Abstract
Bispecific antibodies are proteins that bind two different antigens and may retarget immune cells with a binding moiety specific for a leukocyte marker. A binding event in blood could in principle prevent antibody extravasation and accumulation at the site of disease. In this study, we produced and characterized two tetravalent bispecific antibodies that bind with high affinity to the alternatively-spliced EDB domain of fibronectin, a tumor-associated antigen. The bispecific antibodies simultaneously engaged the cognate antigens (murine T cell co-receptor CD3 and hen egg lysozyme) and selectively accumulated on murine tumors in vivo. The results, which were in agreement with predictions based on pharmacokinetic modeling and antibody binding characteristics, confirmed that bispecific antibodies can reach abluminal targets without being blocked by peripheral blood leukocytes.
Keywords: antibody, biodistribution, bispecific, syngeneic, tumor
Bispecific and multispecific antibodies were proposed over 20 years ago as pharmaceutical agents capable of redirecting the lytic activity of immune cells toward malignant target cells.1,2 These proteins are frequently designed to simultaneously engage in a binding interaction with a surface antigen on a tumor cell and with a surface protein on an immune cell. Indeed, it has been shown in vitro that bispecific antibodies mediate the formation of an artificial immunological synapse by bringing cytotoxic T lymphocytes (CTLs) and tumor cells into close proximity, which results in the selective killing of the target cell.3,4
The potential of bispecific antibodies as cancer therapeutics relates to their ability to mediate repeated and selective rounds of target cell lysis in an MHC- and T cell receptor independent manner. Some bispecific antibodies can be effective in vitro at picomolar concentrations and have been shown to eradicate tumors implanted in mice.5–9 Furthermore, there is emerging evidence that certain bispecific antibodies may provide a substantial benefit to patients with cancer, in particular, those with hematological malignancies.10,11
Strategies used in the 1980s for the construction of dual specificity products involved creation of murine IgG antibodies or F(ab’)2 molecules by fusion of two different hybridoma lines (quadroma) (Fig. 1A), but, because of random pairing of heavy and light chains, the yield of antibodies with the desired bispecificity was extremely low.12 In addition, these constructs had from low antitumor activity and caused side effects due to the presence of the Fc region. The development of diabodies and bispecific T cell engagers (BiTEs; scFv fragments arranged in a tandem array; Figure 1A) greatly improved the process for the preparation of homogenous bispecific antibodies, but these antibody formats may display reduced uptake at the site of disease because of the monovalence of each scFv binding moiety.13–16 Multivalent engagement with both tumor cell and immune cell can be achieved using the tetravalent bispecific “TandAb” format, which consists of a sequential fusion of VH-VL-VH-VL domains of two antibodies, arranged in a single polypeptide (Figure 1B).17,18
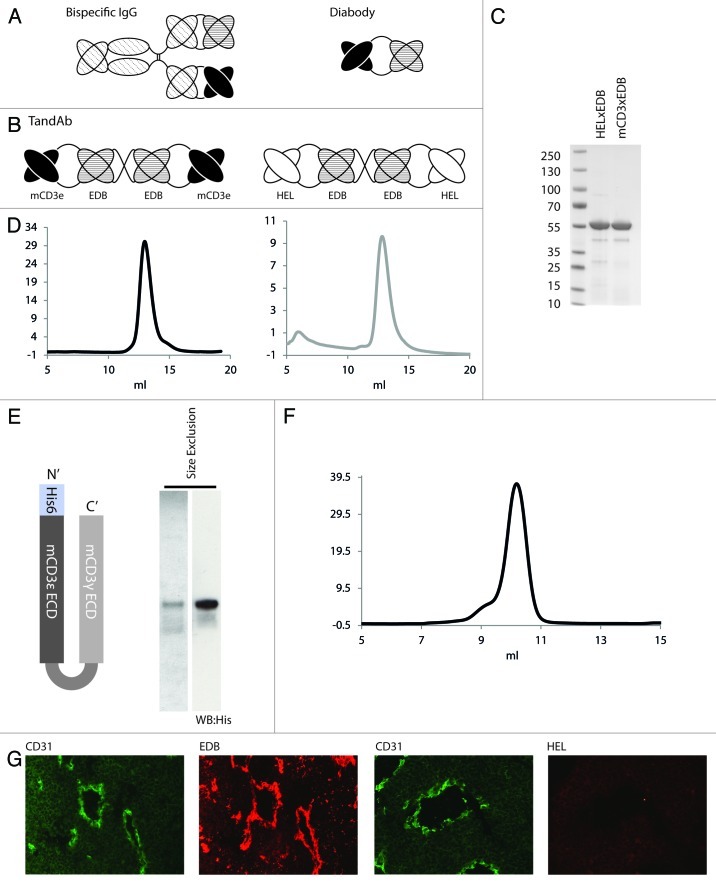
Figure 1. Expression and purification of bispecific TandAb antibodies, recombinant CD3 antigen and localization of EDB in murine tumors. (A) Schematic representation of bispecific antibodies in the IgG and diabody formats. (B) Bispecific antibodies mCD3εxEDB and HELxEDB in the TandAb format used in this study. (C) SDS PAGE of purified TandAbs under non-reducing conditions showing migration at the expected (monomeric) size of 55 kD. (D) Size exclusion profile (Sephadex 200) of purified TandAb dimer after an initial polishing step (data not shown) showing a single peak corresponding to the expected size of 110 kD. (E) Schematic representation of the CD3 antigen (mCD3εγ26) used in this study, Coomassie staining and anti-His western blot of the purified antigen. (F) Size exclusion chromatography of purified mCD3εγ26. (G) Sections of F9 teratocarcinoma tumors excised from 129Sv mice. The first and third panels show immunohistochemical staining of blood vessel endothelial marker CD31 (green). The second panel shows immunohistochemical staining of tumor antigen EDB (red). The fourth panel shows staining of the tumor with antibody against the irrelevant hen egg lysozyme antigen, as negative control. For detection, biotinylated L19 small immunoprotein (SIP), revealed with Alexa594-conjugated streptavidin (Invitrogen) (red), was used. Biotinylated KSF SIP (binding the irrelevant hen egg lysozyme antigen) was used as negative control.
