Abstract
Modulating the binding affinities to IgE or changing the FcγR binding properties of anti-IgE antibodies offers an opportunity to enhance the therapeutic potential of anti-IgE antibodies, but the influence of increased affinity to IgE or reduced Fc effector function on the pharmacological properties of anti-IgE therapies remains unclear. Our studies were designed to characterize the pharmacokinetics, pharmacodynamics and immune-complex distribution of two high-affinity anti-IgE monoclonal antibodies, high-affinity anti-IgE antibody (HAE) 1 and 2, in mice and monkeys. HAE1, also known as PRO98498, is structurally similar to omalizumab (Xolair®), a humanized anti-IgE IgG1 marketed for the treatment of asthma, but differs by 9 amino acid changes in the complementarity-determining region resulting in a 23-fold improvement in affinity. HAE2 is similar to HAE1, but its Fc region was altered to reduce binding to Fcγ receptors. As expected given the decreased binding to Fcγ receptors, systemic exposure to pre-formed HAE2:IgE complexes in mice was greater (six-fold) and distribution to the liver lower (four-fold) compared with HAE1:IgE complexes. In monkeys, systemic exposure to HAE1 was similar to that previously observed for omalizumab in this species, but required comparatively lower serum drug concentrations to suppress free IgE levels. HAE2 treatment resulted in greater exposure and greater increase of total IgE, relative to HAE1, because of decreased clearance of HAE2:IgE complexes. Overall, these data suggest that increased binding affinity to IgE may provide a more effective therapeutic for asthma patients, and that retaining FcγR binding of the anti-IgE antibody is important for elimination of anti-IgE:IgE complexes.
Keywords: pharmacokinetics, pharmacodynamics, Fc-gamma receptor, complex clearance, Immunoglobulin-E, anti-IgE, monoclonal antibody
Introduction
Since its discovery in 1967, the role of human immunoglobulin E (IgE) as a key mediator in the pathophysiology of asthma and allergy has been well-established. When IgE binds to Fc epsilon-receptors (FcεR) on the surface of mast cells and basophils, allergens cross-link the bound IgE, thereby causing degranulation and the subsequent release of inflammatory mediators such as histamine.1,2 Because of the central role that IgE plays in the allergic cascade, recent advancements in the treatment of allergic disease have focused on blocking the binding of IgE to FcεR by use of monoclonal antibodies (mAbs) targeting IgE.
Omalizumab (Xolair®) is a humanized IgG1 mAb directed against the Cε3 domain of IgE approved for the treatment of moderate to severe asthma. In human trials, omalizumab treatment has been shown to reduce serum levels of free IgE and to downregulate the number of FcεR1 cells on basophils in atopic subjects.1 Clinical benefit with omalizumab has been demonstrated when free IgE levels are reduced to less than 20 IU/mL in asthmatic patients.3 To achieve a clinically meaningful reduction in the level of free IgE, however, an individualized tiered dosing table based on baseline levels of free IgE and body weight is required.3 Individuals with high pre-treatment IgE levels (> 700 IU/mL) or those with higher body weights (> 150 kg) often require multiple omalizumab injections or do not qualify for therapy because the dosage required would be too high.4 Due to these limitations, development of a higher-affinity anti-IgE molecule offers the potential to reduce the dose required to suppress free IgE levels and may allow treatment of asthmatic individuals not eligible to receive omalizumab therapy. However, the influence of increased affinity to IgE or reduced Fc effector function on the pharmacological properties of anti-IgE therapies remains unclear.
High affinity anti-IgE antibody-1 (HAE1, also known as PRO98498) is a second generation, higher affinity version of omalizumab that binds to the same epitope on IgE as omalizumab. HAE1 was developed using the same IgG1 framework as omalizumab, but differs from omalizumab by nine amino acid differences in the complementarity-determining region (CDR). In vitro studies with the Fab fragments of HAE1 and omalizumab demonstrated that these nine amino acid changes increased the binding affinity of HAE1 to IgE by approximately 23-fold over that of omalizumab. This improvement in affinity is due primarily to a ~22-fold slower disassociation rate (Koff) of HAE1 for IgE. Based on kinetic binding measurements, the apparent Kd for HAE1 is 0.66 nM compared with 15.5 nM for omalizumab.5 High affinity anti-IgE antibody-2 (HAE2) shares the same Fab fragment and IgG1 framework as HAE1 with the exception of a single-point mutation (D265A) in the Fc region, which results in an ~85-fold reduction in HAE2-IgE complex binding to FcγRI, II, and III relative to wild-type IgG1.6 Importantly, though interaction with FcγR is significantly reduced due to this mutation, results from in vitro studies demonstrate that interaction with FcRn remains intact.6
Data from studies in mice, monkeys and humans have shown that the disposition of omalizumab is influenced by both its IgG1 framework and by complex formation with IgE.7-9 The substantial structural homology between HAE1 and omalizumab and the similarities in their mechanism of action suggest that the absorption, distribution, metabolism, and excretion (ADME) profiles should be similar. The markedly higher affinity of HAE1 for IgE, however, may modify the pharmacodynamic (PD) profile of both free IgE and total IgE (free IgE and IgE complexed with drug) compared with omalizumab. Likewise, the reduced binding of HAE2 to Fcγ receptors could affect the overall disposition profile of the HAE2:IgE complexes compared with omalizumab.
The purpose of our studies was to characterize the pharmacokinetics (PK), pharmacodynamics (PD) and immune-complex distribution of HAE1 and HAE2 in mice and monkeys.
Results
The goals of our studies were to assess the immune complex distribution and the PK/PD of HAE1 and HAE2 in relevant animal models. No adverse events related to immune complex formation were observed in either species. Our initial in vivo assessment of pre-formed anti-IgE:IgE immune complexes was performed in mice because this species was used extensively in assessing the clearance mechanisms utilized for omalizumab:IgE and the model has been well-characterized.10 We chose the cynomolgus monkey to evaluate the PK and PD of HAE1 and HAE2 because both bind to cynomolgus IgE with similar affinity compared with that observed for human IgE,5 and omalizumab and IgE are known to form complexes of various sizes in cynomolgus monkey.8 Additionally, cynomolgus monkey was used extensively in omalizumab non-clinical studies; therefore, conducting PK/PD studies of HAE1 and HAE2 in this species would allow direct comparisons to previous results obtained with omalizumab.
