Abstract
The overall aim of the Global Enteric Multicenter Study–1 (GEMS-1) is to identify the etiologic agents associated with moderate-to-severe diarrhea (MSD) among children <5 years of age, and thereby the attributable pathogen-specific population-based incidence of MSD, to guide investments in research and public health interventions against diarrheal disease. To accomplish this, 9 core assumptions were vetted through widespread consultation: (1) a limited number of etiologic agents may be responsible for most MSD; (2) a definition of MSD can be crafted that encompasses cases that might otherwise be fatal in the community without treatment; (3) MSD seen at sentinel centers is a proxy for fatal diarrheal disease in the community; (4) matched case/control is the appropriate epidemiologic design; (5) methods across the sites can be standardized and rigorous quality control maintained; (6) a single 60-day postenrollment visit to case and control households creates mini-cohorts, allowing comparisons; (7) broad support for GEMS-1 messages can be achieved by incorporating advice from public health spokespersons; (8) results will facilitate the setting of investment and intervention priorities; and (9) wide acceptance and dissemination of the GEMS-1 results can be achieved.
The Global Enteric Multicenter Study–1 (GEMS-1) aims to identify the etiologic agents associated with moderate-to-severe diarrhea (MSD) in 3 pediatric age groups (0–11, 12–23, and 24–59 months of age) and estimate the population-based incidence in 4 sites in sub-Saharan Africa and 3 sites in South Asia. It also intends to identify water/sanitation/hygiene risk factors that may favor the transmission of specific enteric pathogens or factors that may be protective against pathogens that cause MSD. Each GEMS-1 site has an associated defined population under prospective demographic surveillance.
In the first article in this supplement, Levine et al provide the rationale for the GEMS-1 study and its genesis. In this article, we describe certain assumptions that were either fundamental in the design of GEMS-1, critical to its successful implementation, or necessary for the interpretation, widespread dissemination, and advocacy of its results.
ASSUMPTION 1: A LIMITED NUMBER OF ETIOLOGIC AGENTS MAY BE RESPONSIBLE FOR A DISPROPORTIONATELY LARGE FRACTION OF ALL MSD
The genesis of GEMS-1 was largely driven by the fundamental concept that it may be possible to diminish the morbidity and mortality burden caused by diarrheal disease in young children in developing countries if effective vaccines were available and introduced against the main etiologic agents that cause potentially fatal MSD. As reviewed by Levine et al (this supplement), many infectious agents have been purported to have the ability to cause severe and fatal diarrheal disease in young children. If the overall burden of diarrheal disease represents the collective consequence of a large number of pathogens, each making a small contribution, it is unlikely that the vaccine approach would be feasible from the epidemiologic, industrial, financial, or public health perspectives. On the other hand, if only a relatively small number of etiologic agents in infants 0–11 months, toddlers 12–23 months, and preschool children (24–59 months of age) were found to be responsible for a notable proportion of all MSD, then a vaccine-based strategy could be feasible. In undertaking GEMS-1, we were following the assumption that if we could identify a limited number of target pathogens for antidiarrheal vaccine development and/or delivery, a small number of vaccines could protect against a large fraction of the multicausal syndrome known as diarrheal disease. And if those vaccines were implemented widely in the developing world, the slope of decrease of the global morbidity and mortality burden from MSD could be steepened [1].
ASSUMPTION 2: A DEFINITION OF ACUTE MSD CAN BE CRAFTED THAT LIKELY ENCOMPASSES THOSE CASES THAT MIGHT BE FATAL IF THEY WERE NOT READILY TREATED
Fundamental to the GEMS-1 project was creating a definition for MSD that would receive wide acceptance by clinicians and epidemiologists and that would be practical for use in the field and applicable in both sub-Saharan Africa and South Asian settings. As described by Kotloff et al (this supplement), this took considerable discussion among GEMS investigators and clinical consultants on the Steering Committee on Epidemiologic and Clinical Issues (Table 1) who shared their experiences and constraints. Through consensus, a useful definition of MSD was successfully crafted: a child with diarrhea (≥3 abnormally loose stools) within the previous 24 hours with onset within the previous 7 days, following at least 7 days without diarrhea, and accompanied by evidence of clinically significant dehydration (loss of skin turgor, sunken eyes, or a decision by the clinician to administer intravenous fluids), dysentery (blood in the stool), or a clinical decision to hospitalize the child. Thus, the case definition encompasses episodes that might be fatal based on dehydration, dysentery, or systemic toxicosis. Cases progressing to persistent diarrhea, another clinical diarrheal syndrome associated with increased risk of fatality, were detected by means of a simple 14-day follow-up visual aid by which the mother or other caretaker recorded the number of days that diarrhea continued unabated (see Kotloff et al, this supplement). Following completion of the initial 3-year case control study of the etiology and burden of MSD (thereafter referred to as “GEMS-1”), a one-year, carry-on study called “GEMS-1A” was initiated to study diarrhea cases not meeting the definition for MSD. These less severe diarrhea (LSD) cases are being enrolled alongside MSD, with controls defined identically for both groups, enabling comparison of etiology and other outcomes between MSD and LSD.
