Abstract
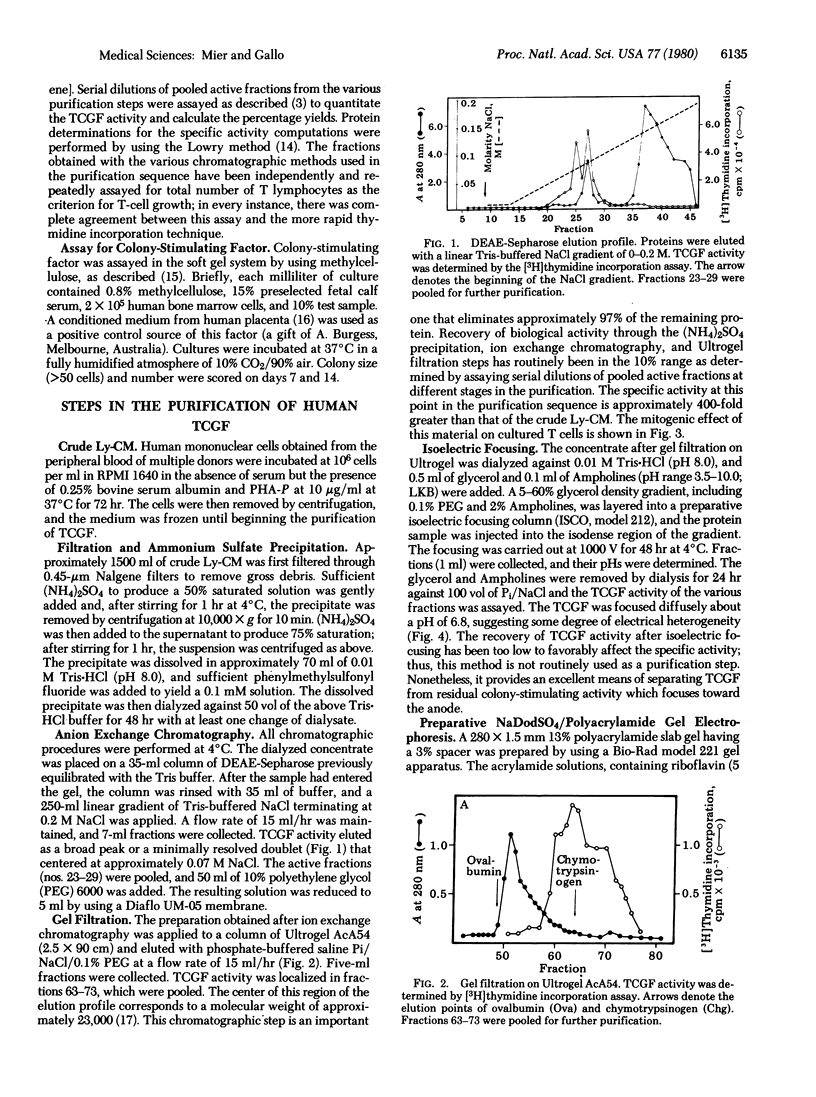
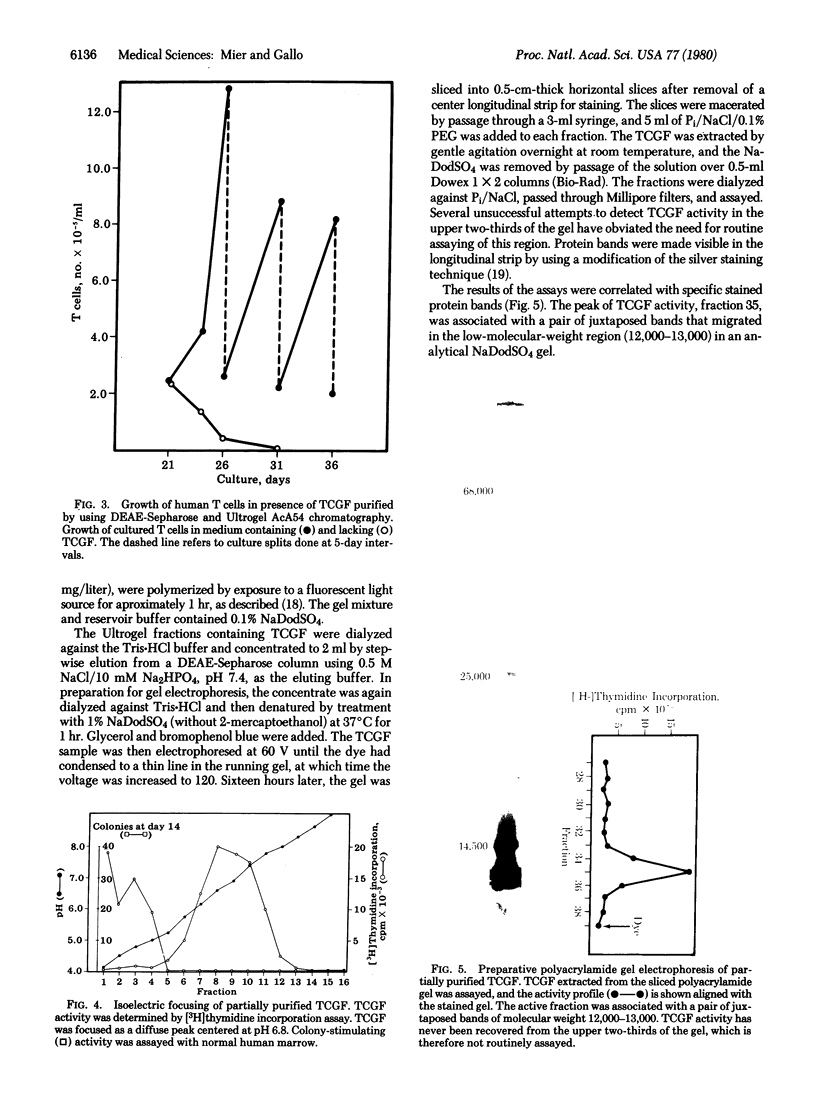
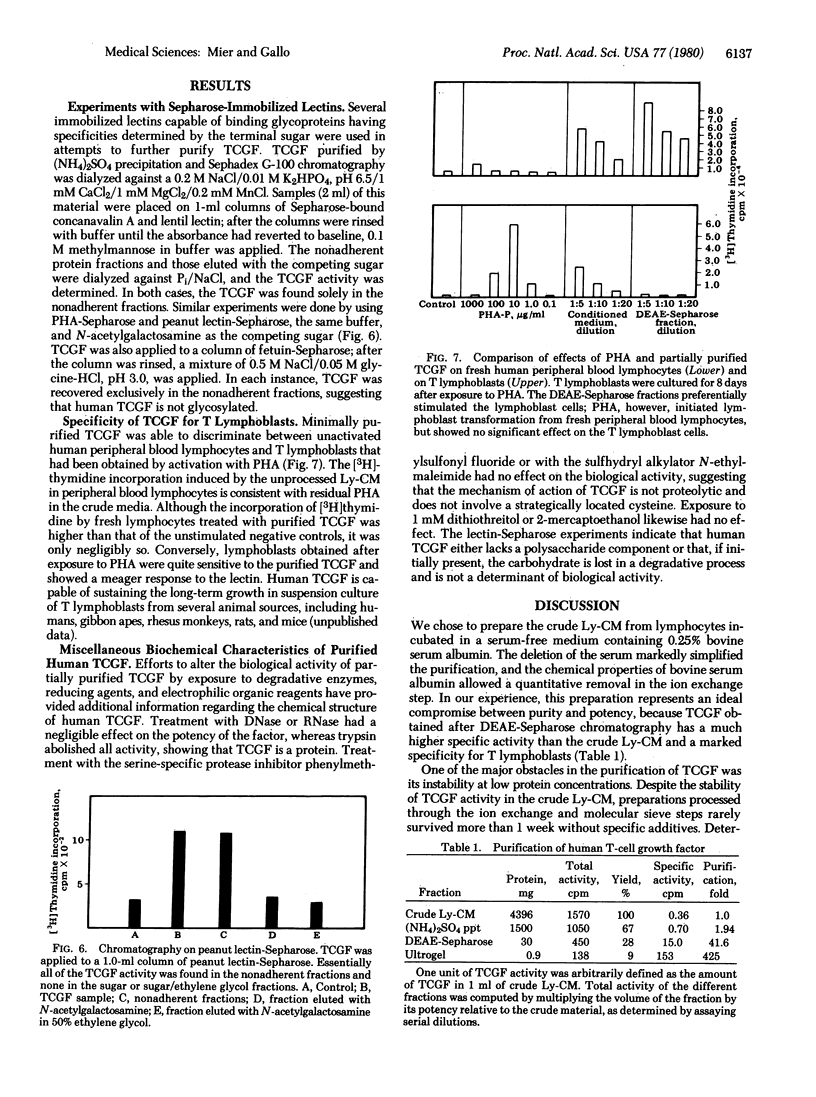
Human T-cell growth factor (TCGF), a mitogenic protein that appears in the media of cultured lymphocytes after phytohemagglutinin-stimulation, has been purified more than 400-fold from serum-free conditioned media by using a sequence of ion exchange chromatography and gel filtration. The purified growth factor elutes as a broad peak from DEAE-Sepharose, focuses diffusely at a pH of about 6.8 on isoelectric focusing (suggesting heterogeneity in electrical charge), has an estimated molecular weight of approximately 23,000 as judged by gel filtration (12,000-13,000 on Na-DodSO4/polyacrylamide gel electrophoresis), is resistant to DNase and RNase, is degraded by trypsin, and does not adhere to any of several lectin-Sepharoses. These characteristics indicate that it is nonglycosylated and protein in nature. The activity of the factor determined by cell counts or [3H]thymidine incorporation in human T lymphoblasts, is stable at room temperature in crude conditioned media, but the partially purified factor requires the addition of albumin or polyethylene glycol to maintain stability. Unlike the crude conditioned media, the purified factor lacks colony-stimulating activity and, unlike lectins, antigens, and crude conditioned media, it does not initiate blastogenesis in peripheral blood lymphocytes but is a selective mitogen for T cells that have undergone blast transformation secondary to exposure to a lectin or antigen. This indicates that the factor is a second signal in the T-cell immune response. The partially purified factor has been used to selectively grow several human T-cell lines, including cells that are cytotoxic to a variety of target cells.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews P. Estimation of the molecular weights of proteins by Sephadex gel-filtration. Biochem J. 1964 May;91(2):222–233. doi: 10.1042/bj0910222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. E., Gillis S., Ferm M. M., Smith K. A. The effect of T cell growth factor on the generation of cytolytic T cells. J Immunol. 1978 Dec;121(6):2168–2173. [PubMed] [Google Scholar]