125I-IgE-HAE1 complex distribution in mice
The plasma concentration-time profile for 125I-IgE-HAE1 complexes was characterized by a rapid distribution phase followed by a slow elimination phase. Generally similar disposition profiles were also observed for 125I-IgE, 125I-IgE-omalizumab and 125I-IgE-HAE2 (Fig. 1). Exposure to 125I-IgE-HAE1 complexes, as determined by the area under the serum concentration-time curve out to day 7 (AUC), was generally similar to that observed for 125I-IgE or 125I-IgE-omalizumab complexes. The mean AUC for 125I-IgE-HAE1 was 14 d * μg/mL compared with 14 and 11 d * μg/mL for 125I-IgE and 125I-IgE-omalizumab, respectively. Comparatively, the AUC for 125I-IgE-HAE2 complexes was 6- to 8-fold greater (88 d * μg/mL) than that observed for the other treatment groups.
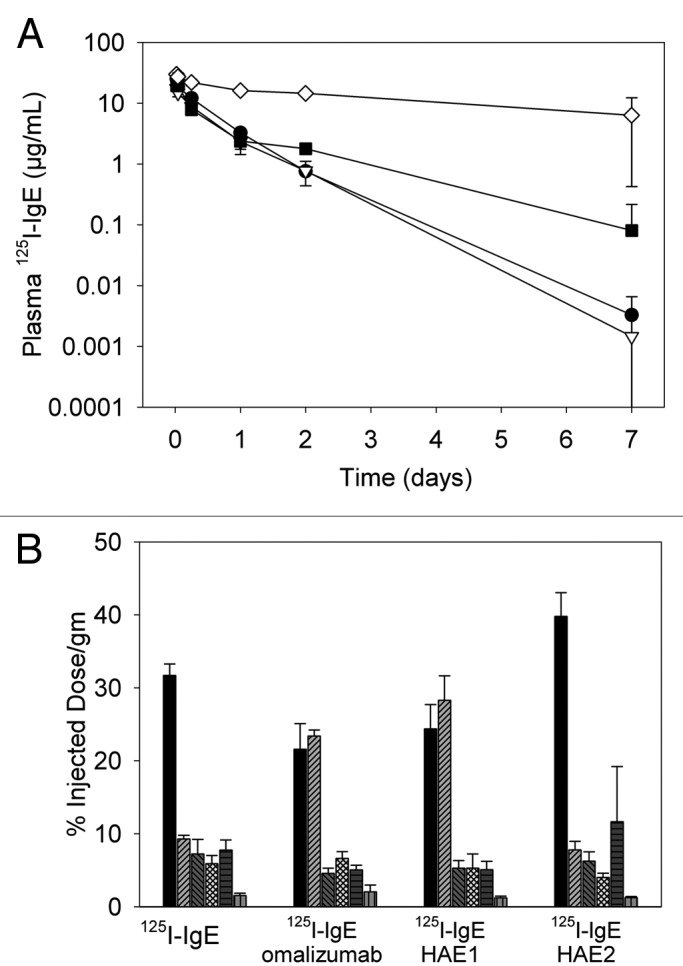
Figure 1. (A) Plasma concentration-time profiles for 125I-IgE alone (1.3 mg/kg) or pre-complexed with 5-fold molar excess of omalizumab, HAE1 or HAE2 (5 mg/kg) following a single intravenous administration. Data represent the mean (± SD) of n = 3 mice per group at each timepoint (closed circle, 125I-IgE; open triangle, 125I-IgE plus omalizumab; closed square, 125I-IgE plus HAE1; open diamond, 125I-IgE plus HAE2). (B) Tissue distribution (% injected dose/gm) for 125I-IgE alone (1.3 mg/kg) or pre-complexed with 5-fold molar excess of omalizumab, HAE1 or HAE2 (5 mg/kg) 30 min following a single intravenous administration. Data represent the mean (± SD) of n = 3 mice per group (black, blood; light gray hatched, liver; dark gray hatched, kidney; light gray checked, spleen; dark gray vertical lines, lung; light gray horizontal lines, stomach).
125I-IgE-HAE1 complexes appeared evenly distributed between blood (24% injected dose per gram tissue (ID/gm)) and liver (28% ID/gm) at 30 min post-dose and uptake in spleen, kidney, lungs and stomach was minor (Fig. 1B). These results appeared similar to those observed for 125I-IgE-omalizumab complexes. Comparatively, when 125I-IgE was administered as a single agent, a larger proportion of signal was retained in blood (32% ID/gm,) with little distribution to liver (9% ID/gm). For 125I-IgE-HAE2, signal in blood was highest, constituting ~40% ID/gm of the injected dose, with uptake into the liver representing only 8% ID/gm. These results reflect an approximate 4-fold reduction in 125I-IgE-HAE2 complex distribution to liver compared with 125I-IgE-HAE1. By 7 d post dose, signal was observed only in animals given 125I-HAE2, where 7% ID/gm was observed in blood and < 2% ID/gm observed for all other tissue (data not shown).
PK/PD in cynomolgus monkeys
Following subcutaneous (SC) administration to cynomolgus monkeys, exposure to both HAE1 and HAE2 increased with dose and exhibited a long elimination phase that appeared similar across dose levels (Fig. 2A and B). Peak concentrations were observed 1–3 d post-dose. For HAE1, the apparent clearance (CL/F) decreased modestly as doses increased (Table 1) and the apparent volume of distribution at steady-state (Vss/F) was approximately equal to plasma volume (range: 37–75 mL/kg).11 Comparatively, for HAE2, the CL/F and Vss/F were significantly reduced (p < 0.05) relative to HAE1. The mean half- life of both HAE1 and HAE2 appeared similar and ranged from 7–12 d.