Table 1.
Steering Committee on Epidemiologic and Clinical Issues
| Members | Affiliation |
|---|---|
| External Members | |
| Fred N. Binka | University of Ghana |
| John D. Clemens | International Vaccine Institute; University of California, Los Angeles (currently) |
| Dani I. Cohen | Tel Aviv University |
| Roger I. Glass | Fogarty International Center |
| Halvor Sommerfelt | University of Bergen |
| Paul D. Stolley | University of Maryland School of Medicine |
| Internal Members | |
| Richard A. Adegbola | Medical Research Council, Gambia |
| Adebayo Akinsola | Medical Research Council, Gambia |
| Pedro L. Alonso | University of Barcelona |
| Sujit K. Battacharya | National Institute of Cholera and Enteric Diseases; Indian Council ofMedical Research; World Health Organization (currently) |
| Robert F. Breiman | Centers for Disease Control and Prevention |
| Sumon K. Das | International Centre for Diarrhoeal Disease Research, Bangladesh |
| Abu S. Faruque | International Centre for Diarrhoeal Disease Research, Bangladesh |
| Philip C Hill | Medical Research Council, Gambia |
| Byomkesh Manna | National Institute of Cholera and Enteric Diseases |
| Eric D. Mintz | Centers for Disease Control and Prevention |
| Tacilta Nhampossa | Centro de Investigaçao em Saude da Manhiça |
| Richard Omore | Centers for Disease Control and Prevention |
| Ciara O'Reilly | Centers for Disease Control and Prevention |
| Debasish Saha | Medical Research Council, Gambia |
| Samba O. Sow | Center for Vaccine Development, Mali |
| Dipika Sur | National Institute of Cholera and Enteric Diseases |
| Anita K. M. Zaidi | Aga Khan University |
ASSUMPTION 3: MSD SEEN AT SENTINEL HOSPITALS AND HEALTH CENTERS IS A PROXY FOR FATAL PEDIATRIC DIARRHEAL DISEASE IN THE COMMUNITY
It was necessary to have a strategy to capture cases of MSD in a practical way. The assumption made by the designers of the GEMS-1 project is that cases of diarrheal disease for which healthcare is sought at hospitals or health centers are likely to be more severe than cases that might be detected at the household level. Thus, the assumption was that passive surveillance at sentinel fixed healthcare facilities would be enriched for MSD cases. Accordingly, the selection of the most appropriate sentinel healthcare facilities was a critical step in the GEMS-1 design. As described by Kotloff et al, a large baseline random survey (Health Care Utilization and Attitudes Survey [HUAS]) carried out within the linked defined population generated the necessary information to identify the most appropriate facilities. This survey also provided information on healthcare preferences to allow adjustment so that population-wide incidence rates could be generated based on extrapolation of data gathered from sentinel health centers (SHCs).
ASSUMPTION 4: THE APPROPRIATE EPIDEMIOLOGIC DESIGN FOR IDENTIFYING THE RELATIVE IMPORTANCE OF PATHOGENS ASSOCIATED WITH MSD IS A MATCHED CASE/CONTROL STUDY
Whereas diarrheal illness among children in developing countries is common, cases of a severity that meet the definition of MSD constitute only a fraction of all pediatric diarrhea cases. Thus, utilizing a prospective cohort design with active household-based surveillance would require a very large cohort. Moreover, active household surveillance would likely modify the true incidence of MSD, since milder forms of diarrhea (when detected) would be treated and the evolution of the illness might be interrupted; that is, without having been detected by household visits, some of those diarrhea cases would have progressed to MSD and would have been detected when care was sought at a fixed healthcare facility. By contrast, a properly designed and executed prospective case/control study can accomplish the objective in a more practical, economical, and cost-effective way. An age-, sex- and village or neighborhood-matched control selection strategy was selected to limit potential confounding by factors that may not be easily controlled for in statistical analysis, thus ensuring the integrity of the results. The advantages and caveats of case/control studies have been reviewed [2, 3]. This type of study must be meticulously designed both with respect to identification of the MSD cases and selection of matched controls. A Steering Committee on Epidemiologic and Clinical Issues replete with individuals with expertise in case/control study design and in the performance of such studies in developing countries (Table 1) was instrumental in influencing the design of GEMS-1 and in attention to details of both case and control selection.
