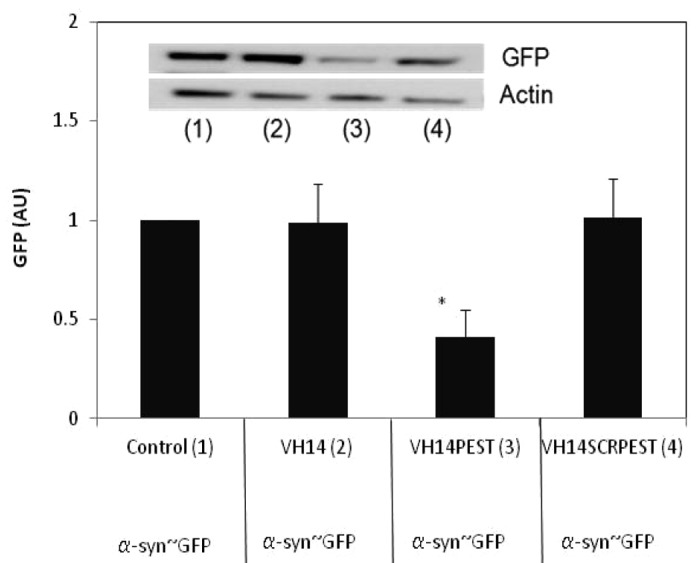
Figure 3. α-syn-GFP is degraded significantly in presence of VH14-PEST when compared with control (empty vector: pcDNA3.1- only treatment), VH14 or VH14-scrambled-PEST treated ST14A cells. ST14A cells were transiently co-transfected with α-syn-GFP and control or VH14 or VH14-PEST or VH14-scrambled-PEST constructs. 48 h. post-transfection, cells were harvested, cell lysates prepared, soluble protein separated and run on western blots. Proteins were identified using GFP antibody, and actin was used as a loading control. Proteins were quantified densitometrically and normalized to actin. At least 6 independent experiments were performed. One-way ANOVA with Minitab statistical software was used to perform statistical significance. (*p < 0.05, **p < 0.01, compared with intrabody-HA)
Discussion
Application of recombinant DNA methods have been used to engineer antibody molecules that retain the high specificity and affinity in single-chain Fv or single domain formats that will allow extensive engineering. This process is now well developed for multifunctional and antibody-drug conjugates used as cancer immunotherapies.19 There have also been notable breakthroughs in using engineered fragments that target infectious agents and toxins.20 There are obviously many critical intracellular targets in addition to those that are soluble or on cell surfaces, particularly in neurodegenerative diseases that currently lack protective therapies. scFv antibodies or VH/VL nanobodies delivered as intracellular antibody (intrabody) genes make ideal candidates for modulation of intracellular processes,21 but progress has been limited due to the problem of insolubility of these fragments in the complex, crowded, unfavorable redox environment of the cytoplasm. Intracellular proteins are cleared via proteasomes, lysosomes, or autophagosomes, each of which utilizes different mechanisms. Simply binding to an abnormal intracellular protein can alter the kinetics of misfolding, production of abnormal cleavage products, or extent of post-translational modification,1,22 but, since even the strongest binding antibody is thermodynamically reversible, toxic products of the target protein will accumulate over time. In this study, we present data showing that for several different intrabody constructs, fusion with the proteasomal targeting motif PEST can enhance the initial solubility of the intrabody, while increasing the turnover of the bound antigen, permanently reducing future availability for toxic forms.
We used this approach to fuse a C-terminal PEST region of mouse ornithine decarboxylase (mODC; amino acids 422–461) to intrabodies against proteins implicated in neurodegeneration.10,23,24 PEST (proline, glutamic acid, serine and threonine) motifs are targeted to the proteasome for rapid turnover. The PEST region is also enriched in polar and negatively charged residues and thus capable of adding negative charge to the fused proteins. Our lab has previously shown that the soluble level of an aggregation-prone intrabody is correlated with the estimated protein charge and hydropathicity at physiological pH.5 Since the net negative charge at physiological pH correlates with increased soluble expression of intrabodies, we hypothesized that the PEST fusion intrabodies would show improved soluble expression. Our theoretical calculations of charge properties are in reasonable agreement with the in situ solubilities, and both PEST and scrambled PEST protein constructs increased the soluble levels of the intrabodies significantly relative to the parent intrabody.
