Abstract
Despite the burden of both malnutrition and tuberculosis in children worldwide, there are few studies on the mechanisms that underlie this relationship. From available research, it appears that malnutrition is a predictor of tuberculosis disease and is associated with worse outcomes. This is supported through several lines of evidence, including the role of vitamin D receptor genotypes, malnutrition's effects on immune development, respiratory infections among malnourished children, and limited work specifically on pediatric tuberculosis and malnutrition. Nutritional supplementation has yet to suggest significant benefits on the course of tuberculosis in children. There is a critical need for research on childhood tuberculosis, specifically on how nutritional status affects the risk and progression of tuberculosis and whether nutritional supplementation improves clinical outcomes or prevents disease.
Tuberculosis remains a significant source of morbidity and mortality among children in resource-limited settings. Of the 9 million new tuberculosis infections each year, 11% are in children [1]. Malnutrition is also highly prevalent in children living in tuberculosis endemic countries and contributes to 2.2 million deaths in children under 5 years of age globally [2]. Poverty, overcrowding, food insecurity, and human immunodeficiency virus (HIV) further set the stage for both malnutrition and poor infection control.
Although the World Health Organization (WHO) states that malnutrition is a significant risk factor for childhood tuberculosis [1], there are limited studies to explain the mechanisms underlying this association. This may be due to the challenges in diagnosing pediatric tuberculosis, difficulty in establishing a causal role of malnutrition on tuberculosis, and an overall low research priority because of the limited infectivity of children. We will review 4 lines of support that serve as the foundation of our understanding of the interaction between pediatric tuberculosis and nutritional status, namely, (1) gene polymorphisms relating vitamin metabolism to risk of tuberculosis, (2) studies investigating immune development among malnourished children, (3) associations between malnutrition and respiratory tract infections in children, and (4) associations between nutritional status and tuberculosis in both animal models and children. Taken together, the evidence suggests that malnutrition affects genetic expression and immune function that predisposes children to tuberculosis progression, and the resulting disease and inflammatory response further worsens the nutritional state. Because of this devastating cycle, understanding the mechanisms that contribute to this precise interaction in children is essential to addressing both epidemics and ascertaining whether nutritional interventions will be of benefit.
METHODS
References were identified through searches on PubMed, Cochrane, Web of Knowledge, and Google Scholar. Inclusion criteria were articles in English related to risk factors, etiology, and management of tuberculosis in relation to nutritional status. PubMed searches included the terms “tuberculosis,” “malnutrition [MeSH],” “nutritional status [MeSH],” “infection,” “pulmonary infection,” “respiratory tract infections [MeSH],” “milk, human/immunology,” and “micronutrient.” Searches were completed with and without the limits “All Infant: birth–23 months, All Child: 0–18 years.” The Cochrane database was searched with the terms “malnutrition” and “tuberculosis.” Web of Knowledge search terms included “malnutrition” and “tuberculosis” and was limited to pediatrics. Lastly, Google Scholar searches included “malnutrition tuberculosis children,” “micronutrient deficiency tuberculosis,” “malnutrition respiratory infections children,” and “micronutrient tuberculosis.”Articles relevant to the topic were reviewed and included in our discussion, as were significant articles cited by these papers.
Genetics of Malnutrition and Tuberculosis
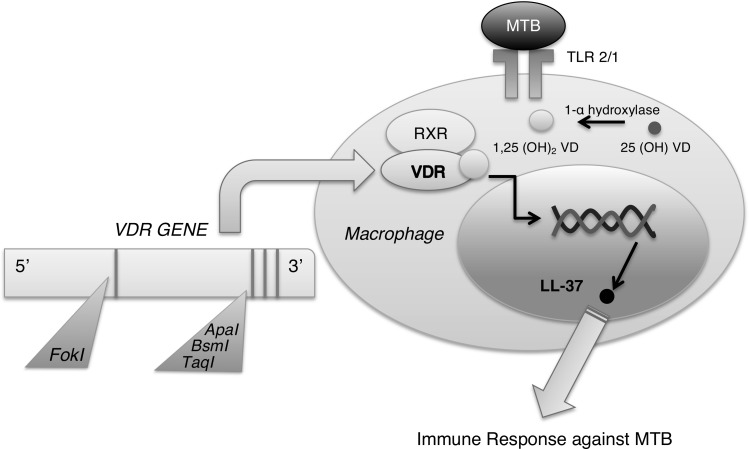
There is growing evidence that genes related to vitamin metabolism contribute to susceptibility to tuberculosis. Specifically, vitamin D provides an exciting example of how genetics may underlie risk of tuberculosis disease (Figure 1). The vitamin D receptor (VDR) is a soluble nuclear receptor found in many immune cells and is believed to play a role in cytokine secretion patterns, maturation of dendritic cells, and effector and regulatory T-cell function [3]. Several VDR gene polymorphisms have been found that can impact tuberculosis risk and outcomes, including BsmI, TaqI, and ApaI at the 3′ end of VDR, and FokI on exon site 2 (Figure 1) [4]. For example, the TaqI Tt and ApaI AA genotype are associated with improved response to therapy and faster time to sputum conversion in tuberculosis patients [4]. On the other hand, TaqI tt, TaqI Bb, TaqI Ff, and BsmI bb have been associated with an increased risk of tuberculosis [4]. Risk or protection may be influenced by ethnic background; a recent meta-analysis was performed on a variety of populations and found that the FokI ff genotype was most significant in the Asian population, whereas there was no effect in Africans or South Americans [5]. Population differences may be related to a variety of factors, including vitamin D status and HIV and tuberculosis prevalence rates.
