Abstract
The recently isolated 28-residue sequence of prosomatostatin, a putative somatostatin precursor from pig hypothalamus and intestine, was synthesized by solid-phase methodology, characterized, and tested in rats for its effects on the release of insulin, glucagon, growth hormone, and prolactin. The synthetic product strongly suppressed plasma levels of insulin, glucagon, and growth hormone, and it appeared to be more active in the pancreas than in the pituitary. It inhibited insulin release about 5 times more effectively than somatostatin on a weight basis. The prohormone also suppressed growth hormone and prolactin levels in vitro. A time-course experiment for the effect of prosomatostatin on growth hormone release in vivo showed a significant suppression of plasma growth hormone for at least 90 min.
Full text
PDF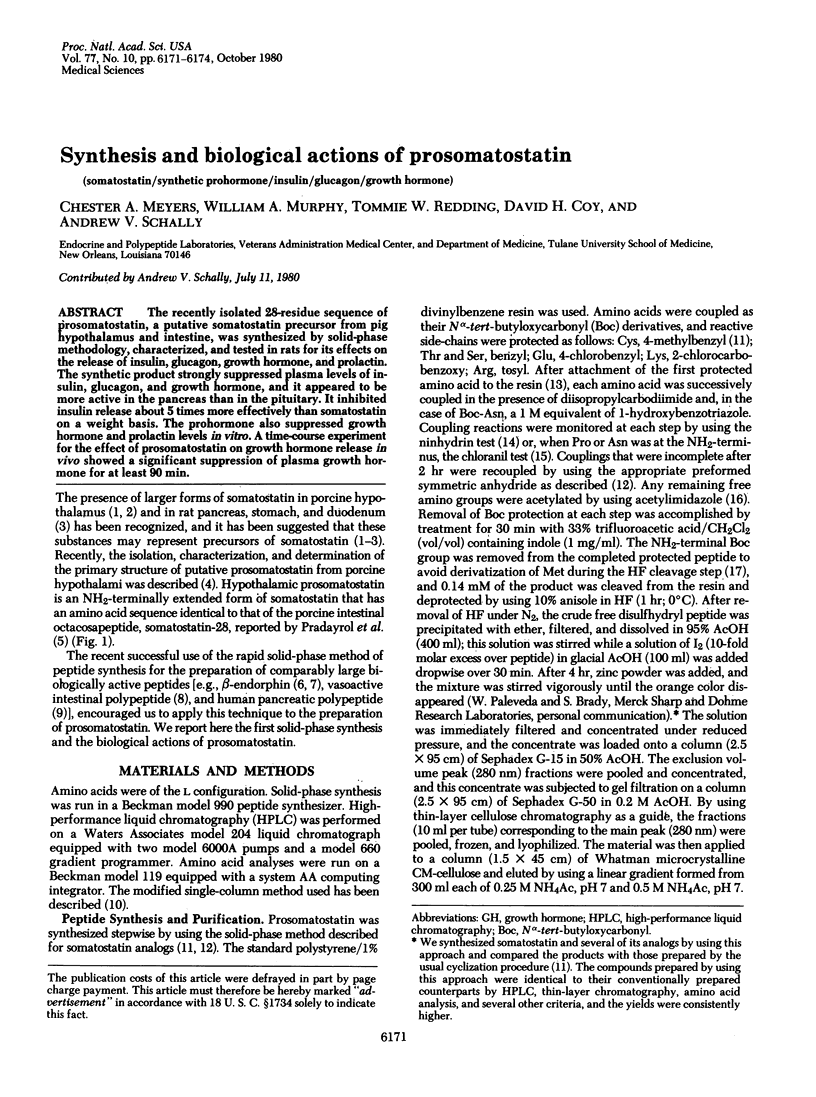

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arimura A., Sato H., Dupont A., Nishi N., Schally A. V. Somatostatin: abundance of immunoreactive hormone in rat stomach and pancreas. Science. 1975 Sep 19;189(4207):1007–1009. doi: 10.1126/science.56779. [DOI] [PubMed] [Google Scholar]
- Coy D. H., Coy E. J., Hirotsu Y., Vilchez-Martinez J. A., Schally A. V., van Nispen J. W., Tesser G. I. Investigation of the role of tryptophan in the luteinizing hormone-releasing hormone. Biochemistry. 1974 Aug 13;13(17):3550–3553. doi: 10.1021/bi00714a022. [DOI] [PubMed] [Google Scholar]
- Coy D. H., Gardner J. Solid-phase synthesis of porcine vasoactive intestinal peptide. Int J Pept Protein Res. 1980 Jan;15(1):73–78. doi: 10.1111/j.1399-3011.1980.tb02552.x. [DOI] [PubMed] [Google Scholar]
- Gordin A., Meyers C., Arimura A., Coy D. H., Schally A. V. An in vivo model for testing inhibition of arginine-induced insulin and glucagon release by somatostatin analogs. Acta Endocrinol (Copenh) 1977 Dec;86(4):833–841. doi: 10.1530/acta.0.0860833. [DOI] [PubMed] [Google Scholar]
- Kaiser E., Colescott R. L., Bossinger C. D., Cook P. I. Color test for detection of free terminal amino groups in the solid-phase synthesis of peptides. Anal Biochem. 1970 Apr;34(2):595–598. doi: 10.1016/0003-2697(70)90146-6. [DOI] [PubMed] [Google Scholar]
- Li C. H., Yamashiro D., Tseng L. F., Loh H. H. Synthesis and analgesic activity of human beta-endorphin. J Med Chem. 1977 Mar;20(3):325–328. doi: 10.1021/jm00213a001. [DOI] [PubMed] [Google Scholar]