- Ben-Sasson S. A., Henkart P. A. Enhancement of weak mixed lymphocyte-type reactions by hydrophilic polymers. J Immunol. 1977 Jul;119(1):227–231. [PubMed] [Google Scholar]
- Blyden G., Handschumacher R. E. Purification and properties of human lymphocyte activating factor (LAF). J Immunol. 1977 May;118(5):1631–1638. [PubMed] [Google Scholar]
- Bonnard G. D., Yasaka K., Jacobson D. Ligand-activated T cell growth factor-induced proliferation: absorption of T cell growth factor by activated T cells. J Immunol. 1979 Dec;123(6):2704–2708. [PubMed] [Google Scholar]
- Burgess A. W., Wilson E. M., Metcalf D. Stimulation by human placental conditioned medium of hemopoietic colony formation by human marrow cells. Blood. 1977 Apr;49(4):573–583. [PubMed] [Google Scholar]
- Chervenick P. A., Boggs D. R. In vitro growth of granulocytic and mononuclear cell colonies from blood of normal individuals. Blood. 1971 Feb;37(2):131–135. [PubMed] [Google Scholar]
- Gillis S., Ferm M. M., Ou W., Smith K. A. T cell growth factor: parameters of production and a quantitative microassay for activity. J Immunol. 1978 Jun;120(6):2027–2032. [PubMed] [Google Scholar]
- Kasakura S. Cytotoxic lymphocytes induced by soluble factors derived from the medium of leukocyte cultures. J Immunol. 1977 Jan;118(1):43–47. [PubMed] [Google Scholar]
- Kurnick J. T., Grönvik K. O., Kimura A. K., Lindblom J. B., Skoog V. T., Sjöberg O., Wigzell H. Long term growth in vitro of human T cell blasts with maintenance of specificity and function. J Immunol. 1979 Apr;122(4):1255–1260. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Maca R. D., Bonnard G. D., Herberman R. B. The suppression of mitogen- and alloantigen-stimulated peripheral blood lymphocytes by cultured human T lymphocytes. J Immunol. 1979 Jul;123(1):246–251. [PubMed] [Google Scholar]