Figure 1.
Role of genetics in tuberculosis susceptibility. The vitamin D receptor (VDR) has an integral role in the tuberculosis immune response through its binding with vitamin D to induce antimicrobial function via LL-37. Several polymorphisms in the VDR gene have also been implicated in tuberculosis susceptibility. Abbreviations: MTB, Mycobacterium tuberculosis; RXR, retinoid X receptor; TLR, Toll-like receptor; VD, vitamin D.
Vitamin D itself is also essential for downstream genetic expression important for the immune response against Mycobacterium tuberculosis. In the innate immune system, activation of Toll-like receptor (TLR) 2/1 by M. tuberculosis antigen presentation leads to the expression of VDR and 1-α vitamin D hydroxylase [4]. The hydroxylase converts 25(OH) vitamin D to its active form 1,25 (OH)2 vitamin D, which binds to VDR. They then form a heterodimer with retinoid X receptor (RXR), creating a complex that translocates to the nucleus to regulate gene transcription (Figure 1). A key protein formed is LL-37, a member of the cathelicidin family, known to have antimicrobial effects against Mycobacterium tuberculosis while recruiting other immune cells to the site of infection [4]. Other functions for vitamin D and VDR are to regulate antigen presentation and processing, phagocytosis, and interleukin (IL)-1 β and tumor necrosis factor α (TNF-α) production essential for the immune response [3].
Studies on vitamin D and VDR genetics illustrate a novel gene-environment model that can help to stratify tuberculosis risk. It should be noted though that these studies were all conducted in adult tuberculosis populations. Further research is necessary to investigate how these polymorphisms influence the risk of tuberculosis in children.
Immune Development and Malnutrition
Defense against M. tuberculosis requires a complex immune response that involves both innate and adaptive immunity [6]. However, in newborns, it is important to appreciate that cell-mediated immunity is incomplete, and they depend mostly on innate immunity and maternal antibodies [7]. Yet, even innate immunity is impaired; evidence suggests that newborns have reduced function in antigen-presenting cells (APCs), neutrophils, and TLRs, and decreased blood complement levels [7]. Moreover, adaptive immunity is thought to be skewed to a helper T cell 2 type response, potentially as a way to reduce a proinflammatory reaction, decrease an allo-immune response against the mother, and promote tolerance of harmless new antigens such as gut flora and food [7]. However, this also places them at considerable risk against intracellular organisms, including tuberculosis, that depend on a Th1 response [6, 7]. Nutrition plays an essential role to develop the appropriate innate and Th1 immune responses against tuberculosis [8].
The mucosal lining is an early site of defense against tuberculosis, where bacterial products trigger TLR signaling pathways in dendritic cells and macrophages to release cytokines and bactericidal defensins and cathelicidins [6, 9]. After birth, a neonate leaves the sterile uterine environment to be rapidly exposed to foreign antigens, which has a large impact on the formation of mucosal innate immunity. Furthermore, the colonization of commensal flora is thought to compete with pathogenic bacteria while shaping TLR responses in the child [7]. Growing evidence demonstrates that early nutrition, in the form of breast milk, has immune properties that modulate inflammation while promoting innate immunity in the mucosa [7]. Breast milk contains a variety of important immune factors including lysozyme, defensins, lactoferrin, soluble CD14, cytokines, complement, and antiviral lipids [10]. Milk trigylcerides, when partially digested from lipases, become monoglyceride and free fatty acid, which can be toxic to many pathogens [10]. Breast milk is also a large source of glycans, which serves as substrate for fermentation and colonization of commensal bacteria, and inhibit pathogen-binding to the mucosal surface [10]. Studies have shown that limited or nonexclusive breastfeeding is associated with an increased risk of respiratory infections [11–13]. A prospective study in Brazil showed that neonates with acute viral bronchiolitis and a shorter length of exclusive breastfeeding had worse clinical outcomes, including increased use of oxygen and longer hospital stay [12]. Therefore, maternal nutrition and her ability to confer protection have large implications on the development of a functional innate immune system in the child.