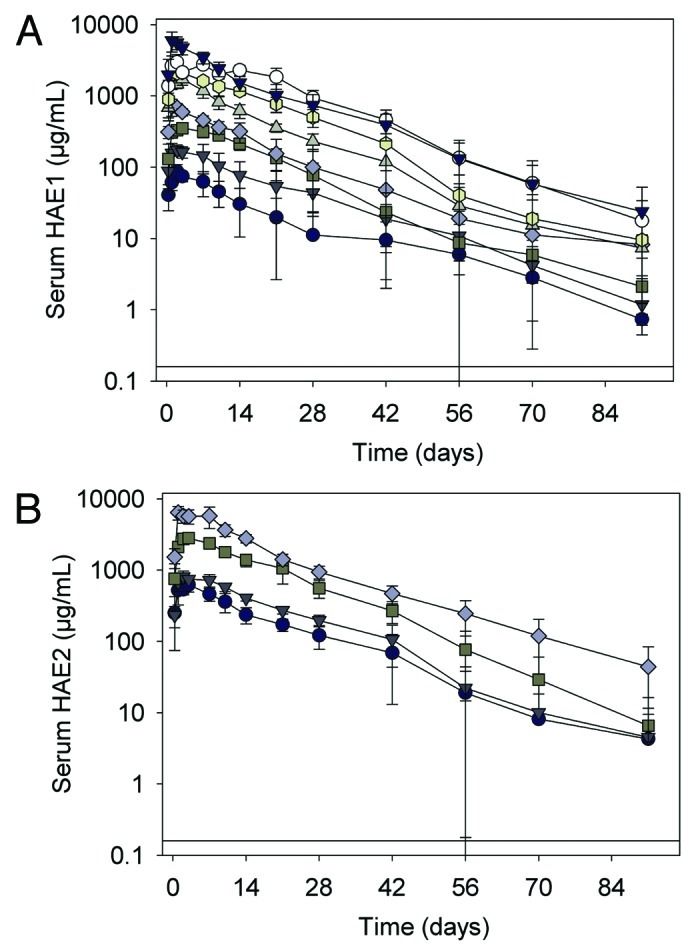
Figure 2. (A) Serum HAE1 concentration-time profiles following a single subcutaneous administration to juvenile cynomolgus monkeys. Data represent the mean ± SD of n = 6 animals/group. Closed circle, HAE1 7.5 mg/kg; closed dark gray triangle, 15 mg/kg; closed square, 30 mg/kg; closed diamond, 50 mg/kg; closed light gray triangle, 75 mg/kg; closed octagon, 100 mg/kg; open circle, 200 mg/kg; closed black triangle, 250 mg/kg. Black solid line represents the limit of quantitation (LOQ) in the HAE1 ELISA (< 0.160 μg/mL). (B) Serum total HAE2 concentration-time profiles following a single subcutaneous administration to juvenile cynomolgus monkeys. Data represent the mean ± SD of n = 6 animals/group. Closed circle, HAE2 30 mg/kg; closed triangle, 50 mg/kg; closed square, 100 mg/kg; closed diamond, 250 mg/kg. Black solid line represents the limit of quantitation (LOQ) in the HAE2 ELISA (< 0.160 μg/mL).
Table 1. Non compartmental pharmacokinetic parameter estimates (mean ± SD) following a single SC bolus of HAE1 or HAE2 to juvenile cynomolgus monkeys.
| Dose (mg/kg) |
CL/F (mL/day/kg) |
Vss/F (mL/kg) |
t1/2, elim (day) |
|---|---|---|---|
| HAE1 | |||
| 7.5 |
7.9 ± 4.3 |
75 ± 10 |
6.8 ± 4.1 |
| 15 |
6.5 ± 5.1 |
68 ± 16 |
7.8 ± 42 |
| 30 |
4.4 ± 0.9 |
62 ± 7.7 |
7.1 ± 4.1 |
| 50 |
5.1 ± 1.7 |
63 ± 7.6 |
8.4 ± 4.7 |
| 75 |
3.1 ± 0.5 a |
42 ± 4.3 b |
6.6 ± 4.7 |
| 100 |
2.5 ± 0.4 b |
37 ± 2.9 b |
9.1 ± 5.1 |
| 200 |
2.8 ± 0.6 b |
52 ± 4.9 b |
10 ± 4.5 |
| 250 | 3.4 ± 0.8 a | 50 ± 10 b | 8.6 ± 6.0 |
| HAE2 | |||
|---|---|---|---|
| 30 |
3.0 ± 1.1 d |
46 ± 10 d |
9.0 ± 4.9 |
| 50 |
3.2 ± 0.5 d |
51 ± 6.2 d |
7.0 ± 4.0 |
| 100 |
1.9 ± 0.2 c |
31 ± 4.5 c, d |
7.6 ± 2.9 |
| 250 | 2.4 ± 0.3 c | 38 ± 3.7 c, d | 12 ± 4.3 |
CL/F, clearance; t1/2,elim, elimination half life; Vss/F, volume of distribution at steady state. Note: n = 6/group. ap ≤ 0.05 compared with HAE1 7.5 and 15 mg/kg dose groups. bp ≤ 0.05 compared with HAE1 7.5, 15, 30 and 50 mg/kg dose groups. cp ≤ 0.05 compared with HAE2 30 and 50 mg/kg dose groups. dp ≤ 0.05 compared with HAE1 at equivalent dose.
Serum total IgE levels were highly variable throughout the study but increased significantly (p < 0.05) following administration of HAE1 or HAE2 and remained elevated relative to placebo for the majority of the study (Fig. 3A and 3B). In animals given HAE1, the mean maximal increase in total IgE was 13- to 43-fold above baseline (IgEmax/Bsl IgE) across dose levels and fell to ~5 to 10-fold above baseline at most dose levels at the end of the study (IgElast/Bsl IgE). In animals given HAE2, the magnitude of the total IgE response was significantly greater than that observed for HAE1 where the maximal increase in total IgE was 30- to 112-fold above baseline (IgEmax/Bsl IgE) and ~20-fold above baseline at the end of the study (IgElast/Bsl IgE). For both HAE1 and HAE2, no clear or significant dose response relationship was seen (Table 2).