Leukocyte antigens frequently targeted by bispecific antibodies for re-directing an immune response against tumor cells include CD3 (a component of the T cell receptor found on T lymphocytes), and the Fc receptors CD64 (constitutively expressed on macrophages and monocytes) and CD16 (found on natural killer cells, neutrophils and macrophages). A multispecific antibody, catumaxomab (Removab), which simultaneously targets the carcinoma marker EpCam, CD3 and Fc receptors), is approved in the European Union for the locoregional treatment of malignant ascites.19 The bispecific T cell engaging antibody blinatumomab (AMG103, formerly MT103) in BiTE format, which simultaneously recognizes the CD3xCD19 antigens, induced tumor regressions in the majority of relapsed non-Hodgkin B cell lymphoma and acute lymphoblastic leukemia (ALL) patients treated with this drug.10,11
The pharmaceutical development of bispecific antibodies is made more difficult by the fact that two binding moieties have to be optimized to achieve biological activity in vivo. The biochemical binding properties of the two antibodies can have a profound effect both on target cell lysis and on pharmacokinetic parameters. For example, objective responses with blinatumomab were only achieved when patients were treated by continuous intravenous infusion for a period of 4–8 weeks, while the administration of the same product by bolus injection did not confer a substantial benefit to patients and led to adverse side effects.10,20 In spite of many years of research and considerable industrial investments in the field, not much is known about the real ability of bispecific antibodies to localize at the site of disease in vivo. Cochlovius et al. reported a biodistribution of 125I-labeled TandAb in Rag2 deficient mice bearing subcutaneous (s.c.) Raji xenografts.21 It is to be noted that Rag2 knockout mice have very few T cells that lack expression of extracellular CD3.22 In 2009, Kontermann and colleagues reported a quantitative biodistribution study in which bispecific antibodies fused to different half-life prolonging moieties were administered to nude mice.23 Although it could be shown that PEGylation increases serum half-life and tumor accumulation of bispecific antibodies, the biodistribution experiments were performed in nude mice, which lack functional T cells, xenografted with human cancer cells. To our knowledge, and as stated by Stork et al., no biodistribution study has been published so far using bispecific antibodies in fully syngeneic immunocompetent models of cancer.23 The use of such models is crucial to assess whether the binding of a bispecific antibody to circulating immune cells inhibits extravasation and prevents a selective accumulation at the site of disease, which is a prerequisite for therapy.
To study the performance of tumor-targeting bispecific antibodies in vivo in an immunocompetent context, we used the L19 antibody, which is specific to the alternatively-spliced EDB domain of fibronectin, a marker of angiogenesis.24,25 The EDB domain is virtually undetectable in normal adult organs (with the exceptions of the endometrium in the proliferative phase and the placenta), but is strongly expressed in the majority of malignancies, with a prominent perivascular pattern of staining (Fig. 1G).24 The sequence of EDB is identical in mouse and man.26,27 The tumor targeting properties of the L19 antibody have been extensively studied in tumor-bearing mice and in patients with cancer.15,28–30 Interestingly, while a large variety of payloads can be delivered to the tumor subendothelial extracellular matrix using the L19 antibody as delivery vehicle,31,32 payloads that prevent extravasation due to receptor blockade, extreme isoelectric point values or large size were found to completely abrogate selective tumor targeting in vivo.33–38 As a T cell specific antigen, we chose the CD3 epsilon (CD3ε) subunit of the T cell receptor (TCR) expressed on the majority of T lymphocytes, which is frequently used as target in pharmaceutical development programs. We used the Armenian hamster antibody 145-2c11, which specifically recognizes the murine CD3ε isoform, and which has previously been used for the study of bispecific antibodies in mice.39–42
To generate a negative control bispecific antibody of irrelevant specificity in the mouse that would not able to engage in a binding interaction with murine T cells, we used the KSF antibody moiety, which is specific to hen egg lysozyme.43 Schematic representations of the TandAb structures for the mCD3εxEDB and HELxEDB antibodies, forming non-covalent homodimers with four binding sites and two antigen specificities are shown in Figure 1B. Both proteins were purified to near homogeneity using Protein A chromatography (Fig. 1C) and eluted as a single peak of expected size in size-exclusion chromatography (Fig. 1D), after an initial polishing step (Fig. S1).44 Figure 1E shows the schematic representation of the domain structures and the biochemical purity data (anti-His western blot) for a recombinant antigen (termed mCD3εγ26) that was used to assess the binding properties of the bispecific antibodies to mCD3ε.45 The L19 moiety, but not the KSF moiety, is capable of recognizing neo-vascular structures in the F9 murine teratocarcinoma model of cancer, chosen for this study, as evidenced by immunofluorescence analysis of tumor sections (Fig. 1F).