Although innate immunity is important against tuberculosis, adaptive immunity, in particular the Th1-type response, is critical against this intracellular bacterium [6]. The thymus plays an important role in T lymphocyte maturation and is greatly affected by prenatal and early child nutrition [8]. Studies have shown that low birth weight or being born in the “hungry” season is associated with decreased thymus size [14, 15]. Echography demonstrates thymic atrophy in malnourished children and is associated with higher infant mortality due to infections [16]. Protein deficiency has especially been implicated; children with protein energy malnutrition (PEM) have reduced thymic size, and tissue samples demonstrate apoptosis of cortical thymocytes, microenvironment changes around lymphoid tissue and epithelial cells, and a decrease in thymulin hormone production and thymocyte proliferation [16, 17]. Inadequate zinc intake may also contribute to thymic dysfunction; mice placed on a zinc-deficient diet for 4 weeks only retain 25% of their original thymus size [16]. In addition to its role in innate immunity, breast milk is also associated with thymus size, correlated with the level of IL-7 in the milk [15]. Moreover, glutamine is one of the most abundant proteins in breast milk, and supplementation in early-weaned mice inoculated with bacillus Calmette-Guérin (BCG) demonstrate increased peripheral and lymph leukocyte and lymphocytes [18]. Thus, T-cell maturation is directly related to nutritional status essential for the tuberculosis response.
Although our understanding of immune development grows, several questions still remain regarding its role in the defense against tuberculosis. For example, despite immature immunity, why do we see a robust Th1 type response to BCG vaccine? This suggests that children are able to overcome this polarization toward Th2 responses through an unknown mechanism [7]. Regardless of our limitations, however, we are able to note that breast milk, protein, and micronutrients have significant roles in the development of innate and cell-mediated immunity, and that these factors are critical for the tuberculosis response. Although studies have not been conducted to determine how dysfunction in immune development impacts risk of pediatric tuberculosis, this evidence supports that without adequate early nutrition, appropriate immune development is greatly impaired and places the child at considerable risk.
Nutrition and the Child With Respiratory Infections
Respiratory infections are among the largest contributors of morbidity and mortality in children. Because of the high frequency, it provides an opportunity to study how malnutrition impacts the outcomes and risk of diseases spread by respiratory droplets, such as tuberculosis.
Malnutrition has been associated with increased risk of respiratory infections. A prospective trial in Bangladeshi children found that being underweight increased the risk of an upper respiratory infection by 13%, and wasting increased it by 20% [19]. Moreover, a prospective 10-month study in nomadic Kenyan children found that wasting predicted risk of acute respiratory infections in the wet season [20]. Malnutrition is also a significant predictor of mortality in children with pneumonia, attributing to 52.3% of pneumonia-related deaths [21].
Several mechanisms may underlie the increased risk and severity of respiratory infections in malnourished children. A prospective study on neonates from the Netherlands found that cord blood vitamin D levels predicted risk of RSV bronchiolitis in the first year of life [22]. Zinc levels were also found to be significantly lower in Bangladeshi children with a lower respiratory tract infection and PEM [23]. In addition, leptin deficiency has been implicated; it is structurally similar to cytokines such as IL-6 and IL-11, and the long isoform of the leptin receptor OB-Rb is similar to the cytokine receptor family gp130 [24]. Leptin leads to the secretion of several cytokines, and animal models demonstrate that elevated leptin during starvation prevents lymphoid tissue atrophy. An ex vivo study of T-lymphocytes from malnourished children in Mexico found that incubation with leptin lead to decreases in IL-4 and IL-10 production and increases in IL-2 and interferon γ (IFN-γ), suggesting a shift to the Th1 response [24]. Thus, malnourished children with respiratory infections may have deficits in cell-mediated immunity and the Th1 type response, both critical for tuberculosis immunity.
Because respiratory infections are prevalent in children, a large body of evidence has emerged on risk factors for infection and poor outcomes, including nutritional status. Although there are limitations in translating the risk of one pathogen to another, it has been observed that poor nutrition is associated with severe deficits in immunity, both innate and cell-mediated. Thus, until further research is conducted on childhood tuberculosis, we can learn from past studies in children that suggest that malnutrition significantly worsens the risk and severity of respiratory disease.
Nutrition and the Child With Tuberculosis Disease
Although a third of the world is infected with tuberculosis, there is only a 10% lifetime risk of progression to disease in HIV-uninfected individuals [9]. Malnutrition is thought to contribute to this progression in children, through possible mechanisms as described above. However, it is difficult to disentangle this process in vivo, for once the child has active disease, the resulting inflammatory and immune response increases metabolic rate, affects synthetic pathways (a so-called anabolic block), and impacts absorption, distribution, and excretion of nutrients, which altogether promotes malnutrition [25]. This is supported with evidence that shows tuberculosis therapy significantly improves anthropometric status and micronutrient levels [25, 26]. Thus, although cross-sectional studies demonstrate nutritional deficiencies in malnourished children with tuberculosis [27], they have a limited role in describing the mechanisms underlying this relationship. Instead, it is more useful to evaluate how the malnourished child develops an immune response against tuberculosis soon after infection, and how any possible dysfunction may promote progression to active disease. Consequently, we depend on experiments in which animals are exposed to a virulent strain of tuberculosis, as well as studies in which malnourished children and animals are “infected” via BCG vaccination.