ASSUMPTION 5: THE CLINICAL AND LABORATORY METHODS ACROSS THE SITES COULD BE STANDARDIZED AND GOOD CLINICAL PRACTICES, GOOD CLINICAL LABORATORY PRACTICES, AND RIGOROUS QUALITY CONTROL COULD BE MAINTAINED THROUGHOUT THE STUDY
There exist institutions in the developing world such as the International Center for Diarrhoeal Diseases Research, Bangladesh (ICDDR,B) in Dhaka and the National Institute for Cholera and Enteric Diseases (NICED) in Kolkata, India, that have exceptional laboratory infrastructure and expertise for the detection of diarrheal pathogens and their characterization using sophisticated techniques. However, in very high-mortality areas of sub-Saharan Africa, there are no comparable venerable institutions with similar track records of sophisticated laboratory competence in detecting and characterizing the broad range of viral, bacterial and protozoal pathogens. On the other hand, there were potential field sites in areas of high young child mortality in sub-Saharan Africa with more general laboratory infrastructure, including where sophisticated techniques were used for the detection of other pathogens. We made the assumption that the capacity of these laboratories could be strengthened and expanded so that they could utilize the wide array of diagnostic technologies (including multiplex polymerase chain reaction) described by Panchalingam et al in this supplement for the detection of a very wide range of diarrheal pathogens.
Of equal importance, the new techniques not only had to be introduced but had to be standardized across all the GEMS sites and a system for continuous quality control had to be assured. The intention was to accomplish this with intensive initial training workshops, additional on-site training and by periodic site visits by an external laboratory supervisor with expertise in quality control and experience working in laboratories in the developing world. At all sites, the GEMS clinical microbiology procedures follow the tenets of Good Clinical Laboratory Practices.
ASSUMPTION 6: MAKING A SINGLE 60-DAY POSTENROLLMENT VISIT TO CASE AND CONTROL HOUSEHOLDS CREATES PROSPECTIVE MINI-COHORTS FOR COMPARISONS
Some diarrheal pathogens can lead to death by dehydration stemming from losses of body water and electrolytes through loose stools and vomiting, and accompanied by diminished intake [4, 5]. When these abnormal losses and diminished intake occur in an infant (whose daily water and electrolyte requirements per kilogram greatly exceed those of an older child or adult), and if prompt and adequate rehydration therapy is not introduced, severe dehydration, renal shutdown, and death can ensue [4, 5]. Some enterotoxigenic bacterial pathogens such as Vibrio cholerae O1 and some strains of enterotoxigenic Escherichia coli can cause such severe purging that even an older child can become severely dehydrated. However, once prompt and appropriate therapy is administered, survival of the patient is virtually assured and within a few days they are back to normal. In contrast, some enteric pathogens that cause pediatric diarrhea invade the intestinal mucosa and cause much destruction accompanied by neutrophilic infiltration. Shigella is the prototype for this type of pathogen, as the mucosal destruction can result in outright bloody diarrhea (ie, dysentery) [6–8]. Other enteric pathogens such as enteropathogenic E. coli (EPEC) and Cryptosporidium cause other forms of mucosal disruption and modification characterized by effacement of enterocytes and pedestal formation [9, 10] or an unusual form of shallow invasion [11]. With pathogens such as Shigella, EPEC, and Cryptosporidium that result in striking pathologic changes in the intestinal mucosa, one cannot help but wonder whether over a more extended period of observation some episodes of diarrheal illness may result in adverse nutritional consequences and death many days beyond the initial acute injury, but by which time the subject may no longer have overt diarrhea. Presumably, cases of diarrheal illness accompanied by significant mucosal destruction or modification may be at particular risk of delayed consequences.