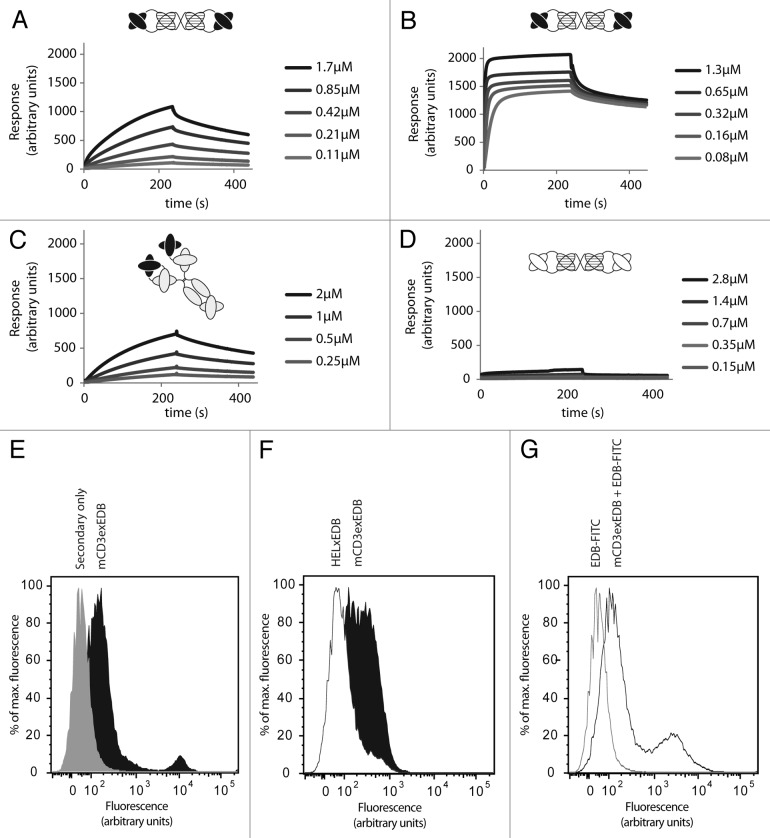
Binding of the mCD3εxEDB antibody to the cognate recombinant mCD3εγ26 (Fig. 2A) and EDB (Fig. 2B) antigens was assayed in vitro by surface plasmon resonance (SPR) on a BIAcore instrument. Simultaneous binding of mCD3εxEDB to immobilized mCD3εγ26 and soluble EDB was further confirmed in a sandwich SPR experiment (Fig. S2). Figure 2C shows association of the parental 145-2c11 IgG antibody as positive control on a mCD3εγ26-coated chip. As expected, HELxEDB did not bind to mCD3εγ26 (Fig. 2D), but bound equally well as mCD3εxEDB to immobilized EDB on a sensor chip (data not shown). In addition, binding of mCD3εxEDB (Fig. 2E), but not HELxEDB (Fig. 2F), to the CD3-positive cells of murine CTLL2 T cell lymphoma could be shown by FACS. Simultaneous binding of the bispecific mCD3εxEDB TandAb to CD3 on the cell surface and to soluble fluorescently-labeled EDB domain could also be detected by FACS using CTLL2 cells (Fig. 2G).
Figure 2. Binding affinity of TandAbs to CD3 and EDB antigens in vitro. (A) Binding of mCD3εxEDB to immobilized mCD3εγ26, and (B) to EDB at indicated concentrations of antibody. (C) Binding of the parental 145-2c11 IgG antibody to mCD3εγ26. (D) Binding profile of negative control HELxEDB antibody to mCD3εγ26. (E) FACS of CTLL2 cells stained with mCD3εxEDB and protein A-Alexa488 conjugate (black) or with Protein A-Alexa488 conjugate alone (gray). (F) Cells stained with 60 ng mCD3εxEDB (black) or HELxEDB (white) and revealed with protein A-Alexa488 conjugate. (G) Cells stained with mCD3εxEDB and EDB-FITC conjugate (black line) or EDB-FITC conjugate alone (gray line).
Figure 3 shows biodistribution results in immunocompetent 129Sv mice bearing F9 murine teratocarcinomas, 24h after injection of 125I radiolabelled mCD3εxEDB TandAb (Fig. 3A) and HELxEDB TandAb (Fig. 3B). Both bispecific antibodies exhibited preferential accumulation at the tumor site, with tumor:organ ratios comparable to the one of the parental L19 antibody in diabody format.16,46,47
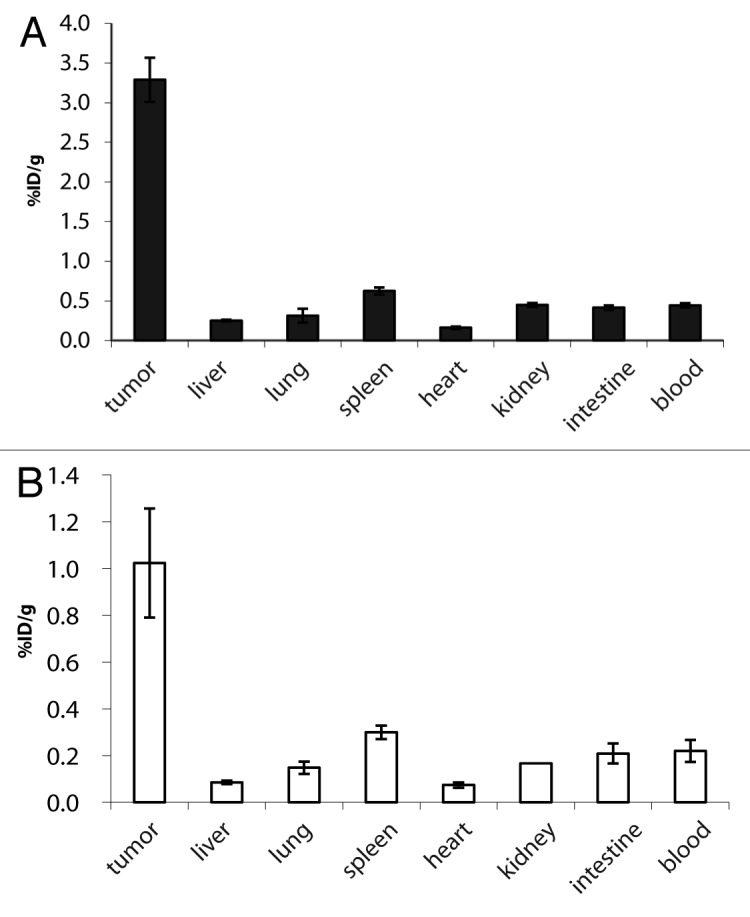
Figure 3. Biodistribution of 125I labeled TandAbs in immunocompetent mice bearing syngeneic tumors. Immunocompetent mice bearing F9 syngeneic tumors were injected with 10 µg (0.09 nmol) 125I radiolabelled (A) mCD3εxEDB TandAb and (B) HELxEDB TandAb. Bars show the mean ± SE percentage injected dose per gram of tissue (%ID/g). Bioreactivity after radioiodination was confirmed by binding to EDB resin (data not shown).