Th-1 Immunity Against Tuberculosis Is Impaired by Malnutrition
Guinea pigs given a low protein diet and then exposed to M. tuberculosis have deficits in mounting an appropriate Th1-type cell-mediated response. This includes decreased lymphocyte proliferation, higher immunoglobulin G levels, and decreased cytokines such as IL-2, TNF-α, and IFN-γ [28]. In addition, there are increases in Fc-γ T cells and transforming growth factor β, considered to have a suppressive effect on function and T-cell proliferation [28]. Consequently, these animals have evidence of worse disease, with higher bacillary load in the lung and spleen [29]. Micronutrients deficiencies such as zinc and vitamin A are also associated with greater bacterial load and worse lesions in the lung [30, 31]. More recently, polyunsaturated fatty acids, in particular the anti-inflammatory omega 3 (n-3) fatty acids, have shown to decrease skin response, increase the bacterial load, and reduce lymphocyte proliferation in tuberculosis-exposed guinea pigs [32]. There is mixed support in mice studies; endogenous release of n-3 fatty acid from transgenic mice demonstrated an increased bacterial load after tuberculosis inoculation, whereas exogenous supplementation provided a level of protection against tuberculosis [33, 34]. Overall, however, we see that nutrition has a profound effect on the Th-1 immune system's ability to defend against tuberculosis soon after infection and thus predisposes the animal to disease progression.
Malnutrition and BCG Vaccination
Studies have shown that children who were vaccinated with BCG had significantly lower tuberculin skin responses if they had severe protein deficiency [17, 35, 36]. Although milder forms of malnutrition may not have deficits in tuberculin response [37], a prospective study among infants vaccinated at birth with BCG showed that mildly or moderately malnourished children still had a decrease in tuberculosis-associated cell-mediated immune responses [38]. This is supported in animal studies, in which protein-deficient animals have a significantly impaired protection from BCG after tuberculosis exposure, as seen with greater bacterial load in the lungs compared with nourished vaccinated animals [39]. This deficit appears to be related to cell-mediated immunity, as it is associated with reduced tuberculin skin reactivity and impaired IL-2, TNF-α, and IFN-γ release from BCG vaccinated, protein-deficient animals [28]. Renourishment of animals returns protection similar to controls, suggesting that protein deficiency serves to affect the function, but not acquisition, of the adaptive immune response against tuberculosis after BCG vaccination [29].
From in vivo studies of children and animals exposed to mycobacterium via inoculation or vaccination, we see that a range of macro- and micronutrients have direct effects on the proper functioning of immune cells that would allow the child to either clear the infection or drive it into a latent state. Consequently, this places children at risk for progression to active disease and further worsening of their malnutrition.
Nutritional Supplementation as Adjuvant Therapy in Tuberculosis
Ultimately, we want to know if nutritional supplementation can improve immune function and clinical outcomes in tuberculosis. Early ecological studies found that during times of food restriction, such as war, tuberculosis morbidity rose significantly and then sharply declined after food supplies returned [17]. However, clinical trials face large challenges, because tuberculosis therapy will cause a rapid drop in bacillary load and improve nutritional status. Consequently, this can overshadow any modest change after supplementation [40]. One promising randomized trial among adults with tuberculosis in Indonesia found that supplementation of zinc and vitamin A resulted in faster sputum conversion time and resolution of lung lesions on chest X-ray [41]. However, more recently, the same group was unable to repeat the results in a more malnourished population with a combined or individual addition of vitamin A and zinc [42].
The few trials on nutritional supplementation for pediatric tuberculosis do not suggest a significant benefit (Table 1) [43]. A study in Brazil showed that zinc supplementation at the time of purified protein derivative (PPD) placement in malnourished children increased the size of induration, suggesting an improvement in cell-mediated immunity [44]. However, an in-vitro study found that in HIV-positive patients, zinc was unable to improve IFN-γ response or increase lymphocyte levels after PPD stimulation [45]. Clinical trials have shown mixed results. Hanekom et al [46] evaluated the response to vitamin A supplementation in 85 South African children at baseline, 6 weeks, and 3 months after initiation of tuberculosis therapy. Supplementation was not associated with a significant improvement in outcomes, including weight change or improvement in respiratory symptoms. Morcos et al [47] conducted a small trial on vitamin D supplementation among children ages 1.5–13 years old and noted clinical and radiographic improvement in the supplementation group but did not demonstrate differences in vitamin D levels or weight gain at the end of therapy. The most comprehensive trial was conducted recently by Mehta et al [48] among 255 children from Tanzania 6 weeks to 5 years of age with active tuberculosis. The children were randomized to receive a daily multivitamin or placebo for 8 weeks after initiation of therapy. Overall, there was no difference in weight after 8 weeks, and there was also no effect in terms of CD4, CD8, and CD3 T-cell subsets.