- Merril C. R., Switzer R. C., Van Keuren M. L. Trace polypeptides in cellular extracts and human body fluids detected by two-dimensional electrophoresis and a highly sensitive silver stain. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4335–4339. doi: 10.1073/pnas.76.9.4335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizel S. B., Oppenheim J. J., Rosenstreich D. L. Characterization of lymphocyte-activating factor (LAF) produced by the macrophage cell line, P388D1. I. Enhancement of LAF production by activated T lymphocytes. J Immunol. 1978 May;120(5):1497–1503. [PubMed] [Google Scholar]
- Morgan D. A., Ruscetti F. W., Gallo R. Selective in vitro growth of T lymphocytes from normal human bone marrows. Science. 1976 Sep 10;193(4257):1007–1008. doi: 10.1126/science.181845. [DOI] [PubMed] [Google Scholar]
- Rosenberg S. A., Spiess P. J., Schwarz S. In vitro growth of murine T cells. I. Production of factors necessary for T cell growth. J Immunol. 1978 Nov;121(5):1946–1950. [PubMed] [Google Scholar]
- Ruscetti F. W., Morgan D. A., Gallo R. C. Functional and morphologic characterization of human T cells continuously grown in vitro. J Immunol. 1977 Jul;119(1):131–138. [PubMed] [Google Scholar]
- Strausser J. L., Rosenberg S. A. In vitro growth of cytotoxic human lymphocytes. I. Growth of cells sensitized in vitro to alloantigens. J Immunol. 1978 Oct;121(4):1491–1495. [PubMed] [Google Scholar]
- Watson J., Aarden L. A., Lefkovits I. The purification and quantitation of helper T cell-replacing factors secreted by murine spleen cells activated by concanavalin A. J Immunol. 1979 Jan;122(1):209–215. [PubMed] [Google Scholar]
- Zarling J. M., Bach F. H. Continuous culture of T cells cytotoxic for autologous human leukaemia cells. Nature. 1979 Aug 23;280(5724):685–688. doi: 10.1038/280685a0. [DOI] [PubMed] [Google Scholar]