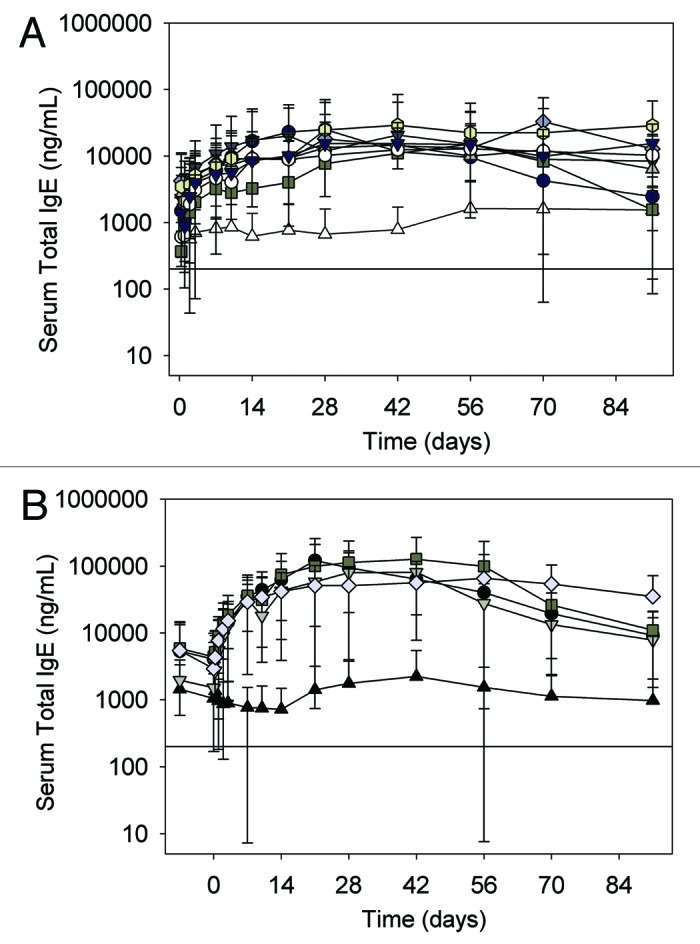
Figure 3. (A) Serum total IgE concentration-time profiles following a single subcutaneous administration of HAE1 to juvenile cynomolgus monkeys. Data represent the mean ± SD of n = 6 animals/group. Open triangle, control; Closed circle, HAE1 7.5 mg/kg; closed dark gray triangle, 15 mg/kg; closed square, 30 mg/kg; closed diamond, 50 mg/kg; closed light gray triangle, 75 mg/kg; closed octagon, 100 mg/kg; open circle, 200 mg/kg; closed black triangle, 250 mg/kg. Black solid line represents the limit of quantitation (LOQ) in the Total igE ELISA (< 200 ng/mL). (B) Serum total IgE concentration-time profiles following a single subcutaneous administration of HAE2 to juvenile cynomolgus monkeys. Data represent the mean ± SD of n = 6 animals/group. Closed triangle, control; Closed circle, HAE2 30 mg/kg; closed triangle, 50 mg/kg; closed square, 100 mg/kg; closed diamond, 250 mg/kg. Black solid line represents the limit of quantitation (LOQ) in the Total igE ELISA (< 200 ng/mL).
Table 2. Pharmacodynamic parameters (mean ± SD) following a single SC dose of HAE1 or HAE2 to juvenile cynomolgus monkeys.
| Dose (mg/kg) |
Bsl Avg IgE (ng/mL) |
IgEmax/Bsl IgE Ratio |
IgElast/Bsl IgE Ratio |
|---|---|---|---|
| HAE1 | |||
| Control |
656 ± 707 |
7.5 ± 7.6 |
2.4 ± 1.9 |
| 7.5 |
1310 ± 1290 |
30 ± 33 a |
5.3 ± 9.7 |
| 15 |
2730 ± 3850 |
34 ± 32 a |
4.3 ± 5.4 |
| 30 |
585 ± 798 |
26 ± 15 a |
6.5 ± 10 |
| 50 |
3040 ± 5190 |
13 ± 13 |
0.9 ± 0.3 |
| 75 |
2510 ± 3380 |
31 ± 28 a |
7.6 ± 11 |
| 100 |
2790 ± 4730 |
43 ± 43 a |
32 ± 50 |
| 200 |
623 ± 355 |
35 ± 25 a |
13 ± 14 |
| 250 | 1250 ± 864 | 13 ± 8.5 | 5.6 ± 8.8 |
| HAE2 | |||
|---|---|---|---|
| Control |
1830 ± 2630 |
1.9 ± 0.7 |
1.0 ± 0.7 |
| 30 |
4680 ± 7320 |
89 ± 68 b, c |
21 ± 39 |
| 50 |
1730 ± 1300 |
58 ± 24 b, c |
18 ± 40 |
| 100 |
5120 ± 6410 |
112 ± 136 b |
15 ± 27 |
| 250 | 4190 ± 6690 | 30 ± 20 b | 20 ± 22 a |
Bsl Avg IgE, mean baseline total IgE concentration; IgElast/Bsl IgE, last observed total IgE concentration divided by Bsl Avg IgE; IgEmax/Bsl IgE, maximum observed total IgE concentration divided by Bsl Avg IgE. Note: Data represent the mean (±SD) of n = 6/group. ap < 0.05 compared with Placebo. bp < 0.0001 compared with Placebo. cp < 0.05 compared with HAE1 at equivalent dose.
The free IgE response following administration of HAE1 was highly variable across treatment groups with levels substantially reduced relative to placebo in some animals while remaining unchanged in others. As a result, mean free IgE concentration-time profiles showed a clear reduction in free IgE concentrations only at the higher dose levels (≥ 50 mg/kg) and a clear dose response was not observed (Fig. 4A). Upon closer examination of the PK/PD relationship in individual animals, however, we observed that the reduction in free IgE showed a strong dependence on the molar relationship between HAE1 and total IgE concentrations in serum. In animals with high HAE1 concentrations relative to total IgE (molar ratio > 1:1), free IgE concentrations fell sharply. In animals with low HAE1 concentrations relative to total IgE (molar ratio < 1:1), minimal change in free IgE was observed (Fig. 4B). By evaluating results in this manner, it is clear that maximal suppression of free IgE levels can be achieved when the molar ratio of HAE1 to total IgE is greater than ~1:1.