Table 1.
Summary of Micronutrients Important for Immunity Against Tuberculosis
| Name | Function | Supplementation for Childhood Tuberculosis |
|---|---|---|
| Vitamin D | Macrophage function, proper phagocytosis, lysosomal fusion | Radiological improvement, but no difference in serum levels or weight change [47] |
| Vitamin A | Regulates innate immunity, T and B lymphocyte function, and maintains mucosal epithelium | No improvement in weight or respiratory symptoms [46] |
| Vitamin E | Antioxidant properties that may reduce oxidative stress on T lymphocytes | When included in a multivitamin for children, did not improve weight gain [48] |
| Zinc | Widespread effect on immunity, and deficiency can lead to lymphopenia, poor lymphocyte functioning, thymic atrophy, impaired cell mediated immunity and shift to the Th2 response. Also, essential for metallo-enyzme formation and creation of free radicals | Improves tuberculin response, though no studies on treatment outcomes [44] |
| Selenium | Cell and humoral immunity, utilized in creation of metallo-enzymes | No known studies in children |
| Iron | Innate immunity such as neutrophil and natural killer function, T-cell maturation, and deficiency can result in shift toward Th2 response | No known studies in children |
In summary, there is insufficient evidence to support the use of macro- or micronutrient supplementation for children with active tuberculosis at this time. However, there are several limitations in interpreting supplementation trials, including variable dose concentration, adherence issues, and lack of complementing food sources. In addition, although clinical trials have been unable to demonstrate differences in micronutrient levels or nutritional status at the conclusion of tuberculosis therapy, individuals early in treatment on supplements have faster improvements in micronutrient levels and clinical indicators [46, 49]. A substudy of the Hanekom et al [50] trial also found that vitamin A supplementation in children may help in reducing soluble CD30 (sCD30) levels, suggesting a shift toward Th1 type responses important against tuberculosis. Thus, more comprehensive studies are required to further elucidate how nutritional supplementation may benefit the risk and outcomes of tuberculosis in children. Furthermore, while an early study in Harlem, New York, demonstrated the preventative value of nutritional supplementation on tuberculosis prevalence among household contacts, greater research is required on the role of nutrition on prevention of tuberculosis disease [17, 40].
CONCLUSION
Studies of the role of nutrition on childhood tuberculosis are significantly limited. Yet we continue to classify malnourished children as high risk for tuberculosis and support overall nutritional supplementation for children with tuberculosis. However, the mechanisms underlying the association between malnutrition and childhood tuberculosis remain unclear. Using the available evidence, we suggest a model detailing the known information between nutritional status, immune function, and risk of tuberculosis disease. However, it is equally important to recognize the severe gaps in our knowledge (Table 2). We require greater prospective studies that evaluate how nutritional status impacts the risk of tuberculosis, while conducting further randomized controlled trials on the use of supplementation in tuberculosis therapy. In resource-limited settings, tuberculosis in children is a major cause of morbidity and mortality, and a large reservoir for continued transmission of infection. As our basic understanding of the interaction between tuberculosis and malnutrition grows, it is important that we seek to apply these advances to the welfare of this vulnerable population.
Table 2.
Research Priorities in Childhood Tuberculosis and Malnutrition
| 1. The role of VDR genetic polymorphisms on susceptibility of tuberculosis in children |
| 2. The relationship between early malnutrition, immune development , and tuberculosis -specific responses |
| 3. Prospective studies on how malnutrition predicts tuberculosis disease |
| 4. Longitudinal studies on the role of malnutrition on the efficacy of BCG and novel tuberculosis vaccines |
| 5. Supplementation for both adjuvant therapy and tuberculosis prevention in children |
Abbreviations: BCG, bacillus Calmette-Guérin; VDR, vitamin D receptor.
Notes
Acknowledgments. We thank Dr Robert Salata and Dr Christina Lancioni for their critical review of the article. We also greatly thank Dr Henry Boom, Dr Harriet Mayanja-Kizza, Dr Moses Joloba, the Makerere University-Case Western Reserve University Research Collaboration and the Tuberculosis Research Unit for their guidance and support.
Authors'Contributions. The authors contributed equally to the literature search and writing of the article. Tables and figures were designed by D. J.
Financial support. This project has been funded in whole or in part by the Tuberculosis Research Unit (TBRU), established with Federal funds from the United States National Institutes of Allergy and Infectious Diseases & the United States National Institutes of Health and Human Services, under Contract No. HHSN266200700022C/NO1-AI-70022.