- Markley L. D., Dorman L. C. A comparative study of terminating agents for use in solid-phase peptide synthesis. Tetrahedron Lett. 1970 May;(21):1787–1790. doi: 10.1016/s0040-4039(01)98083-9. [DOI] [PubMed] [Google Scholar]
- Meyers C. A., Coy D. H., Huang W. Y., Schally A. V., Redding T. W. Highly active position eight analogues of somatostatin and separation of peptide diastereomers by partition chromatography. Biochemistry. 1978 Jun 13;17(12):2326–2331. doi: 10.1021/bi00605a011. [DOI] [PubMed] [Google Scholar]
- Meyers C., Arimura A., Gordin A., Fernandez-Durango R., Coy D. H., Schally A. V., Drouin J., Ferland L., Beaulieu M., Labrie F. Somatostatin analogs which inhibit glucagon and growth hormone more than insulin release. Biochem Biophys Res Commun. 1977 Jan 24;74(2):630–636. doi: 10.1016/0006-291x(77)90349-7. [DOI] [PubMed] [Google Scholar]
- Noble R. L., Yamashiro D., Li C. H. Synthesis of a nonadecapeptide corresponding to residues 37-55 of ovine prolactin. 1 Detection and isolation of the sulfonium form of methionine-containing peptides. J Am Chem Soc. 1976 Apr 14;98(8):2324–2328. doi: 10.1021/ja00424a055. [DOI] [PubMed] [Google Scholar]
- Pradayrol L., Jörnvall H., Mutt V., Ribet A. N-terminally extended somatostatin: the primary structure of somatostatin-28. FEBS Lett. 1980 Jan 1;109(1):55–58. doi: 10.1016/0014-5793(80)81310-x. [DOI] [PubMed] [Google Scholar]
- Schally A. V., Dupont A., Arimura A., Redding T. W., Nishi N., Linthicum G. L., Schlesinger D. H. Isolation and structure of somatostatin from porcine hypothalami. Biochemistry. 1976 Feb 10;15(3):509–514. doi: 10.1021/bi00648a009. [DOI] [PubMed] [Google Scholar]
- Schally A. V., Huang W. Y., Chang R. C., Arimura A., Redding T. W., Millar R. P., Hunkapiller M. W., Hood L. E. Isolation and structure of pro-somatostatin: a putative somatostatin precursor from pig hypothalamus. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4489–4493. doi: 10.1073/pnas.77.8.4489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vale W., Grant G., Amoss M., Blackwell R., Guillemin R. Culture of enzymatically dispersed pituitary cells: functional validation of a method. Endocrinology. 1972 Aug;91(2):562–572. doi: 10.1210/endo-91-2-562. [DOI] [PubMed] [Google Scholar]