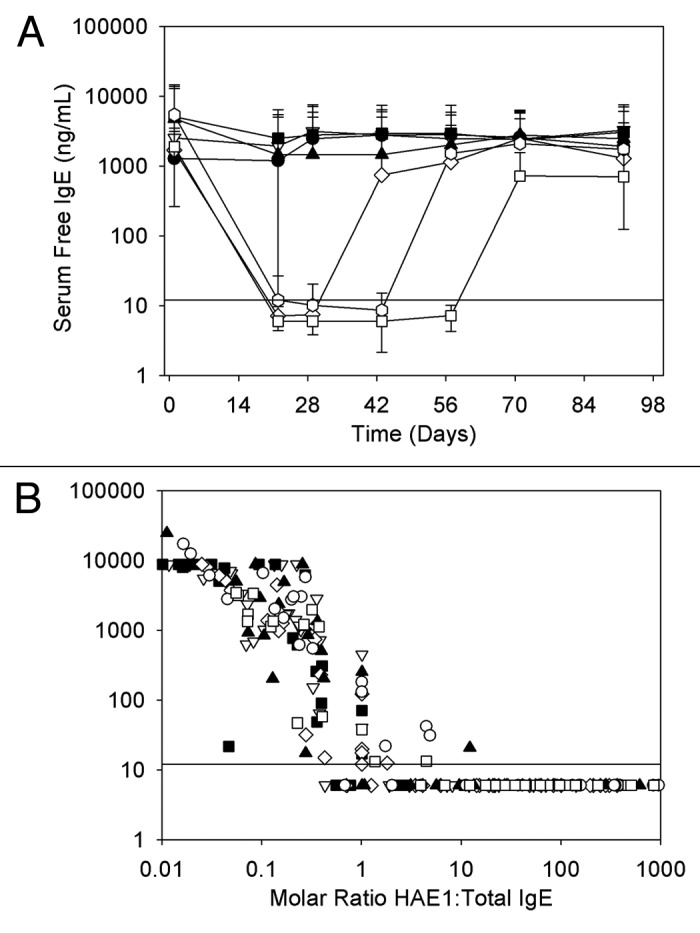
Figure 4. (A) Serum free IgE concentration-time profiles following a single subcutaneous administration of HAE1 to juvenile cynomolgus monkeys. Data represent the mean (± SD) of n = 6 animals/group (closed circle, placebo; open triangle, 7.5 mg/kg; closed square, 15 mg/kg; open diamond, 30 mg/kg; closed triangle, 50 mg/kg; open circle, 100 mg/kg; open square, 250 mg/kg; solid line represents the limit of quantitation (LOQ) in the free IgE ELISA (< 12 ng/mL). To illustrate the extent of reduction in free IgE levels during the course of treatment, LOQ samples were assigned a value of 6 ng/mL. This value was used in determining the mean free IgE concentration shown here. (B) The relationship of serum free IgE to the molar ratio of serum HAE1 to total IgE following a single subcutaneous administration of HAE1 to juvenile cynomolgus monkeys. Data points represent results from individual animals (closed circle, placebo; open triangle, 7.5 mg/kg; closed square, 15 mg/kg; open diamond, 30 mg/kg; closed triangle, 50 mg/kg; open circle, 100 mg/kg; open square, 250 mg/kg; solid line represents the limit of quantitation (LOQ) in the free IgE ELISA (< 12 ng/mL). To illustrate the extent of reduction in free IgE levels during the course of treatment, LOQ samples were assigned a value of 6 ng/mL.
Discussion
Omalizumab and IgE complex formation and disposition has been well-characterized, but the affect of increasing affinity to IgE or reducing Fc effector function on anti-IgE:IgE immune complex formation and clearance has not been evaluated. Liu et al. demonstrated that omalizumab and IgE formed complexes of various sizes in vitro due to two potential omalizumab binding sites on IgE.7 The size of the anti-IgE:IgE complexes formed were dependent on the molar ratio of the interacting components, with hexamers formed when omalizumab was in equal molar ratio with IgE and trimers observed when omalizumab was in 5-fold molar excess to IgE. Complex size distribution was also evaluated for both HAE1 and HAE2 prior to conducting our in vivo studies. Results from these studies confirmed complex formation with IgE (data not shown) and that complex size and distribution were consistent with that observed for omalizumab using size exclusion chromatography.7
In the mouse complex distribution study, a five-fold molar excess of anti-IgE to 125I-IgE resulted in the formation of primarily trimeric complexes with a smaller amount of hexamers across treatment groups. Following IV administration of these complexes, relatively minor differences in the plasma concentration-time profiles were observed between 125I-IgE-omalizumab and 125I-IgE-HAE1 (Fig. 1A), suggesting faster elimination of omalizumab complexes. Since the 125I-IgE-omalizumab profile appeared similar to that of the 125I-IgE profile, we attribute these results to increased dissociation of omalizumab from the 125I-IgE over time relative to HAE1. However, since the differences in the estimates of systemic exposure (AUC0–7) were not significant between 125I-IgE-omalizumab (11 d * µg/mL) and 125I-IgE-HAE1 (14 d * µg/mL), the overall results from this study suggest that binding affinity had minimal impact on IgE-anti-IgE complex disposition in this species.
The systemic exposure to 125I-IgE-HAE2 was six- to eight-fold greater compared with 125I-IgE-HAE1 or 125I-IgE-omalizumab and distribution to the liver was significantly reduced. It is well-established that clearance of most immune complexes is mediated via interaction with FcγR in liver and that inhibition of this pathway reduces clearance of immune complexes in mice.12-14 Therefore, since HAE2 binding to FcγR is ~85-fold reduced compared with wild-type IgG1, the reduced clearance and distribution to the liver observed for 125I-IgE-HAE2 complexes add support to the hypothesis that the primary mode of elimination of 125I-IgE-HAE1 complexes is via FcγR.
In cynomolgus monkeys, the PK of HAE1 was characterized by a long half-life and a slow apparent clearance (CL/F) that showed a modest reduction with increasing dose. These results are similar to those observed for omalizumab in cynomolgus monkeys.9 At HAE1 doses less than 50 mg/kg, CL/F ranged from 4–8 mL/day/kg whereas at doses greater than 50 mg/kg, CL/F decreased to ~3 mL/day/kg across dose levels. This nonlinearity in the apparent clearance is attributed at least partially to target-mediated clearance due to complexation with IgE. Because the assay used to evaluate anti-IgE levels measured total drug, including both free anti-IgE monomer and anti-IgE complexed with IgE, the reported apparent clearance of HAE1 was a sum of the clearance of both free antibody and antibody-IgE complex. At doses above 50 mg/kg, the clearance of HAE1 (~3 mL/day/kg) was similar to that observed for a typical IgG1 with linear kinetics in cynomolgus monkey.15 In this case, HAE1 is likely in large molar excess with IgE, and therefore the rate of clearance mostly reflects the nonspecific clearance processes of free drug, largely mediated by the reticuloendothelial system (RES).
At lower dose levels, where the ratio of free HAE1 to IgE was lower, faster clearance was observed, which suggests that the kinetics may be influenced by a target-mediated process due to HAE1-IgE complex interaction with the FcγR pathway. It is well-established that the Fcγ pathway plays a role in the clearance of immune complexes, and a previous study with omalizumab in cynomolgus monkey showed that the clearance of omalizumab-IgE immune complexes is dependent on the number of Fcγ domains present.10 The noncompartmental methods used make it difficult to deconvolute differences in PK properties between free HAE1 and HAE1 complexed with IgE, but the fact that HAE1 clearance appears to plateau at higher doses (> 50 mg/kg) may suggest a saturation of the FcγR pathway. The comparatively faster overall clearance observed for HAE1 relative to HAE2 also suggests that this pathway is active to some extent across all dose levels.