An important component of GEMS is a large case/control study. Onto that platform we have inserted the performance of a single visit to the households of cases and controls approximately 60 days after enrollment. At that time, follow-up anthropometric measurements are made (in particular, length/height) to determine whether the linear growth of cases over that period are the same or different from their matched controls, and whether growth impediments were associated with infection with specific enteropathogens. At the time of this 60-day follow-up visit, we are also ascertaining whether the child (whether case or control) is still alive. Our assumption is that the risk of death may be greater over that approximately 60-day period for the cases than for their matched controls. If such an effect is discovered, intensive analyses of the data would be undertaken to attempt to discover important determinants, including baseline differences, host risk factors, and possible associations with specific pathogens.
ASSUMPTION 7: BROAD SUPPORT AND “BUY-IN” TO THE CONCEPT OF GEMS-1 CAN BE ACHIEVED BY INCORPORATING THE BEST ADVICE FROM MANY MEMBERS OF THE PUBLIC HEALTH, MICROBIOLOGIC, EPIDEMIOLOGIC, PEDIATRIC, NUTRITION SCIENCE, BIOSTATISTICAL, AND DISEASE CONTROL COMMUNITIES IN VARIOUS PARTICIPATORY AND ADVISORY ROLES
The GEMS is a historical, complex, highly ambitious multicenter project that is unlikely to be repeated. Therefore, it is critical that there be wide conceptual support for the concept and methods. Since the results of GEMS will indicate that some pathogens appear to be more important than others for MSD, champions of one or another pathogen may be disappointed by the results even as other champions are elated by the recognition of how their pathogen of interest has fared. Therefore, prior to the initiation of the studies themselves, it was critical that the epidemiologic, clinical microbiologic, data management, and statistical analysis methods be vetted by a broad array of experts and stakeholders. For this reason, the process of designing the GEMS clinical protocol, and selection of the pathogens to be detected and with what laboratory methods, was painstaking and iterative, and was accomplished with the assistance of the Steering Committee on Epidemiologic and Clinical Issues (Table 1) and a Steering Committee on Microbiological Issues (Table 2). Advice and guidance on anthropometric and nutritional aspects of GEMS were provided by a Steering Committee on Nutritional Issues (Table 3), while the statistical strategies of analyzing the data were greatly influenced by a Steering Committee on Biostatistical Issues (Table 4). Each of these steering committees included widely recognized authorities who collectively provided broad expertise. Annual investigators’ meetings were convened, either at GEMS sites in Africa (Mali and Mozambique) or Asia (India) or in the United States (Baltimore, Seattle, Philadelphia, and Boston). Some members of the various steering committees were invited to the investigators’ meetings to provide liaison to their respective steering committees.
Table 2.
Steering Committee on Microbiological Issues
| Members | Affiliation |
|---|---|
| External Members | |
| Roger I. Glass | Fogarty International Center |
| Patrick R. Murray | National Institutes of Health; BD Diagnostics (currently) |
| Philippe J. Sansonetti | Institut Pasteur |
| Duncan A. Steele | World Health Organisation; PATH; Bill and Melinda Gates Foundation (currently) |
| Internal Members | |
| Martin Antonio | Medical Research Council |
| Anowar Hossain | International Centre for Diarrhoeal Diseaese Research, Bangladesh |
| Eric R. Houpt | University of Virginia |
| Inacio M. Mandomando | Centro de Investigaçao em Saude da Manhiça |
| Benjamin Ochieng | Centers for Disease Control and Prevention |
| Joseph Oundo | Centers for Disease Control and Prevention |
| William A. Petri | University of Virginia |
| Valeria Prado | University of Chile |
| T. Ramamurthy | National Institute of Cholera and Enteric Diseases |
| Ann-Mari Svennerholm | University of Göteborg |
| Boubou Tamboura | Center for Vaccine Development, Mali |
| Roberto Vidal | University of Chile |
| Anita K. M. Zaidi | Aga Khan University |
Table 3.
Steering Committee on Nutritional Issues
| Member | Affiliation |
|---|---|
| Claudio E. Lanata | Instituto de Investigacíon Nutricional |
| Reynaldo Martorell | Emory University |
| Rebecca J. Stoltzfus | Cornell University |
Table 4.