To approximate the fraction of the protein that was being degraded via the proteasome, we used the specific proteasomal inhibitor epoxomicin. This inhibition greatly increased soluble expression of all intrabody constructs. The increase in intrabody-SCRPEST protein in the presence of epoxomicin might mean that the scrambled sequence has retained some PEST activity. It seems more likely, however, that some fraction of these proteins normally may be degraded by the proteasome, with PEST serving to increase the rate. Just as the PEST and scrambled PEST fusions influence the soluble expression of the intrabodies, it is possible that structure and sequence of intrabodies may influence how much and where the PEST and scrambled PEST fused intrabodies may be degraded. This may explain why 10H scFv, which has a sequence and solubility that differ from the others in this series, also shows a smaller effect of the PEST and SCRPEST.
In contrast to previous studies23,25 where heterologous transfer of mODC-PEST to stable proteins such as GFP or Luciferase results in a significant reduction of intracellular half-life, the transfer of mODC-PEST degron to several of our intrabodies has not greatly reduced intracellular levels.10,26 It is critical to consider the effect of the fusion on the steady-state level of intrabody protein because long half-life has been reported to be as or more critical than affinity for intracellular function.22 Our lab recently showed that fusing the mODC PEST degron (aa 422–661) to the anti-huntingtin C4 scFv intrabody27,28 reduced the target mutant huntingtin exon 1 protein fragments by 80–90% relative to C4 scFv alone,10 but there was only a 25% decrease of C4 scFv-PEST intrabody protein levels in either the presence or absence of antigen. This is a relatively modest decrease in level, and the clinical target in vivo (huntingtin) is known to be present only at low levels, suggesting that the fusion will enhance, rather than impair, clinical utility.
In the present study, a diverse set of anti-α-syn-PEST intrabodies do not appear to be significantly destabilized by the mODC PEST degron. In fact, given the intrinsic insolubility of the constructs in this study, the overall soluble expression is greatly increased. These data are in contrast to the data published on anti-β-galactosidase 13R4 scFv-PEST intrabody,26 where the mODC-PEST fusion rendered the intrabody-PEST protein itself unstable, and subsequent binding to its β-gal target stabilized the complex. We have now shown that 6 different intrabodies fused to the mODC-PEST degron do not result in a destabilization of the intrabody-PEST fusion protein, although two of them do enhance the turnover of their monomeric targets. For α-syn, intrabody levels may be even more critical than for huntingtin, since the target protein is present at relatively high levels. The PEST fusion approach may therefore be critically improving clinical functionality. Using an alternative approach for a camelid VHH intrabody against botulinum neurotoxin, a direct fusion with a targeted F-box sequence was able to enhance toxin turnover even at the low levels induced by the fusion.20 This F-box approach may require very high affinity, plus intrinsically soluble intrabodies, but is also worth considering for empirical testing.
The lack of instability of the fusion intrabodies in the absence of target, while accelerating clearance of antigen complexes such as huntingtin or α-syn, with variable turnover of the fusion intrabody itself, may be due to intrabody folding that partially buries the critical regions of the degron that have been described by Coffino and colleagues.28,29 One explanation consistent with these observations is that, upon binding of antigen, the proteasomal targeting motif becomes available, and the complex migrates to the site of degradation. Given that proteins must further unfold in order to enter the proteasome, it is possible the antigen enters first, and that some of the intact intrabody is released. Alternatively, when the intrabody transiently dissociates, proximity to the proteasome may favor degradation of antigen. Future structural studies will be necessary to explore detailed mechanisms.
Conclusion
Our data clearly demonstrate that, while the details of the precise effects of PEST fusions on intrabodies are dependent upon the individual sequences, there is a high probability that fusion to PEST (or possibly similar negatively charged retargeting sequences) can dramatically improve solubility and functionality of intrabodies that are otherwise difficult to use intracellularly. Almost all promising intrabody constructs are thus worth testing empirically as PEST fusions in a relevant assay system.