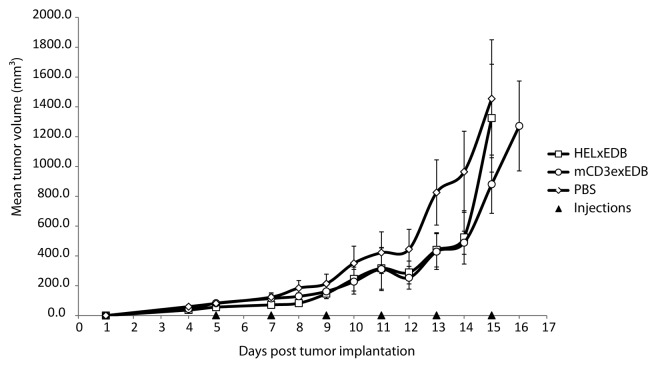
Having demonstrated the ability of mCD3xEDB to accumulate at the tumor site and to simultaneously engage in a binding interaction with its cognate target antigens in vitro, we tested the therapeutic potential of this TandAb in vivo. We did not anticipate that the targeted redirection of a T cell response to the sub-endothelial extracellular matrix would mediate a therapeutic effect, but we could not exclude that small amounts of EDB(+)fibronectin on the surface of tumor cells would be sufficient to elicit an anti-cancer response.48 The results of a therapy experiment in 129Sv mice bearing murine F9 teratocarcinomas (n = 6 per treatment group) are shown in Figure 4. Mice were injected i.v. in the tail vein with 40 µg mCD3εxEDB, HELxEDB or saline solution every 48h for 6 injections in total. Therapy was stopped at days 15 and 16 because treatment with mCD3εxEDB did not result in a statistically significant anti-tumor effect compared with control treatments.
Figure 4. Therapy with mCD3εxEDB TandAb in mice. Mean tumor volume ± SD (mm3) is shown over time in 129Sv mice (n = 6 per treatment group) implanted s.c. with F9 tumor cells. Mice were injected i.v. in the tail vein with 40 µg TandAb mCD3εxEDB, HELxEDB or saline solution every 48 h for 6 injections in total.
Our study shows that TandAb mCD3εxEDB could preferentially accumulate at the tumor site, without being trapped in blood by circulating T cells. These findings are compatible with a pharmacokinetic prediction, based on a two-compartment model and on the knowledge of kinetic binding parameters, concentrations and affinity constants. The velocity of complex formation between two molecular ligands A (target) and B (antibody) in solution can be described by the following differential equation:36
where [AB] is the concentration of the complex and [A] and [B] the concentrations of the free ligands, kon the kinetic association constant and koff the kinetic dissociation constant. Neglecting blood clearance and assuming that ligand B is present in large molar excess to A and that koff is negligibly low in comparison to kon, Equation 1 can be written as:
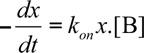 |
where x is the fraction of unbound ligand A. The solution to this differential equation has the form of an exponential rate of change:
where x(t) is the fraction of unbound target antigen A at the time t and x(0) the initial amount of unbound antigen A. From Equation 3 we can derive the equation describing the time T1/2 required for semi-saturation of the target antigen by the antibody:
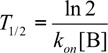 |
The BIAcore binding curves for the interaction between mCD3εxEDB and its cognate mCD3εγ26 antigen allowed the determination of kinetic binding parameters kon (1.1 × 104 M−1.s−1) and koff (2.0 × 10−3 s−1). From these values, an apparent mean Kd of 200 ± 78 nM could be derived [Kd = (koff/kon)]. The high Kd value indicates that CD3 antigen molecules on the surface of circulating T lymphocytes would not be saturated by antibody administered at submicromolar concentrations in biodistribution experiments. Furthermore, knowledge of blood TandAb concentration and the kon value (1.1 × 104 M−1.s−1) predicts an association kinetic between bispecific antibody and circulating T cell that is slow, compared with the antibody extravasation and localization to EDB(+) fibronectin in the tumor neo-vasculature.
In summary, we produced and characterized novel bispecific antibodies that recognize the alternatively-spliced EDB domain of fibronectin in vitro and in vivo. The use of an antibody moiety specific to the murine mCD3 antigen allowed the execution of biodistribution studies in syngeneic immunocompetent models of cancer, revealing that the bispecific antibodies were capable of selective localization at site of disease. A pharmacokinetic model, based on the knowledge of antibody and antigen concentrations, as well as of kinetic binding parameters, indicates that the use of bispecific antibodies at concentrations below the dissociation constant Kd for the mCD3 binding moiety is compatible with an efficient antibody extravasation and antigen targeting in vivo, without trapping effects by circulating T cells. Previous predictions have stated that the use of bispecific antibodies with low affinity to CD3 may be preferable for therapeutic applications because leukocyte trapping and undesired T cell activation may be avoided.49 Our results show the impact of Kd on the targeting performance of a bispecific antibody in a setting where a relatively high Kd for the CD3 binding interaction (200 ± 78 nM) is permissive to a good antibody accumulation at the tumor site in vivo.
Materials and Methods
Construction of bispecific antibodies
TandAbs were genetically assembled by successive overlap PCR in the order VH2c11-Linker10aa-VLL19-Linker12aa-VHL19-Linker10aa-VL2c11 and cloned into the pcDNA3.1 expression vector downstream of a mammalian excretion signal sequence. In the first step, cDNA sequences of VHL19, VLL19, VH2c11and VL2c11 were amplified with the following primer pairs (italic indicates overhangs, underline indicates BamHI restriction cutting site) respectively (1) 5′GGTGGATCCGG CGGTGGCTCGGAGGTGCAGCTGTTGGAGTCTGGGG3′ and (2) 5′C AGGGAACCCTGGTCACCGTCTCGAGTTCCGCCAAGACCACCCCCAAGCTGGG3′; (3) 5′T CCGCCAAGACCACCCCCAAGCTGGGCGGCGAAATTGTGTTGACGCAGTCTCCAGG3′ and (4) 5′GCCAAGGGACCAAGGTGGAAATCAAAGGTGGAGGCTCAGGCGGTGGATCC GGC3′; (5) 5′CCTGTTCCTCGTCGCTGTGGCTACAGGTGTGCACTCGGAGGTGCAGCTGG TGGAGTCTGGGGG3′ and (6) 5′GGTCACCGTCTCCTCATCCGCCAAGACCACCCCCAAG CTGGG3′; (7) 5′TCCGCCAAGACCACCCCCAAGCTGGGCGGCGACATCCAGATGAC CCAGTCTCCATC3′ and (8) 5′CTGGCACCAAGCTGGAAATCAAACGGGAACAAAAA CTCATCTCAGAAGAGG3′. In the second step, VH2c11-Linker10aa-VLL19 and VHL19-Linker10aa-VL2c11 fragments were assembled using the primer pairs (9) 5′CCCAAGCTTGTCGACCATGGGCTGGAGCCTGATCCTCCTGTTCCTCGTCGCTGTGGC3′ and (4); (10) 5′GAACAAAAACTCATCTCAGAAGAGGATCTGAATGGGGCCGCATA GGCGGCCGCAACG3′ and (1). Both fragments were subsequently digested and ligated into one at the BamHI restriction site before insertion into the vector.