Steering Committee on Biostatistical Issues
| Member | Affiliation |
|---|---|
| Barry I. Graubard | National Cancer Institute |
| Lawrence H. Moulton | Johns Hopkins University |
| William K. Pan | Johns Hopkins University |
| Peter Smith | London School of Tropical Medicineand Hygiene |
| Janet Wittes | Statistics Collaborative |
Once the collection of field and laboratory data were completed and data cleaning was under way toward achieving locked datasets, and once the analytical approaches had been agreed upon, a large independent external committee was formed, the GEMS International Strategic Advisory Committee (GEMS-ISAC; Table 5), to provide a fresh, very high-level overview of the project and to provide strategic advice. The idea for establishing the ISAC was conceived at the Bill & Melinda Gates Foundation by Thomas Brewer and Niranjan Bose. The ISAC, with Professor George Griffin of St George's Hospital Medical School as senior co-chair and with Dean Fred Binka of the University of Ghana and Professor Zulfqar Bhutta as the other two co-chairs, brings together approximately 20 internationally recognized leaders in public health, microbiology, epidemiology, nutrition, statistics, clinical infectious diseases, pediatrics, diarrheal diseases, environmental health (water/sanitation/hygiene), and environmental engineering, from both industrialized and developing countries.
Table 5.
International Strategic Advisory Committee
| Member | Affiliation |
|---|---|
| George E. Armah | University of Ghana |
| Zulfiqar A. Bhuttaa | Aga Khan University |
| Fred N. Binkaa | University of Ghana |
| Robert E. Black | Johns Hopkins University |
| A. Louis Bourgeois | PATH |
| Philip J. Cooper | Liverpool School of Tropical Medicine |
| Alejandro Cravioto | International Centre for Diarrhoeal Disease Research, Bangladesh |
| Valerie A. Curtis | London School of Hygiene and Tropical Medicine |
| Gordon Dougan | Wellcome Trust Sanger Institute |
| Kenneth C. Earhart | Centers for Disease Control and Prevention |
| Adenike Grange | Independent |
| George E. Griffinb | St George's, University of London |
| Gangadeep Kang | Christian Medical College |
| Claudio E. Lanata | Instituto de Investigacíon Nutricional |
| Reynaldo Martorell | Emory University |
| G. Balakrish Nair | National Institute of Cholera and Enteric Diseases |
| Miguel O'Ryan | University of Chile |
| Philippe J. Sansonetti | Pasteur Institute |
| Peter Smith | London School of Tropical Medicine and Hygiene |
a Co-chair.
b Principal co-chair.
The GEMS has been fortunate at all stages of the project, from conception to analyses of data, to have a series of program officers at the Bill & Melinda Gates Foundation who have been extraordinarily invaluable in supporting the project in every way possible. These include Thomas Brewer, Niranjan Bose, and Duncan Steele in the later years and Regina Rabinovich and Jan Agosti in the early years of the project.
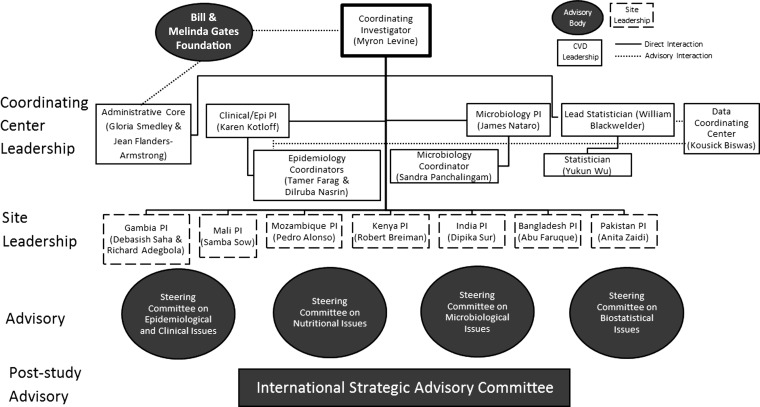
An organogram of the management of the GEMS is shown in Figure 1. Also shown in this figure is the interface and point in the project the various committees interacted with the GEMS leadership and the GEMS investigators.
Figure 1.
Organogram for the Global Enteric Multicenter Study-1 (GEMS-1). This flowchart shows the organizational structure between the GEMS-1 coordinating center leadership, based at the Center for Vaccine Development, and the site principal investigators, based on the ground in the 7 country sites. It also shows the roles played by the various advisory bodies. Abbreviations: CVD, Center for Vaccine Development; PI, principal investigator.
ASSUMPTION 8: RESULTS WILL FACILITATE THE SETTING OF INVESTMENT & INTERVENTION PRIORITIES
It is the hope and expectation of the GEMS investigators that the results of the GEMS will provide decision makers in international agencies, government development agencies, research funding agencies, philanthropic organizations, and public health implementers in developing countries an evidence base on the etiology and burden of more severe forms of diarrheal disease in developing countries, insights on MSD-associated mortality, and MSD-associated nutritional consequences.