Materials and methods
Cell culture and transfection
ST14A is a rat striatal progenitor cell line with high transfectability and many neuronal characteristics.5 Cells maintain relatively constant morphology during passaging, which is a favorable characteristic to reduce variable effects of macromolecular crowding on soluble expression of the intrabody. ST14A cells were cultured according to the standard protocol, and were mycoplasma free.10 Cells were transiently transfected with intrabody plasmids using Jet PEI DNA transfection reagent (Polyplus Transfection Inc.) as previously described.10 Culture medium was changed 4 h after transfection. Specific proteasome inhibitor epoxomicin (9 μM per well) or the vehicle DMSO were applied to the cells 12 h prior to the harvest.10 Cells were harvested 48 h post-transfection. To assay effects of the intrabody-PEST fusion on antigen turnover, α-syn~GFP plasmids, which use a (Gly4Ser)4 spacer to ensure individual folding of syn and GFP,3 were co-transfected with intrabody plasmids.
cDNA, previously described by Butler and Messer,10 encoding C4 scFv -HA [GenBank accession number EU 490426 for scFv-C4; hemagglutinin (HA) epitope tag with amino acid sequence YPYDVPDYA to visualize intrabodies] and C4 scFv-HA-PEST (standard and scrambled from aas 422–461 mODC) was used to subclone the D10, 10H. D5E scFv, and VH14 single domain nanobody, with and without PEST or PEST scrambled, using XbaI, NotI and HindIII restriction sites with standard cloning techniques (XbaI NotI for intrabody; NotI, HindIII for HA or HA-PEST). The resulting product was ligated into pcDNA3.1- with the use of restriction sites XbaI and HindIII. All expressed plasmids were prepared using Endofree Plasmid Maxi kit by Qiagen, and were confirmed by DNA sequencing.
Cell fractionation, western blot and quantification
Transiently transfected ST14A cells and the medium were collected from 6 well culture plates by trypsinization and subsequent centrifugation. Cells were washed twice with 1× PBS. Soluble and insoluble proteins were extracted as previously described.10 Briefly, whole cell lysates were extracted at 4°C in 30 μL/well RIPA lysis buffer (50 mM Tris pH 7.5, 150 mM NaCl, 1% NP40, 0.25% sodium deoxycholate, 0.1% SDS) with added complete protease inhibitor cocktail (Roche). Soluble and insoluble proteins were separated by microcentrifugation at 13000 rpm for 15 min, 4°C. Biorad RC-DC protein assay kit (Bio-Rad laboratories) was used to quantify soluble protein in the supernatant. 5× denaturing sample buffer (125 mM Tris, 4% SDS, 20% glycerol, 5% 2-mercaptoethanol, 0.02% bromophenol blue, pH 6.8) was used to make 1 mg/ml protein containing soluble lysates. The lysates were boiled at 95°C for 5 min prior to loading on the gels. Fifteen μg soluble protein was resolved by SDS-PAGE and transferred onto PVDF membranes (PerkinElmer) at 24 V for 30 min. HA tagged intrabodies were probed with monoclonal anti-HA (1:3000, Covance) and monoclonal anti-actin (as a loading control, 1:2000, Sigma) primary antibodies. HRP-conjugated goat anti-mouse IgG secondary antibody was used (1:2000, Santa Cruz Biotechnology) to label the proteins, which were then detected by ECL kit (PerkinElmer). GFP rabbit antibody (Cell Signaling 1:1000) was used to probe α-syn~GFP, with HRP-conjugated goat anti-rabbit secondary. Bands were quantified using densitometry and normalized with actin. Soluble expression of intrabody constructs in presence and absence of selective proteasome inhibitor epoxomicin was ascertained using western blots. ImageJ densitometry software (http://rsb.info.nih.gov/ij/) was used to quantify soluble proteins. Multiple exposures were taken to quantitate the protein levels from unsaturated bands in presence and absence of proteasome inhibitor, all normalized within lanes.
Statistical analysis was performed by one-way ANOVA with Minitab or Statview statistical software. All assays represent at least three independent replications.
Physico-chemical parameters
Protein Calculator v3.3, the tool developed by Chris Putnam at the Scripps Research Institute (www.scripps.edu/~cdputnam/protcalc) and the Protparam tool at the ExPASy proteomics server of the Swiss Institute of Bioinformatics were used to calculate physico-chemical parameters for different α-syn intrabody constructs, including isoelectric point, net charge at physiological pH (7.4) and grand average of hydropathicity (GRAVY).5 Hydrophobic and hydrophilic properties of amino acids are used to formulate the hydropathy scale and GRAVY score indicates hydropathic nature of protein. PEST-FIND algorithm was used to calculate scores of PEST and scrambled PEST motifs.8,10
Accession numbers: Sequence data have been deposited in GenBank as follows: D10-AY550177; 10H-JX430806; VH14-JX430807; D5E-JX442980
Acknowledgments
We thank Abigail Snyder-Keller and Kevin Manley for valuable discussions and Elena Cattaneo (University of Milano, Italy) for the rat ST14A cell line. We also thank Dr. Michael Sierks (ASU) for important discussions about the intrabodies and the Wadsworth Center Applied Genomic Technologies Core for sequencing. This work was supported by the Michael J. Fox Foundation and NIH/NINDS (NS053912, NS 073415 and NS061257).