Serum total IgE increased significantly following administration of HAE1, which is consistent with results observed for omalizumab in both cynomolgus monkeys9 and in humans.16 Also similar to omalizumab, this increase is attributed to complex formation with IgE and redistribution of IgE from extravascular sites. Because the clearance of free IgE is more rapid than that of the anti-IgE, an accumulation of total IgE (free IgE plus IgE:anti-IgE complexes) is seen due to complex formation. The slower clearance of the complex relative to free IgE is likely due to interaction with the FcRn receptor, which may have a limited ability to rescue the complex from lysosomal degradation.17
Although IgE values were highly variable, the maximum observed IgE concentration was ~10- to 40-fold above the initial baseline concentration (IgEmax/bsl IgE) in monkeys administered HAE1 and ~30- to 110-fold above baseline levels in those receiving HAE2. In contrast, for omalizumab, a maximum increase in total IgE of six to nine-fold above baseline concentrations has been reported in cynomolgus monkey.9 This increase in the magnitude of total IgE response for both HAE1 and HAE2 compared with omalizumab likely reflects the increased binding affinity for IgE.18 The significantly greater increase in total IgE observed for HAE2 compared with HAE1 is likely again directly related to reduced interaction of IgE-HAE2 complexes with FcγR, thereby decreasing clearance of these complexes via this pathway.
In contrast to the increase in total IgE observed following administration of HAE1, levels of free IgE decreased. The response was highly variable from animal to animal, and so a clear dose response was not observed. Closer evaluation of the overall PK/PD relationship, however, demonstrated a clear relationship between the amount of free IgE suppression and the molar ratio of HAE1 to total IgE. At molar ratios close to 1:1 and under conditions of drug excess, there was a sharp drop in free IgE to undetectable levels in the free IgE ELISA (< 12 ng/mL). In comparison in humans, omalizumab must be given at a molar excess of 15–20 to 1 relative to baseline IgE to achieve adequate levels of free IgE suppression.16 Therefore, these data suggest that HAE1 may require a lower molar ratio of drug to IgE to suppress free IgE concentrations to levels considered therapeutic, which is consistent with our assumption that a higher-affinity anti-IgE molecule may offer the potential to reduce the dose required to suppress free IgE levels.
To evaluate the potential for improved dosing with HAE1 in humans, a mechanism-based PKPD model was developed to characterize the relationships between HAE1 PK and free and total IgE dynamics.5 The model was initially based on an existing PK/PD model for omalizumab17,19 together with the in vitro binding affinity of HAE1 and was subsequently refined with PK/PD data from a Phase 1 study with HAE1. The refined model predicted that the molar ratio required for HAE1 to reduce free IgE to therapeutic levels would be ~5:1, which is ~3-fold lower than that observed for omalizumab. Furthermore, it was predicted that HAE1 would lower free IgE more quickly at lower serum concentrations and maintain the suppression for a longer duration than omalizumab at comparable dose levels. Subsequently, data from a Phase 1 study with HAE1 has since demonstrated that a lower HAE1 dose (90 mg) does achieve greater suppression of free IgE in humans relative to omalizumab at 150 mg.20 These results support the hypothesis that the higher affinity of HAE1 translates into greater in vivo potency; however, these data should be interpreted with caution because the analysis was performed across studies. Furthermore, there is likely an upper limit to the increased potency that can be achieved by increasing binding affinity because the improvement may be offset by the turnover rate of the IgE.21
In this report, we evaluated the effects of increasing the antigen affinity or reducing Fc effector function of an anti-IgE antibody on its complex formation, disposition, PK, and PD. Although the PK observed for HAE1 appeared similar to that reported for omalizumab in cynomolgus monkeys, a significantly greater increase in total IgE was observed. More importantly, increasing the binding affinity of HAE1 appeared to reduce the amount of antibody required to suppress free IgE concentrations to levels considered therapeutic. Results with HAE2, which differs from HAE1 in only a single amino acid residue critical for FcγR binding, support the role of effector function in the clearance of immune complexes and confirm previous reports that inhibition of immune complex interaction, specifically with FcγR, can influence complex clearance. Taken together, the data support the expectation that increasing the binding affinity of an anti-IgE mAb may prove useful in treating patients with pretreatment IgE levels or body weights that fall outside the dosing requirements for omalizumab, and that retaining the effector function of an anti-IgE antibody is important for elimination of anti-IgE:IgE complexes.
Materials and Methods
Test material
Human IgE U266 was purified at Genentech as previously described.7 Omalizumab, HAE1 (also known as PRO98498) and HAE2 are humanized IgG1 mAbs manufactured by Genentech at production scale in stably transfected Chinese hamster ovary (CHO) cell lines. Omalizumab and omalizumab vehicle were formulated in 261 mM sucrose, 15 mM histidine, and 0.03% polysorbate 20. HAE1 and HAE2 and their vehicle were formulated in 20 mM histidine, with 200 mM arginine chloride and 0.04% (w/v) polysorbate 20. Human IgE was iodinated with 1 mCi of 125Iodine (125I; Perkin Elmer) using Iodogen (Pierce), according to manufacturer’s instructions.
Anti-IgE-IgE complex distribution study in mice
The mouse complex distribution study was approved by a Genentech Institutional Animal Care and Use Committee (IACUC). Twenty-four CD-1 mice (Charles River Laboratories) in each of four groups were administered a single intravenous (IV) bolus dose of 125I-hIgE (1.3 mg/kg) or pre-formed complexes of 125I-hIgE with 5-fold molar excess of omalizumab, HAE1 or HAE2 (5 mg/kg). A total of 3 mice per group were euthanized at each time point and whole blood collected out to 7 d post-dose. Tissues (liver, kidney, spleen, lung and stomach) were collected at 30 min and 7 d post-dose. The 30 min time point was selected to evaluate uptake toward the end of the distribution phase. The 7 d time point was chosen to evaluate if any accumulation into tissues could still be observed. To block non-specific uptake of iodine in the thyroid, all mice were given a single intraperitoneal bolus dose of sodium iodide (5 mg/kg) at 24 h and 1 h before administration of the test materials. The amount of radioactivity associated with each organ or trichloroacetic acid (TCA) precipitated plasma was determined using a gamma counter (Wallac 1470; PerkinElmer Life Sciences). Serum 125I-IgE concentrations were estimated based upon the specific activity of the injected dose. Tissue distribution data were transformed to the percentage of total dose/gm (% ID/gm) by dividing the radioactivity (cpm) observed in each sample by the amount of total radioactivity (cpm) administered and then normalized to the weight of the tissue (gm).