The GEMS data on etiology of diarrheal disease in developing countries can also serve as a resource for groups that estimate mortality and disease burden (such as the World Health Organization [WHO] Child Health Epidemiology Reference Group, investigators at the Institute for Health Metrics and Evaluation, and similar groups), and groups that are concerned with food safety (such as the Foodborne Disease Burden Epidemiology Reference Group of WHO and the Food and Agriculture Organization) and groups preparing investment cases for diarrheal disease vaccines (eg, the Shigella and ETEC Investment Case efforts at PATH).
ASSUMPTION 9: WIDE ACCEPTANCE AND DISSEMINATION OF THE GEMS-1 RESULTS CAN BE ACHIEVED
Once the GEMS data have been fully analyzed and initial publications prepared, it is critical to disseminate short summaries of the most salient points and press releases in simple transparent language to convey the results to lay audiences, community leaders, politicians, trend setters, opinion makers, and the general population. Principal investigators at many of the GEMS sites have already begun to sensitize political leaders in their government of the importance of the GEMS data. For example, since rotavirus vaccines are expected to be introduced into the routine infant immunization schedule of the Expanded Programme on Immunization for a number of high-mortality developing countries in sub-Saharan Africa and Asia, the GEMS data can be exceedingly helpful to assist advocacy efforts.
Notes
Financial support. This study was funded by the Bill & Melinda Gates Foundation (grant number 38874).
Supplement sponsorship. This article was published as part of the supplement entitled “The Global Enteric Multicenter Study (GEMS),” sponsored by the Bill & Melinda Gates Foundation.
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Levine MM. Enteric infections and the vaccines to counter them: future directions. Vaccine. 2006;24:3865–73. doi: 10.1016/j.vaccine.2006.03.039. [DOI] [PubMed] [Google Scholar]
- 2.Schulz KF, Grimes DA. Case-control studies: research in reverse. Lancet. 2002;359:431–4. doi: 10.1016/S0140-6736(02)07605-5. [DOI] [PubMed] [Google Scholar]
- 3.Rothman KJ, Greenland S. Modern epidemiology. 3rd ed. Philadelphia: Lippincott Williams & Wilkins; 2008. [Google Scholar]
- 4.Booth IW, Levine MM, Harries JT. Oral rehydration therapy in acute diarrhoea in childhood. J Pediatr Gastroenterol Nutr. 1984;3:491–9. doi: 10.1097/00005176-198409000-00003. [DOI] [PubMed] [Google Scholar]
- 5.Levine MM, Pizarro D. Advances in therapy of diarrheal dehydration: oral rehydration. Adv Pediatr. 1984;31:207–34. [PubMed] [Google Scholar]
- 6.Sansonetti PJ. War and peace at mucosal surfaces. Nat Rev Immunol. 2004;4:953–64. doi: 10.1038/nri1499. [DOI] [PubMed] [Google Scholar]
- 7.Cossart P, Sansonetti PJ. Bacterial invasion: the paradigms of enteroinvasive pathogens. Science. 2004;304:242–8. doi: 10.1126/science.1090124. [DOI] [PubMed] [Google Scholar]
- 8.Levine MM, DuPont HL, Formal SB, et al. Pathogenesis of Shigella dysenteriae 1 (Shiga) dysentery. J Infect Dis. 1973;127:261–70. doi: 10.1093/infdis/127.3.261. [DOI] [PubMed] [Google Scholar]
- 9.Moon HW, Whipp SC, Argenzio RA, Levine MM, Giannella RA. Attaching and effacing activities of rabbit and human enteropathogenic Escherichia coli in pig and rabbit intestines. Infect Immun. 1983;41:1340–51. doi: 10.1128/iai.41.3.1340-1351.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rothbaum RJ, Partin JC, Saalfield K, McAdams AJ. An ultrastructural study of enteropathogenic Escherichia coli infection in human infants. Ultrastruct Pathol. 1983;4:291–304. doi: 10.3109/01913128309140582. [DOI] [PubMed] [Google Scholar]
- 11.Elliott DA, Clark DP. Cryptosporidium parvum induces host cell actin accumulation at the host-parasite interface. Infect Immun. 2000;68:2315–22. doi: 10.1128/iai.68.4.2315-2322.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]