Cell lines and mice
For the production of the bispecific antibodies, Chinese hamster ovary cells (CHO-S; Invitrogen) in suspension were used. The tumor cell line used for the therapy study was the murine teratocarcinoma cell line F9 [CRL-1720, American Type Culture Collection (ATCC)]. For flow cytometry experiments, the murine cytotoxic T cell line CTLL2 [TIB-214, American Type Culture Collection (ATCC)] was used. CHO-S cells in suspension were cultured in shaker incubators (37°C) in PowerCHO-2CD medium containing 8 mM Ultraglutamine and HT supplement. F9 cells were cultured on 0.1% gelatin-coated tissue flasks in Dulbecco’s modified Eagle Medium (Gibco) supplemented with 10% Fetal Calf Serum (FCS) incubated at 37°C and 5% CO2. CTLL2 cells were cultured at 37°C and 5% CO2 in RPMI-1640 medium (Gibco) supplemented with 10% FCS (Invitrogen, Germany), Antibiotic-Antimycotic (Gibco), Ultraglutamine, 1 mM Na-Pyruvate, 50 µM β-mercaptoethanol, human IL2 (20 units/ml) (Sino Biological). Female 129/SvEv mice were obtained from Charles River.
Protein expression and purification
Bispecific antibodies were expressed using transient gene expression. For 200 mL of production, 200.106 CHO-S cells were resuspended in 100 mL ProCHO4 (Lonza). Plasmid DNA (250 µg) was mixed with 150 mM NaCl to reach a final volume of 5 mL. Five milliliter of 250 mg/ml 25-kDa linear polyethylene imine (PEI; 1 mg/mL solution in water at pH 7.0; Polysciences) in 150 mM NaCl were added to the DNA NaCl solution and allowed to stand at room temperature for 10 min. The solution containing the PEI-DNA complexes was then added to the cells and gently mixed. The transfected cultures were incubated in a shaker incubator at 37°C. At 4 h post-transfection, the transfected culture was diluted with 100 mL Power-CHO-2CD medium and then incubated at 31°C in a shaking incubator for 6 d. The fusion proteins were purified from the cell culture medium by protein A affinity chromatography and then dialyzed against phosphate buffered saline (PBS, 6 mg/ml NaCl, 3.12 mg/ml NaH2PO4, 5.34 mg/ml Na2HPO4, 300 mM NaOH, pH 7.4).
Construction and bacterial expression of mCD3eg26 and EDB antigens
The mCD3εγ26 construct is an N-terminally His-tagged heterodimer consisting of the murine CD3 epsilon (mCD3ε) and gamma (mCD3γ) chains linked by a 26 amino acid linker (GSADDAKKDAAKKDDAKKDDAKKDGS) as first reported by Kim et al..45 The mCD3ε and mCD3γ cDNA was purchased from Sino Biological Inc. The mCD3εγ26 nucleotide sequence was assembled by overlap PCR using the following primers: (1) 5′CCCCCATATGGACGATGCCGAGAACATTGAATAC3′ (2) 5′TTTCTTTGCATCGT CCTTTTTGGCTGCGTCCTTTTTGGCGTCGTCAGCTGACCCGTACGTGTTTTTATTTGAGGCTG3′; (3) 5′GCAGCCAAAAAGGACGATGCAAAGAAAGACGATGCCAAGAAGGAC GGCAGCCAGACAAATAAAGCAAAGAATTTGG3′ (4) 5′CTGCAAGTGTATTACAGA ATGTAGGCGGCCGC3′ and inserted into the pET28b bacterial vector (Invitrogen) using the NheI and NotI restriction enzymes. The expression of the recombinant EDB domain was performed using a pQE12 vector encoding the exons 7, B, 8 and 9 of fibronectin.27 The insert containing plasmids was electroporated into BL-21 E.coli and expression was performed in 200 ml LB medium containing 1 mM IPTG (added when the culture reached OD6000.6), for 5 h at 37°C. Bacterial cells were lysed by sonication in Tris-HCL pH8 buffer and unlysed cells were removed by centrifugation at 5,000 g for 15 min at 4°C. Lysate was then centrifuged at 25,000 g for 1h at 4°C to pellet inclusion bodies. mCD3εγ26 was purified from the supernatant soluble lysate fraction by immobilized nickel affinity chromatography (IMAC) using Ni-NTA (Quiagen), followed by a polishing step by size-exclusion chromatography (Sephadex 75). The purity of the resulting 30 kD antigen was confirmed by Coomassie staining, anti-His western blot and size exclusion chromatography (Sephadex 75).
Conjugation of EDB to FITC
Protein was incubated 32 h with 10-fold molar excess Fluorescein isothiocyanate (FITC) after pH was adjusted to 8.4 by addition of 500 mM NaHCO3. Excess FITC was removed by buffer exchange column (PD-10, GE Healthcare) according to manufacturer’s protocol and protein fractions were collected by elution with PBS.
Flow cytometry analysis
105 CTLL2 cells were resuspended in a 96-well U-bottom plate in 200 μl per well FACS buffer (5% FBS, 2 mM EDTA). Cells were pelleted and incubated on ice for 1 h with bispecific antibodies in PBS. Cells were then washed 3 times with FACS buffer and incubated 30 min with 200 µl of a 1:600 dilution of protein-A-Alexa488 conjugate (Invitrogen, Life Technologies) or FITC-conjugated EDB (0.1 mg/ml). Cells were then again washed 3 times with FACS buffer and analyzed on a FACS Canto II Flow Cytometer (BD Biosciences).