Single dose PK/PD study in cynomolgus monkeys
The cynomolgus monkey PK/PD study was approved by the IACUC at Shin Nippon Biomedical Laboratories. Juvenile cynomolgus monkeys (Macaca fascicularis) aged 8–10 mo were randomized to dose groups (n = 3 per gender per group) based on their mean IgE values prior to dosing. The study was conducted in two dosing cohorts and animals from both cohorts were compared directly. In cohort 1, animals received a single SC dose of placebo (control 1) or HAE1 at 7.5, 15, 30, 50, 100, or 200 mg/kg with a 72-h observation period before increasing the dose to the next level. In cohort 2, animals were dosed SC with either placebo (control 2), HAE1 at 75 or 250 mg/kg or HAE2 at 30, 50, 100 or 250 mg/kg. Blood samples were collected for serum total HAE1 or HAE2 and plasma total or free IgE analysis out to 90 d post-dose. Because of sample volume limitations, collection of samples for plasma free IgE analysis did not occur until study day 22.
Bioanalytical analysis of pharmacokinetic and pharmacodynamic samples in cynomolgus monkeys
Serum HAE1/HAE2
The concentration of total HAE1 or HAE2 was determined in an ELISA with a detection limit of 0.16 μg/mL. In each sample, HAE1- or HAE2-IgE complexes were disassociated using 3M KSCN in acetate buffer at room temperature (rt) for 30 min and then neutralized with assay diluent. HAE1 or HAE2 antibodies were then captured using a mouse mAb directed against anti-IgE (AME7; Genentech) and detected with another mouse mAb directed against anti-IgE (AME2; Genentech) conjugated to horseradish peroxidase (HRP). Tetramethyl benzidine (TMB) peroxidase was used as the substrate for HRP. The optical density (OD) was read at 450 nm (reference 650 nm) using SoftMax® Pro Software (Molecular Devices Corporation).
Serum Total IgE
The concentration of total IgE was determined in an ELISA with a detection limit of 200 ng/mL. IgE was captured with a polyclonal goat anti-human IgE and detected with biotinylated polyclonal goat anti-human IgE, followed by streptavidin:HRP. TMB peroxidase was used as the substrate for HRP. The optical density (OD) was read as above.
Serum Free IgE
Serum Free IgE concentrations were evaluated only in samples collected from animals given placebo or HAE1. The concentration of free IgE in cynomolgus monkey serum was determined using an electro-chemiluminescent assay (ECLA). The limit of detection was 12 ng/mL. Free IgE was captured using recombinant non-human primate FcεR1-IgG receptor (rFcεR1-IgG, Genentech) and a sample dilution of 1:4 was chosen because it was found to be the optimal condition in which to minimize serum interference while still maintaining HAE1:IgE complex stability.
Briefly, biotin-labeled rFcεR1-IgG (880 ng/mL) was added to streptavidin-coated magnetic beads (110 µg/mL) in assay diluent (50 mM Hepes, 150 mM NaCl, 2% fish gelatin, 2% polysorbate 20, 0.05% ProClin 300, pH 7.2) and incubated at rt (1–2 h). The biotin-rFcεR1-IgG/streptavidin-magnetic bead complex was then removed from the non-bound rFcεR1-IgG by using a KingFisher magnetic bead washer (ThermoFisher Scientific) and then transferred to plates containing either cynomolgus monkey IgE standards, controls, or serum diluted serum samples and incubated at rt in the dark for 1h. After a wash step, streptavidin-magnetic beads were collected and transferred to 96-well plates containing 200 µL/well of ruthenium-labeled goat anti-human (hu) IgE antibody (880 ng/mL) in 50% FBS and 50% assay diluent and incubated at rt with agitation in the dark (16–22 h). The level of free IgE was quantitated by aspirating 150 µL/well from the ruthenium-goat anti-human IgE-containing plates and analyzing for electrochemiluminescence at 620 nm wavelength using a BioVeris M384 instrument (BioVeris Corp.).
Pharmacokinetic analysis
PK parameters were estimated by non-compartmental methods using WinNonlin version 3.2 (Pharsight Corporation). In mice, analysis was based on the mean plasma 125I-IgE concentration profile of each treatment (n = 3 mice/group at each time point) using an intravascular input model on data acquired through Day 7. In cynomolgus monkeys, serum anti-IgE concentration-time data from each animal were analyzed using an extravascular input model. Estimates of clearance were based on area under the serum concentration-time curve extrapolated to infinity (AUClast + Clast/λz) with the terminal slope (λz) calculated by the log-linear regression of the last 3–9 data points.
Pharmacodynamic analysis
Serum total and serum free IgE levels were measured to evaluate treatment-related changes in cynomolgus monkeys.
Total IgE evaluation
Due to a large amount of variability in baseline IgE concentrations, which ranged from values below assay limit of detection (< 200 ng/mL) to 19,000 ng/mL, total IgE responses were assessed based on total IgE concentrations normalized to baseline values (Table 2). Therefore, so as not to bias our results toward only those animals with measurable total IgE concentrations at baseline and to include all animals in our analyses, concentrations below the limit of detection (< 200 ng/mL) were interpreted as 0.5 × 200 ng/mL (100 ng/mL) and used for graphic and summary presentation in addition to statistical analysis.
Free IgE evaluation
Because of the limit in blood volume that could be obtained from animals, evaluation of the relationship between the molar ratio of serum HAE1 concentration/serum total IgE to that of free IgE concentration could not begin until study day 22, which was the first day that free IgE samples could be collected. Serum free IgE assessment was performed only in samples collected from animals given placebo or HAE1.
Statistical data analysis
In the cynomolgus monkey PK/PD study, we investigated possible differences in PK or PD parameters between treatment groups by two-way analysis of variance (ANOVA) using treatment, dose, and their interaction as factors in the model. Post hoc pair-wise comparisons were performed between treatment groups at equivalent doses (vehicle, 30, 50, and 250 mg/kg). To investigate possible differences among doses within a single treatment, a one-way ANOVA was performed separately in a model with group as the factor. Post hoc pair-wise comparisons were performed among the HAE1 groups and among the HAE2 groups. Results of tests were cited as statistically significant at the conventional 5% level (p < 0.05).