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.World Health Organization (WHO) Guidance for national tuberculosis programmes on the management of tuberculosis in children. Geneva: World Health Organization; 2006. [PubMed] [Google Scholar]
- 2.Black RE, Allen LH, Bhutta ZA, et al. Maternal and child undernutrition: global and regional exposures and health consequences. Lancet. 2008;371:243–60. doi: 10.1016/S0140-6736(07)61690-0. [DOI] [PubMed] [Google Scholar]
- 3.Walker VP, Modlin RL. The vitamin D connection to pediatric infections and immune function. Pediatr Res. 2009;65:106R–113R. doi: 10.1203/PDR.0b013e31819dba91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Luong K, Nguyen LT. Impact of vitamin D in the treatment of tuberculosis. Am J Med Sci. 2011;341:493–8. doi: 10.1097/MAJ.0b013e3182070f47. [DOI] [PubMed] [Google Scholar]
- 5.Gao L, Tao Y, Zhang L, Jin Q. Vitamin D receptor genetic polymorphisms and tuberculosis: updated systematic review and meta-analysis. Int J Tuberc Lung Dis. 2010;14:15–23. [PubMed] [Google Scholar]
- 6.Basu Roy R, Whittaker E, Kampmann B. Current understanding of the immune response to tuberculosis in children. Curr Opin Infect Dis. 2012;25:250–7. doi: 10.1097/QCO.0b013e3283529af9. [DOI] [PubMed] [Google Scholar]
- 7.Levy O. Innate immunity of the newborn: basic mechanisms and clinical correlates. Nat Rev Immunol. 2007;7:379–90. doi: 10.1038/nri2075. [DOI] [PubMed] [Google Scholar]
- 8.Shennan DH, Kibel MA. Tuberculosis. In: Stanfield P, Brueton M, Chan M, Parkin M, Waterston T, editors. Diseases of Children in the Subtropics and Tropics. 4th ed. London: Arnold; 1991. pp. 519–52. [Google Scholar]
- 9.Lawn SD, Zumla AI. Tuberculosis. Lancet. 2011;378:57–72. doi: 10.1016/S0140-6736(10)62173-3. [DOI] [PubMed] [Google Scholar]
- 10.Newburg DS, Walker WA. Protection of the neonate by the innate immune system of developing gut and of human milk. Pediatr Res. 2007;61:2–8. doi: 10.1203/01.pdr.0000250274.68571.18. [DOI] [PubMed] [Google Scholar]
- 11.Coles CL, Fraser D, Givon–Lavi N, et al. Nutritional status and diarrheal illness as independent risk factors for alveolar pneumonia. Am J Epidemiol. 2005;162:999–1007. doi: 10.1093/aje/kwi312. [DOI] [PubMed] [Google Scholar]
- 12.Dornelles CT, Piva JP, Marostica PJ. Nutritional status, breastfeeding, and evolution of infants with acute viral bronchiolitis. J Health Popul Nutr. 2007;25:336–43. [PMC free article] [PubMed] [Google Scholar]
- 13.Wayse V, Yousafzai A, Mogale K, Filteau S. Association of subclinical vitamin D deficiency with severe acute lower respiratory infection in Indian children under 5 y. Eur J Clin Nutr. 2004;58:563–7. doi: 10.1038/sj.ejcn.1601845. [DOI] [PubMed] [Google Scholar]
- 14.Moore SE, Prentice AM, Wagatsuma Y, et al. Early-life nutritional and environmental determinants of thymic size in infants born in rural Bangladesh. Acta Paediatr. 2009;98:1168–75. doi: 10.1111/j.1651-2227.2009.01292.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ngom PT, Collinson AC, Pido-Lopez J, Henson SM, Prentice AM, Aspinall R. Improved thymic function in exclusively breastfed infants is associated with higher interleukin 7 concentrations in their mothers’ breast milk. Am J Clin Nutr. 2004;80:722–8. doi: 10.1093/ajcn/80.3.722. [DOI] [PubMed] [Google Scholar]
- 16.Savino W, Dardenne M, Velloso LA, Dayse Silva-Barbosa S. The thymus is a common target in malnutrition and infection. Br J Nutr. 2007;98:S11–6. doi: 10.1017/S0007114507832880. [DOI] [PubMed] [Google Scholar]
- 17.Scrimshaw NS, Taylor CE, Gordon JE. Interactions of nutrition and infection. Monogr Ser World Health Organ. 1968;57:3–329. [PubMed] [Google Scholar]
- 18.Rogero MM, Tirapegui J, Vinolo MA, et al. Dietary glutamine supplementation increases the activity of peritoneal macrophages and hemopoiesis in early-weaned mice inoculated with Mycobacterium bovis bacillus Calmette-Guerin. J Nutr. 2008;138:1343–8. doi: 10.1093/jn/138.7.1343. [DOI] [PubMed] [Google Scholar]