Immunofluorescent staining
For immunofluorescence staining, F9 tumors were excised, embedded in cryo-embedding medium (Thermo Scientific) and stored at -80°C. Cryostat sections (10 mm) of tumors were fixed in ice-cold acetone, rehydrated with PBS and blocked with 10% fetal bovine serum (Invitrogen) in PBS. Rat-anti-mouse CD31 antibody (BD Biosciences) and was used for staining endothelial cells from blood vessels. Rat-Alexa488 (Invitrogen) coupled secondary antibody was used for detection. Biotinylated L19 small immunoproteins (SIP), revealed with Alexa594-conjugated streptavidin (Invitrogen) was used to stain EDB. Slides were mounted with fluorescent mounting medium (Dako) and analyzed with an Axioskop2 mot plus microscope (Zeiss).
Surface plasmon resonance
Bispecific antibody solutions were filtered (using 0.22 µm PVDF filters) and their binding properties analyzed using a BIAcore3000 instrument (BIAcore) and an antigen coated chip, which was prepared by covalent coupling of mCD3εγ26 (at different concentrations) to a CM5 sensor chip (GE Healthcare) according to the manufacturer’s protocol. The 145-2c11 IgG antibody used as positive control was purchased from eBiosciences.
Biodistribution
Tumor-bearing mice were obtained by subcutaneous injection of F9 teratocarcinoma cells (107) in the flank of 11–13 weeks old female 129/SvEv mice. Five days after tumor implantation, 10 µg (0.09 nmol) 125I radiolabelled mCD3εxEDB TandAb and HELxEDB TandAbs were injected into the lateral tail vein of 129/SvEv (Charles River) mice bearing subcutaneous implanted F9 murine teratocarcinoma cells. Bioreactivity after radioiodination was confirmed by binding to EDB resin. Mice were sacrificed 24 h after injection. Organs were weighed and radioactivity was counted using a Packard Cobra gamma counter. Radioactivity content of representative organs was expressed as the percentage of the injected dose per gram of tissue (%ID/g ± SE).
Therapy experiment
129Sv mice aged between 11–13 weeks were injected s.c. with 107 F9 teratocarcinoma cells. Five days after tumor implantation, when tumors were clearly palpable, mice were randomized in different treatment groups (n = 6 per treatment group). Therapy was performed by injection in the tail vein. 129SvEv mice were injected 6 times, every 48 h with 40 µg mCD3εxEDB or HELxEDB TandAb or saline (PBS). Experiments were performed under a project license granted by the Veterinaeramt des Kantons Zuerich, Switzerland (169/2008).
Supplementary Material
Acknowledgments
Financial contribution from the ETH Zürich, the Swiss National Science Foundation, the Kommission für Technologie und Innovation, the Swiss Cancer League and Philochem AG is gratefully acknowledged.
Disclosure of Potential of Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/mabs/article/22271
References
- 1.Staerz UD, Kanagawa O, Bevan MJ. Hybrid antibodies can target sites for attack by T cells. Nature. 1985;314:628–31. doi: 10.1038/314628a0. [DOI] [PubMed] [Google Scholar]
- 2.Perez P, Hoffman RW, Shaw S, Bluestone JA, Segal DM. Specific targeting of cytotoxic T cells by anti-T3 linked to anti-target cell antibody. Nature. 1985;316:354–6. doi: 10.1038/316354a0. [DOI] [PubMed] [Google Scholar]
- 3.Stamova S, Feldmann A, Cartellieri M, Arndt C, Koristka S, Apel F, et al. Generation of single-chain bispecific green fluorescent protein fusion antibodies for imaging of antibody-induced T cell synapses. Anal Biochem. 2012;423:261–8. doi: 10.1016/j.ab.2011.12.042. [DOI] [PubMed] [Google Scholar]
- 4.Offner S, Hofmeister R, Romaniuk A, Kufer P, Baeuerle PA. Induction of regular cytolytic T cell synapses by bispecific single-chain antibody constructs on MHC class I-negative tumor cells. Mol Immunol. 2006;43:763–71. doi: 10.1016/j.molimm.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 5.Hoffmann P, Hofmeister R, Brischwein K, Brandl C, Crommer S, Bargou R, et al. Serial killing of tumor cells by cytotoxic T cells redirected with a CD19-/CD3-bispecific single-chain antibody construct. Int J Cancer. 2005;115:98–104. doi: 10.1002/ijc.20908. [DOI] [PubMed] [Google Scholar]
- 6.Schlereth B, Fichtner I, Lorenczewski G, Kleindienst P, Brischwein K, da Silva A, et al. Eradication of tumors from a human colon cancer cell line and from ovarian cancer metastases in immunodeficient mice by a single-chain Ep-CAM-/CD3-bispecific antibody construct. Cancer Res. 2005;65:2882–9. doi: 10.1158/0008-5472.CAN-04-2637. [DOI] [PubMed] [Google Scholar]
- 7.Weiner GJ, Hillstrom JR. Bispecific anti-idiotype/anti-CD3 antibody therapy of murine B cell lymphoma. J Immunol. 1991;147:4035–44. [PubMed] [Google Scholar]
- 8.Demanet C, Brissinck J, Van Mechelen M, Leo O, Thielemans K. Treatment of murine B cell lymphoma with bispecific monoclonal antibodies (anti-idiotype x anti-CD3) J Immunol. 1991;147:1091–7. [PubMed] [Google Scholar]
- 9.Demanet C, Brissinck J, Leo O, Moser M, Thielemans K. Role of T-cell subsets in the bispecific antibody (anti-idiotype x anti-CD3) treatment of the BCL1 lymphoma. Cancer Res. 1994;54:2973–8. [PubMed] [Google Scholar]