Glossary
Abbreviations:
- scFv
single-chain Fv
- VH or VL
single-domain
- PD
Parkinson disease
- PEST regions
prolyl (P), glutamyl (E), aspartyl (D), seryl (S) and threonyl (T) residues
- mODC (amino acids 422-461)
mouse ornithine decarboxylase
- α-syn
Alpha-synuclein
- GFP
green fluorescent protein
- HA
hemagglutinin
- NAC
non-amyloid component
- SCRPEST
scrambled PEST
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/mabs/article/21696
References
- 1.Miller TW, Messer A. Intrabody applications in neurological disorders: progress and future prospects. Mol Ther. 2005;12:394–401. doi: 10.1016/j.ymthe.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 2.Messer A, Lynch SM, Butler DC. Developing intrabodies for the therapeutic suppression of neurodegenerative pathology. Expert Opin Biol Ther. 2009;9:1189–97. doi: 10.1517/14712590903176387. [DOI] [PubMed] [Google Scholar]
- 3.Huston JS, Levinson D, Mudgett-Hunter M, Tai MS, Novotný J, Margolies MN, et al. Protein engineering of antibody binding sites: recovery of specific activity in an anti-digoxin single-chain Fv analogue produced in Escherichia coli. Proc Natl Acad Sci U S A. 1988;85:5879–83. doi: 10.1073/pnas.85.16.5879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Butler DC, McLear JA, Messer A. Engineered antibody therapies to counteract mutant huntingtin and related toxic intracellular proteins. Prog Neurobiol. 2012;97:190–204. doi: 10.1016/j.pneurobio.2011.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kvam E, Sierks MR, Shoemaker CB, Messer A. Physico-chemical determinants of soluble intrabody expression in mammalian cell cytoplasm. Protein Eng Des Sel. 2010;23:489–98. doi: 10.1093/protein/gzq022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ellis RJ. Macromolecular crowding: an important but neglected aspect of the intracellular environment. Curr Opin Struct Biol. 2001;11:114–9. doi: 10.1016/S0959-440X(00)00172-X. [DOI] [PubMed] [Google Scholar]
- 7.Wörn A, Plückthun A. An intrinsically stable antibody scFv fragment can tolerate the loss of both disulfide bonds and fold correctly. FEBS Lett. 1998;427:357–61. doi: 10.1016/S0014-5793(98)00463-3. [DOI] [PubMed] [Google Scholar]
- 8.Rechsteiner M, Rogers SW. PEST sequences and regulation by proteolysis. Trends Biochem Sci. 1996;21:267–71. [PubMed] [Google Scholar]
- 9.Rogers S, Wells R, Rechsteiner M. Amino acid sequences common to rapidly degraded proteins: the PEST hypothesis. Science. 1986;234:364–8. doi: 10.1126/science.2876518. [DOI] [PubMed] [Google Scholar]
- 10.Butler DC, Messer A. Bifunctional anti-huntingtin proteasome-directed intrabodies mediate efficient degradation of mutant huntingtin exon 1 protein fragments. PLoS One. 2011;6:e29199. doi: 10.1371/journal.pone.0029199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Emadi S, Barkhordarian H, Wang MS, Schulz P, Sierks MR. Isolation of a human single chain antibody fragment against oligomeric alpha-synuclein that inhibits aggregation and prevents alpha-synuclein-induced toxicity. J Mol Biol. 2007;368:1132–44. doi: 10.1016/j.jmb.2007.02.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Emadi S, Kasturirangan S, Wang MS, Schulz P, Sierks MR. Detecting morphologically distinct oligomeric forms of alpha-synuclein. J Biol Chem. 2009;284:11048–58. doi: 10.1074/jbc.M806559200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhou C, Emadi S, Sierks MR, Messer A. A human single-chain Fv intrabody blocks aberrant cellular effects of overexpressed alpha-synuclein. Mol Ther. 2004;10:1023–31. doi: 10.1016/j.ymthe.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 14.Emadi S, Liu R, Yuan B, Schulz P, McAllister C, Lyubchenko Y, et al. Inhibiting aggregation of alpha-synuclein with human single chain antibody fragments. Biochemistry. 2004;43:2871–8. doi: 10.1021/bi036281f. [DOI] [PubMed] [Google Scholar]