- 19.Zaman K, Baqui AH, Yunus M, Sack RB, Chowdhury HR, Black RE. Malnutrition, cell–mediated immune deficiency and acute upper respiratory infections in rural Bangladeshi children. Acta Paediatr. 1997;86:923–7. doi: 10.1111/j.1651-2227.1997.tb15171.x. [DOI] [PubMed] [Google Scholar]
- 20.Shell–Duncan B, Wood JW. The evaluation of delayed-type hypersensitivity responsiveness and nutritional status as predictors of gastrointestinal and acute respiratory infection: a prospective field study among traditional nomadic Kenyan children. J Trop Pediatr. 1997;43:25–32. doi: 10.1093/tropej/43.1.25. [DOI] [PubMed] [Google Scholar]
- 21.Caulfield LE, de Onis M, Blössner M, Black RE. Undernutrition as an underlying cause of child deaths associated with diarrhea, pneumonia, malaria, and measles. Am J Clin Nutr. 2004;80:193–8. doi: 10.1093/ajcn/80.1.193. [DOI] [PubMed] [Google Scholar]
- 22.Belderbos ME, Houben ML, Wilbrink B, et al. Cord blood vitamin D deficiency is associated with respiratory syncytial virus bronchiolitis. Pediatrics. 2011;127:e1513–20. doi: 10.1542/peds.2010-3054. [DOI] [PubMed] [Google Scholar]
- 23.Shakur MS, Malek MA, Bano N, Rahman M, Ahmed M. Serum and hair zinc in severely malnourished Bangladeshi children associated with or without acute lower respiratory infection. Indian J Pediatr. 2009;76:609–14. doi: 10.1007/s12098-009-0109-y. [DOI] [PubMed] [Google Scholar]
- 24.Rodríguez L, Graniel J, Ortiz R. Effect of leptin on activation and cytokine synthesis in peripheral blood lymphocytes of malnourished infected children. Clin Exp Immunol. 2007;148:478–85. doi: 10.1111/j.1365-2249.2007.03361.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Macallan DC. Malnutrition in tuberculosis. Diagn Microbiol Infect Dis. 1999;34:153–7. doi: 10.1016/s0732-8893(99)00007-3. [DOI] [PubMed] [Google Scholar]
- 26.Ramachandran G, Santha T, Garg R, et al. Vitamin A levels in sputum-positive pulmonary tuberculosis patients in comparison with household contacts and healthy ‘normals. Int J Tuberc Lung Dis. 2004;8:1130–3. [PubMed] [Google Scholar]
- 27.Williams B, Williams AJ, Anderson ST. Vitamin D deficiency and insufficiency in children with tuberculosis. Pediatr Infect Dis J. 2008;27:941–2. doi: 10.1097/INF.0b013e31817525df. [DOI] [PubMed] [Google Scholar]
- 28.Cegielski JP, McMurray DN. The relationship between malnutrition and tuberculosis: evidence from studies in humans and experimental animals. Int J Tuberc Lung Dis. 2004;8:286–98. [PubMed] [Google Scholar]
- 29.McMurray DN, Mintzer CL, Tetzlaff CL, Carlomagno MA. The influence of dietary protein on the protective effect of BCG in guinea pigs. Tubercle. 1986;67:31–9. doi: 10.1016/0041-3879(86)90029-2. [DOI] [PubMed] [Google Scholar]
- 30.Gopalan C. Importance of nutritional factors in tuberculosis. Indian J Tuberculosis. 1957;4:105–126. [Google Scholar]
- 31.McMurray DN, Carlomagno MA, Cumberland PA. Respiratory infection with attenuated Mycobacterium tuberculosis H37Ra in malnourished guinea pigs. Infect Immun. 1983;39:793–9. doi: 10.1128/iai.39.2.793-799.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McFarland CT, Fan YY, Chapkin RS, Weeks BR, McMurray DN. Dietary polyunsaturated fatty acids modulate resistance to Mycobacterium tuberculosis in guinea pigs. J Nutr. 2008;138:2123–8. doi: 10.3945/jn.108.093740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bonilla DL, Fan YY, Chapkin RS, McMurray DN. Transgenic mice enriched in omega-3 fatty acids are more susceptible to pulmonary tuberculosis: impaired resistance to tuberculosis in fat-1 mice. J Infect Dis. 2010;201:399–408. doi: 10.1086/650344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jordao L, Lengeling A, Bordat Y, et al. Effects of omega-3 and -6 fatty acids on Mycobacterium tuberculosis in macrophages and in mice. Microbes Infect. 2008;10:1379–86. doi: 10.1016/j.micinf.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 35.Satyanarayana K, Bhaskaram P, Seshu VC, Reddy V. Influence of nutrition on postvaccinial tuberculin sensitivity. Am J Clin Nutr. 1980;33:2334–7. doi: 10.1093/ajcn/33.11.2334. [DOI] [PubMed] [Google Scholar]