- 10.Bargou R, Leo E, Zugmaier G, Klinger M, Goebeler M, Knop S, et al. Tumor regression in cancer patients by very low doses of a T cell-engaging antibody. Science. 2008;321:974–7. doi: 10.1126/science.1158545. [DOI] [PubMed] [Google Scholar]
- 11.Topp MS, Kufer P, Gökbuget N, Goebeler M, Klinger M, Neumann S, et al. Targeted therapy with the T-cell-engaging antibody blinatumomab of chemotherapy-refractory minimal residual disease in B-lineage acute lymphoblastic leukemia patients results in high response rate and prolonged leukemia-free survival. J Clin Oncol. 2011;29:2493–8. doi: 10.1200/JCO.2010.32.7270. [DOI] [PubMed] [Google Scholar]
- 12.Milstein C, Cuello AC. Hybrid hybridomas and their use in immunohistochemistry. Nature. 1983;305:537–40. doi: 10.1038/305537a0. [DOI] [PubMed] [Google Scholar]
- 13.Holliger P, Prospero T, Winter G. “Diabodies”: small bivalent and bispecific antibody fragments. Proc Natl Acad Sci USA. 1993;90:6444–8. doi: 10.1073/pnas.90.14.6444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kontermann RE. Bispecific Antibodies. Springer Berlin, 2011. [Google Scholar]
- 15.Borsi L, Balza E, Bestagno M, Castellani P, Carnemolla B, Biro A, et al. Selective targeting of tumoral vasculature: comparison of different formats of an antibody (L19) to the ED-B domain of fibronectin. Int J Cancer. 2002;102:75–85. doi: 10.1002/ijc.10662. [DOI] [PubMed] [Google Scholar]
- 16.Viti F, Tarli L, Giovannoni L, Zardi L, Neri D. Increased binding affinity and valence of recombinant antibody fragments lead to improved targeting of tumoral angiogenesis. Cancer Res. 1999;59:347–52. [PubMed] [Google Scholar]
- 17.Kipriyanov SM, Moldenhauer G, Schuhmacher J, Cochlovius B, Von der Lieth CW, Matys ER, et al. Bispecific tandem diabody for tumor therapy with improved antigen binding and pharmacokinetics. J Mol Biol. 1999;293:41–56. doi: 10.1006/jmbi.1999.3156. [DOI] [PubMed] [Google Scholar]
- 18.Kipriyanov SM. Generation of bispecific and tandem diabodies. Methods Mol Biol. 2009;562:177–93. doi: 10.1007/978-1-60327-302-2_14. [DOI] [PubMed] [Google Scholar]
- 19.Seimetz D, Lindhofer H, Bokemeyer C. Development and approval of the trifunctional antibody catumaxomab (anti-EpCAM x anti-CD3) as a targeted cancer immunotherapy. Cancer Treat Rev. 2010;36:458–67. doi: 10.1016/j.ctrv.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 20.Nagorsen D, Kufer P, Zugmaier G, Baeuerle P. Dosage regimen for administering a CD19xCD3 bispecific antibody (Patent Nr.WO2011051307A1). In: WIPO/OMPI, ed., 2011. [Google Scholar]
- 21.Cochlovius B, Kipriyanov SM, Stassar MJ, Schuhmacher J, Benner A, Moldenhauer G, et al. Cure of Burkitt’s lymphoma in severe combined immunodeficiency mice by T cells, tetravalent CD3 x CD19 tandem diabody, and CD28 costimulation. Cancer Res. 2000;60:4336–41. [PubMed] [Google Scholar]
- 22.Shinkai Y, Koyasu S, Nakayama K, Murphy KM, Loh DY, Reinherz EL, et al. Restoration of T cell development in RAG-2-deficient mice by functional TCR transgenes. Science. 1993;259:822–5. doi: 10.1126/science.8430336. [DOI] [PubMed] [Google Scholar]
- 23.Stork R, Campigna E, Robert B, Müller D, Kontermann RE. Biodistribution of a bispecific single-chain diabody and its half-life extended derivatives. J Biol Chem. 2009;284:25612–9. doi: 10.1074/jbc.M109.027078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Castellani P, Viale G, Dorcaratto A, Nicolo G, Kaczmarek J, Querze G, et al. The fibronectin isoform containing the ED-B oncofetal domain: a marker of angiogenesis. Int J Cancer. 1994;59:612–8. doi: 10.1002/ijc.2910590507. [DOI] [PubMed] [Google Scholar]
- 25.Pini A, Viti F, Santucci A, Carnemolla B, Zardi L, Neri P, et al. Design and use of a phage display library. Human antibodies with subnanomolar affinity against a marker of angiogenesis eluted from a two-dimensional gel. J Biol Chem. 1998;273:21769–76. doi: 10.1074/jbc.273.34.21769. [DOI] [PubMed] [Google Scholar]
- 26.Zardi L, Carnemolla B, Siri A, Petersen TE, Paolella G, Sebastio G, et al. Transformed human cells produce a new fibronectin isoform by preferential alternative splicing of a previously unobserved exon. EMBO J. 1987;6:2337–42. doi: 10.1002/j.1460-2075.1987.tb02509.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fattorusso R, Pellecchia M, Viti F, Neri P, Neri D, Wüthrich K. NMR structure of the human oncofoetal fibronectin ED-B domain, a specific marker for angiogenesis. Structure. 1999;7:381–90. doi: 10.1016/S0969-2126(99)80051-3. [DOI] [PubMed] [Google Scholar]
- 28.Santimaria M, Moscatelli G, Viale GL, Giovannoni L, Neri G, Viti F, et al. Immunoscintigraphic detection of the ED-B domain of fibronectin, a marker of angiogenesis, in patients with cancer. Clin Cancer Res. 2003;9:571–9. [PubMed] [Google Scholar]
- 29.Sauer S, Erba PA, Petrini M, Menrad A, Giovannoni L, Grana C, et al. Expression of the oncofetal ED-B-containing fibronectin isoform in hematologic tumors enables ED-B-targeted 131I-L19SIP radioimmunotherapy in Hodgkin lymphoma patients. Blood. 2009;113:2265–74. doi: 10.1182/blood-2008-06-160416. [DOI] [PubMed] [Google Scholar]