Glossary
Abbreviations:
- ADME
absorption, distribution, metabolism and excretion
- AUC
area under the serum concentration-time curve
- Bsl
baseline
- CDR
complementarity determining region
- CL/F
apparent clearance
- FcεR
Fc-epsilon receptor
- FcγR
Fc-gamma receptor
- IgE
Immunoglobulin-E
- IgElast
last observed IgE concentration
- IgEmax
maximum observed IgE concentration
- HAE1
high-affinity anti-IgE antibody-1
- HAE2
high-affinity anti-IgE antibody-2
- IACUC
institutional animal care and use committee
- Kd
equilibrium dissociation constant
- PK
pharmacokinetics
- PD
pharmacodynamics
- t1/2 elim
elimination half-life
- Vss/F
apparent volume of distribution at steady-state
- %ID/gm
percent injected-dose divided by gram tissue weight
- RES
reticuloendothelial system
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/mabs/article/22216
References
- 1.MacGlashan DW, Jr., Bochner BS, Adelman DC, Jardieu PM, Togias A, McKenzie-White J, et al. Down-regulation of Fc(ε)RI expression on human basophils during in vivo treatment of atopic patients with anti-IgE antibody. J Immunol. 1997;158:1438–45. [PubMed] [Google Scholar]
- 2.Macglashan D., Jr. IgE and FcεRI regulation. Ann N Y Acad Sci. 2005;1050:73–88. doi: 10.1196/annals.1313.009. [DOI] [PubMed] [Google Scholar]
- 3.Hochhaus G, Brookman L, Fox H, Johnson C, Matthews J, Ren S, et al. Pharmacodynamics of omalizumab: implications for optimised dosing strategies and clinical efficacy in the treatment of allergic asthma. Curr Med Res Opin. 2003;19:491–8. doi: 10.1185/030079903125002171. [DOI] [PubMed] [Google Scholar]
- 4.Lowe PJ, Tannenbaum S, Gautier A, Jimenez P. Relationship between omalizumab pharmacokinetics, IgE pharmacodynamics and symptoms in patients with severe persistent allergic (IgE-mediated) asthma. Br J Clin Pharmacol. 2009;68:61–76. doi: 10.1111/j.1365-2125.2009.03401.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Putnam WS, Li J, Haggstrom J, Ng C, Kadkhodayan-Fischer S, Cheu M, et al. Use of quantitative pharmacology in the development of HAE1, a High-Affinity Anti-IgE Monoclonal Antibody. AAPS J. 2008;10:425–30. doi: 10.1208/s12248-008-9045-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shields RL, Namenuk AK, Hong K, Meng YG, Rae J, Briggs J, et al. High resolution mapping of the binding site on human IgG1 for FcγR1, FcγRIII, and FcRn and design of IgG1 variants with improved binding for the FcγR. J Biochem. 2001;276:6591–604. doi: 10.1074/jbc.M009483200. [DOI] [PubMed] [Google Scholar]
- 7.Liu J, Lester P, Builder S, Shire SJ. Characterization of complex formation by humanized anti-IgE monoclonal antibody and monoclonal human IgE. Biochemistry. 1995;34:10474–82. doi: 10.1021/bi00033a020. [DOI] [PubMed] [Google Scholar]
- 8.Fox JA, Hotaling TE, Struble C, Ruppel J, Bates DJ, Schoenhoff MB. Tissue distribution and complex formation with IgE of an anti-IgE antibody after intravenous administration in cynomolgus monkeys. J Pharmacol Exp Ther. 1996;279:1000–8. [PubMed] [Google Scholar]
- 9.Schoenhoff M, Bates D, Rupel J, Fei D, Fox JA, Thomas D, et al. Pharmacokinetics/dynamics following administration of a recombinant humanized monoclonal anti IgE antibody in the cynomolgus monkey . J Allergy Clin Immunol. 1995;95:356CS. [Google Scholar]
- 10.Fox JA, Reitz B, Hagler K, Hsei V, Keller G, Ryan A, et al. Pharmacokinetics and clearance mechanisms of anti-IgE:IgE monoclonal and polyclonal complexes. Pharm Res. 1997;14:217S. [Google Scholar]
- 11.Davies B, Morris T. Physiological parameters in laboratory animals and humans. Pharm Res. 1993;10:1093–5. doi: 10.1023/A:1018943613122. [DOI] [PubMed] [Google Scholar]
- 12.Sancho J, González E, Escanero JF, Egido J. Binding kinetics of monomeric and aggregated IgG to Kupffer cells and hepatocytes of mice. Immunology. 1984;53:283–9. [PMC free article] [PubMed] [Google Scholar]
- 13.Kurlander RJ, Ellison DM, Hall J. The blockade of Fc receptor-mediated clearance of immune complexes in vivo by a monoclonal antibody (2.4G2) directed against Fc receptors on murine leukocytes. J Immunol. 1984;133:855–62. [PubMed] [Google Scholar]
- 14.Clarkson SB, Kimberly RP, Valinsky JE, Witmer MD, Bussel JB, Nachman RL, et al. Blockade of clearance of immune complexes by an anti-Fc γ receptor monoclonal antibody. J Exp Med. 1986;164:474–89. doi: 10.1084/jem.164.2.474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Deng R, Iyer S, Theil FP, Mortensen DL, Fielder PJ, Prabhu S. Projecting human pharmacokinetics of therapeutic antibodies from nonclinical data: what have we learned? MAbs. 2011;3:61–6. doi: 10.4161/mabs.3.1.13799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Casale TB, Bernstein IL, Busse WW, LaForce CF, Tinkelman DG, Stoltz RR, et al. Use of an anti-IgE humanized monoclonal antibody in ragweed-induced allergic rhinitis. J Allergy Clin Immunol. 1997;100:110–21. doi: 10.1016/S0091-6749(97)70202-1. [DOI] [PubMed] [Google Scholar]
- 17.Hayashi N, Tsukamoto Y, Sallas WM, Lowe PJ. A mechanism-based binding model for the population pharmacokinetics and pharmacodynamics of omalizumab. Br J Clin Pharmacol. 2007;63:548–61. doi: 10.1111/j.1365-2125.2006.02803.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meno-Tetang GML, Lowe PJ. On the prediction of the human response: a recycled mechanistic pharmacokinetic/pharmacodynamic approach. Basic Clin Pharmacol Toxicol. 2005;96:182–92. doi: 10.1111/j.1742-7843.2005.pto960307.x. [DOI] [PubMed] [Google Scholar]
- 19.Sun Y-N. Advanced methods of PK/PD Systems Analysis. Presented at the Biomedical Simulations Resource Workshop, Marina Del Rey CA, June 22-23, 2001. [Google Scholar]
- 20.Boswell CA, Deng R, Lin K, Putnam WS, Lei C, Theil FP, et al. In vitro-in vivo correlations of pharmacokinetics, pharmacodynamics and metabolism for antibody therapeutics. Pharmacodynamic and Metabolic Outcomes of Therapeutic Proteins and Peptides, 2009; New York: Informa Healthcare 15-52. [Google Scholar]
- 21.Agoram BM, Martin SW, van der Graaf PH. The role of mechanism-based pharmacokinetic-pharmacodynamic (PK-PD) modelling in translational research of biologics. Drug Discov Today. 2007;12:1018–24. doi: 10.1016/j.drudis.2007.10.002. [DOI] [PubMed] [Google Scholar]