- 36.Harland PS, Brown RE. Tuberculin sensitivity following B.C.G. vaccination in undernourished children. East Afr Med J. 1965;42:233–8. [PubMed] [Google Scholar]
- 37.Chadha VK, Jitendra R, Kumar P, Gupta J, Umadevi Relationship of nutritional status with tuberculin sensitivity. Indian J Pediatr. 2009;76:605–7. doi: 10.1007/s12098-009-0094-1. [DOI] [PubMed] [Google Scholar]
- 38.McMurray DN, Loomis SA, Casazza LJ, Rey H, Miranda R. Development of impaired cell-mediated immunity in mild and moderate malnutrition. Am J Clin Nutr. 1981;34:68–77. doi: 10.1093/ajcn/34.1.68. [DOI] [PubMed] [Google Scholar]
- 39.McMurray DN, Carlomagno MA, Mintzer CL, Tetzlaff CL. Mycobacterium bovis BCG vaccine fails to protect protein-deficient guinea pigs against respiratory challenge with virulent Mycobacterium tuberculosis. Infect Immun. 1985;50:555–9. doi: 10.1128/iai.50.2.555-559.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McMurray DN, Cegielski JP. HIV/AIDS, TB, and Nutrition: Scientific inquiry into the nutritional influences on human immunity with special reference to HIV infection and active TB in South Africa. Pretoria, South Africa: Academy of Sciences of South Africa; 2007. The influence of nutrition on the risk and outcomes of tuberculosis; pp. 153–69. Academy of Sciences of South Africa Consensus Panel on Nutrition, HIV/AIDS. [Google Scholar]
- 41.Karyadi E, West CE, Schultink W, et al. A double-blind, placebo-controlled study of vitamin A and zinc supplementation in persons with tuberculosis in Indonesia: effects on clinical response and nutritional status. Am J Clin Nutr. 2002;75:720–7. doi: 10.1093/ajcn/75.4.720. [DOI] [PubMed] [Google Scholar]
- 42.Pakasi TA, Karyadi E, Suratih NM, et al. Zinc and vitamin A supplementation fails to reduce sputum conversion time in severely malnourished pulmonary tuberculosis patients in Indonesia. Nutr J. 2010;9:41. doi: 10.1186/1475-2891-9-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sinclair D, Abba K, Grobler L, Sudarsanam TD. Nutritional supplements for people being treated for active tuberculosis. Cochrane Database Syst Rev. 2011;11:CD006086. doi: 10.1002/14651858.CD006086.pub3. [DOI] [PubMed] [Google Scholar]
- 44.Cuevas LE, Almeida LM, Mazunder P, et al. Effect of zinc on the tuberculin response of children exposed to adults with smear-positive tuberculosis. Ann Trop Paediatr. 2002;22:313–9. doi: 10.1179/027249302125001967. [DOI] [PubMed] [Google Scholar]
- 45.Green JA, Lewin SR, Wightman F, Lee M, Ravindran TS, Paton NI. A randomised controlled trial of oral zinc on the immune response to tuberculosis in HIV-infected patients. Int J Tuberc Lung Dis. 2005;9:1378–84. [PubMed] [Google Scholar]
- 46.Hanekom WA, Potgieter S, Hughes EJ, Malan H, Kessow G, Hussey GD. Vitamin A status and therapy in childhood pulmonary tuberculosis. J Pediatr. 1997;131:925–7. doi: 10.1016/s0022-3476(97)70046-5. [DOI] [PubMed] [Google Scholar]
- 47.Morcos MM, Gabr AA, Samuel S, et al. Vitamin D administration to tuberculosis children and its value. Boll Chim Farm. 1998;137:157–64. [PubMed] [Google Scholar]
- 48.Mehta S, Mugusi FM, Bosch RJ, et al. A randomized trial of multivitamin supplementation in children with tuberculosis in Tanzania. Nutr J. 2011;10:120. doi: 10.1186/1475-2891-10-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Das BS, Devi U, Mohan Rao C, Srivastava VK, Rath PK, Das BS. Effect of iron supplementation on mild to moderate anaemia in pulmonary tuberculosis. Br J Nutr. 2003;90:541–50. doi: 10.1079/bjn2003936. [DOI] [PubMed] [Google Scholar]
- 50.Hanekom WA, Hussey GD, Hughes EJ, Potgieter S, Yogev R, Check IJ. Plasma-soluble CD30 in childhood tuberculosis: Effects of disease severity, nutritional status, and vitamin A therapy. Clin Diagn Lab Immunol. 1999;6:204–8. doi: 10.1128/cdli.6.2.204-208.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]