- 30.Erba PA, Sollini M, Orciuolo E, Traino C, Petrini M, Paganelli G, et al. Radioimmunotherapy with radretumab in patients with relapsed hematologic malignancies. J Nucl Med. 2012;53:922–7. doi: 10.2967/jnumed.111.101006. [DOI] [PubMed] [Google Scholar]
- 31.Pasche N, Neri D. Immunocytokines: a novel class of potent armed antibodies. Drug Discov Today. 2012;17:583–90. doi: 10.1016/j.drudis.2012.01.007. [DOI] [PubMed] [Google Scholar]
- 32.Neri D, Bicknell R. Tumour vascular targeting. Nat Rev Cancer. 2005;5:436–46. doi: 10.1038/nrc1627. [DOI] [PubMed] [Google Scholar]
- 33.Ebbinghaus C, Ronca R, Kaspar M, Grabulovski D, Berndt A, Kosmehl H, et al. Engineered vascular-targeting antibody-interferon-gamma fusion protein for cancer therapy. Int J Cancer. 2005;116:304–13. doi: 10.1002/ijc.20952. [DOI] [PubMed] [Google Scholar]
- 34.Melkko S, Halin C, Borsi L, Zardi L, Neri D. An antibody-calmodulin fusion protein reveals a functional dependence between macromolecular isoelectric point and tumor targeting performance. Int J Radiat Oncol Biol Phys. 2002;54:1485–90. doi: 10.1016/S0360-3016(02)03927-5. [DOI] [PubMed] [Google Scholar]
- 35.Niesner U, Halin C, Lozzi L, Günthert M, Neri P, Wunderli-Allenspach H, et al. Quantitation of the tumor-targeting properties of antibody fragments conjugated to cell-permeating HIV-1 TAT peptides. Bioconjug Chem. 2002;13:729–36. doi: 10.1021/bc025517+. [DOI] [PubMed] [Google Scholar]
- 36.Halin C, Niesner U, Villani ME, Zardi L, Neri D. Tumor-targeting properties of antibody-vascular endothelial growth factor fusion proteins. Int J Cancer. 2002;102:109–16. doi: 10.1002/ijc.10674. [DOI] [PubMed] [Google Scholar]
- 37.Halin C, Gafner V, Villani ME, Borsi L, Berndt A, Kosmehl H, et al. Synergistic therapeutic effects of a tumor targeting antibody fragment, fused to interleukin 12 and to tumor necrosis factor alpha. Cancer Res. 2003;63:3202–10. [PubMed] [Google Scholar]
- 38.Gafner V, Trachsel E, Neri D. An engineered antibody-interleukin-12 fusion protein with enhanced tumor vascular targeting properties. Int J Cancer. 2006;119:2205–12. doi: 10.1002/ijc.22101. [DOI] [PubMed] [Google Scholar]
- 39.Leo O, Foo M, Sachs DH, Samelson LE, Bluestone JA. Identification of a monoclonal antibody specific for a murine T3 polypeptide. Proc Natl Acad Sci USA. 1987;84:1374–8. doi: 10.1073/pnas.84.5.1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Alegre ML, Tso JY, Sattar HA, Smith J, Desalle F, Cole M, et al. An anti-murine CD3 monoclonal antibody with a low affinity for Fc gamma receptors suppresses transplantation responses while minimizing acute toxicity and immunogenicity. J Immunol. 1995;155:1544–55. [PubMed] [Google Scholar]
- 41.Amann M, Brischwein K, Lutterbuese P, Parr L, Petersen L, Lorenczewski G, et al. Therapeutic window of MuS110, a single-chain antibody construct bispecific for murine EpCAM and murine CD3. Cancer Res. 2008;68:143–51. doi: 10.1158/0008-5472.CAN-07-2182. [DOI] [PubMed] [Google Scholar]
- 42.Schlereth B, Kleindienst P, Fichtner I, Lorenczewski G, Brischwein K, Lippold S, et al. Potent inhibition of local and disseminated tumor growth in immunocompetent mouse models by a bispecific antibody construct specific for Murine CD3. Cancer Immunol Immunother. 2006;55:785–96. doi: 10.1007/s00262-005-0082-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fiechter M, Frey K, Fugmann T, Kaufmann PA, Neri D. Comparative in vivo analysis of the atherosclerotic plaque targeting properties of eight human monoclonal antibodies. Atherosclerosis. 2011;214:325–30. doi: 10.1016/j.atherosclerosis.2010.11.027. [DOI] [PubMed] [Google Scholar]
- 44.Silacci M, Brack S, Schirru G, Mårlind J, Ettorre A, Merlo A, et al. Design, construction, and characterization of a large synthetic human antibody phage display library. Proteomics. 2005;5:2340–50. doi: 10.1002/pmic.200401273. [DOI] [PubMed] [Google Scholar]
- 45.Kim KS, Sun ZY, Wagner G, Reinherz EL. Heterodimeric CD3epsilongamma extracellular domain fragments: production, purification and structural analysis. J Mol Biol. 2000;302:899–916. doi: 10.1006/jmbi.2000.4098. [DOI] [PubMed] [Google Scholar]
- 46.Tarli L, Balza E, Viti F, Borsi L, Castellani P, Berndorff D, et al. A high-affinity human antibody that targets tumoral blood vessels. Blood. 1999;94:192–8. [PubMed] [Google Scholar]
- 47.Demartis S, Tarli L, Borsi L, Zardi L, Neri D. Selective targeting of tumour neovasculature by a radiohalogenated human antibody fragment specific for the ED-B domain of fibronectin. Eur J Nucl Med. 2001;28:534–9. doi: 10.1007/s002590100480. [DOI] [PubMed] [Google Scholar]
- 48.Borsi L, Balza E, Carnemolla B, Sassi F, Castellani P, Berndt A, et al. Selective targeted delivery of TNFalpha to tumor blood vessels. Blood. 2003;102:4384–92. doi: 10.1182/blood-2003-04-1039. [DOI] [PubMed] [Google Scholar]
- 49.Bortoletto N, Scotet E, Myamoto Y, D’Oro U, Lanzavecchia A. Optimizing anti-CD3 affinity for effective T cell targeting against tumor cells. Eur J Immunol. 2002;32:3102–7. doi: 10.1002/1521-4141(200211)32:11<3102::AID-IMMU3102>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.