- 15.Giasson BI, Murray IV, Trojanowski JQ, Lee VM. A hydrophobic stretch of 12 amino acid residues in the middle of alpha-synuclein is essential for filament assembly. J Biol Chem. 2001;276:2380–6. doi: 10.1074/jbc.M008919200. [DOI] [PubMed] [Google Scholar]
- 16.Periquet M, Fulga T, Myllykangas L, Schlossmacher MG, Feany MB. Aggregated alpha-synuclein mediates dopaminergic neurotoxicity in vivo. J Neurosci. 2007;27:3338–46. doi: 10.1523/JNEUROSCI.0285-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lynch SM, Zhou C, Messer A. An scFv intrabody against the nonamyloid component of alpha-synuclein reduces intracellular aggregation and toxicity. J Mol Biol. 2008;377:136–47. doi: 10.1016/j.jmb.2007.11.096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dudgeon K, Famm K, Christ D. Sequence determinants of protein aggregation in human VH domains. Protein Eng Des Sel. 2009;22:217–20. doi: 10.1093/protein/gzn059. [DOI] [PubMed] [Google Scholar]
- 19.Wheeler YY, Chen SY, Sane DC. Intrabody and intrakine strategies for molecular therapy. Mol Ther. 2003;8:355–66. doi: 10.1016/S1525-0016(03)00183-7. [DOI] [PubMed] [Google Scholar]
- 20.Kuo CL, Oyler GA, Shoemaker CB. Accelerated neuronal cell recovery from Botulinum neurotoxin intoxication by targeted ubiquitination. PLoS One. 2011;6:e20352. doi: 10.1371/journal.pone.0020352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rondon IJ, Marasco WA. Intracellular antibodies (intrabodies) for gene therapy of infectious diseases. Annu Rev Microbiol. 1997;51:257–83. doi: 10.1146/annurev.micro.51.1.257. [DOI] [PubMed] [Google Scholar]
- 22.Zhu Q, Zeng C, Huhalov A, Yao J, Turi TG, Danley D, et al. Extended half-life and elevated steady-state level of a single-chain Fv intrabody are critical for specific intracellular retargeting of its antigen, caspase-7. J Immunol Methods. 1999;231:207–22. doi: 10.1016/S0022-1759(99)00158-1. [DOI] [PubMed] [Google Scholar]
- 23.Li X, Zhao X, Fang Y, Jiang X, Duong T, Fan C, et al. Generation of destabilized green fluorescent protein as a transcription reporter. J Biol Chem. 1998;273:34970–5. doi: 10.1074/jbc.273.52.34970. [DOI] [PubMed] [Google Scholar]
- 24.Leclerc GM, Boockfor FR, Faught WJ, Frawley LS. Development of a destabilized firefly luciferase enzyme for measurement of gene expression. Biotechniques. 2000;29:590–1, 594-6, 598 passim. doi: 10.2144/00293rr02. [DOI] [PubMed] [Google Scholar]
- 25.Leclerc GM, Boockfor FR, Faught WJ, Frawley LS. Development of a destabilized firefly luciferase enzyme for measurement of gene expression. Biotechniques. 2000;29:590–1, 594-6, 598 passim. doi: 10.2144/00293rr02. [DOI] [PubMed] [Google Scholar]
- 26.Sibler AP, Courtête J, Muller CD, Zeder-Lutz G, Weiss E. Extended half-life upon binding of destabilized intrabodies allows specific detection of antigen in mammalian cells. FEBS J. 2005;272:2878–91. doi: 10.1111/j.1742-4658.2005.04709.x. [DOI] [PubMed] [Google Scholar]
- 27.Lecerf JM, Shirley TL, Zhu Q, Kazantsev A, Amersdorfer P, Housman DE, et al. Human single-chain Fv intrabodies counteract in situ huntingtin aggregation in cellular models of Huntington’s disease. Proc Natl Acad Sci U S A. 2001;98:4764–9. doi: 10.1073/pnas.071058398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li X, Coffino P. Distinct domains of antizyme required for binding and proteolysis of ornithine decarboxylase. Mol Cell Biol. 1994;14:87–92. doi: 10.1128/mcb.14.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang M, MacDonald AI, Hoyt MA, Coffino P. Proteasomes begin ornithine decarboxylase digestion at the C terminus. J Biol Chem. 2004;279:20959–65. doi: 10.1074/jbc.M314043200. [DOI] [PubMed] [Google Scholar]