Abstract
Mycobacterium tuberculosis, the causative agent of tuberculosis, resides and replicates within susceptible hosts by inhibiting host antimicrobial mechanisms. Prostaglandin E2 (PGE2), produced by M. tuberculosis–infected macrophages, exerts a variety of immunomodulatory functions via 4 receptors (EP1–EP4), each mediating distinct PGE2 functions. Here, we show that M. tuberculosis infection selectively upregulates EP2 messenger RNA expression in CD4+ T cells. We found that EP2 deficiency in mice increases susceptibility to M. tuberculosis infection, which correlated with reduced antigen-specific T-cell responses and increased levels of CD4+CD25+Foxp3+ T-regulatory cells. These findings have revealed an important role for EP2 in host immune defense against tuberculosis. As a G protein-coupled receptor, EP2 could serve as a target for immunotherapy of tuberculosis.
(See the editorial commentary by Kaushal, on pages 1803–5.)
Tuberculosis continues to be a major health problem worldwide, affecting >9 million people and killing >1 million people annually [1]. Mycobacterium tuberculosis, the causative agent of tuberculosis, modulates host immunity to delay immune responses and to facilitate chronic infection. Delayed generation of antigen-specific T cells, which depends heavily on the multiplicity of infection, and reduced homing of infected antigen-presenting dendritic cells (DCs) to draining lymph nodes are common features of M. tuberculosis infection in both humans and mice [2].
Virulent M. tuberculosis interferes with host eicosanoid metabolism as a survival strategy. Eicosanoid bioactive molecules, such as prostaglandin E2 (PGE2), are produced by antigen-presenting cells and play an important role in the outcome of polarization of immune responses [3]. Mycobacterium tuberculosis modulates host innate immune responses by subverting PGE2-mediated effects on cytokine and antibody production, phagocytosis, and cell multiplication [4, 5]. Interestingly, PGE2 exhibits both stimulatory and inhibitory effects on DCs depending on its site of activity and the type of receptor engaged [3]. Therefore, manipulation of PGE2 responses may serve as an effective strategy for immunomodulation of M. tuberculosis infection.
PGE2 stimulates DCs and macrophages and promotes DC migration from tissue to lymph nodes [3]. Consistent with these findings, some reports have suggested that PGE2 induces inflammatory responses in lungs, promoting vasodilation, vascular permeability, and bronchodilation [4]. However, following migration of DCs to lymph nodes, PGE2 can suppress the maturation and antigen-presenting functions of these cells [3]. Furthermore, it has been suggested that PGE2 elicits anti-inflammatory responses and induces immune suppression in part by decreasing T-cell proliferation, interleukin 2 production, and interleukin 2 receptor expression [3]. In addition, PGE2 inhibits activation-induced cell death in effector T cells by preventing CD95L upregulation in response to T-cell receptor (TCR) engagement [6]. We have recently shown that PGE2 is particularly critical for preventing activation-induced cell death in T helper 2 (Th2) cells [7]. Taken together, these studies have provided evidence that PGE2 plays a major role in modulating adaptive immune responses.
PGE2 has four distinct receptors (EP1–EP4) that vary widely in expression and downstream intracellular signaling pathways, leading to an increase or attenuation of inflammatory and protective immune responses [3]. It is well documented that PGE2 suppresses DC functions through an EP2/EP4-dependent mechanism [3], whereas it acts via the EP2 receptor on T regulatory cells (Tregs) to inhibit their expansion [8]. Consistent with these findings, signaling through EP2 exhibited a potent antagonism of transforming growth factor beta (Tgf-β)–mediated activities in normal and malignant mammary epithelial cells [9].
Previous studies have shown that PGE2 levels increase during the course of M. tuberculosis infection and that the outcome of adaptive immune responses against this microorganism is influenced by PGE2 levels and by the stage of disease progression [5]. The relatively low levels of PGE2 that are present during the early stages of M. tuberculosis infection counteract bacterial growth in a manner dependent on inducible nitric oxide synthase, whereas the high PGE2 levels that are present during late stages of infection permit disease progression [5]. Consequently, inhibition of PGE2 synthesis during the early phase of M. tuberculosis infection enhances bacterial burden, with a concomitant increase in inflammation and expression of tumor necrosis factor α, interleukin 1α, and interferon γ (IFN-γ) and a nearly complete abrogation of inducible nitric oxide synthase expression [5].
Infection with virulent M. tuberculosis induces lipoxin A4 (LXA4) production and suppression of PGE2 synthesis in macrophages, which leads to necrosis of infected macrophages and increased disease [4]. Consistent with these findings, macrophages from PGE2 synthase-deficient (PGES−/−) mice fail to control virulent M. tuberculosis replication in vitro [4]. Furthermore, PGES−/− animals exhibit a 10-fold increase in M. tuberculosis burden in their lungs, as compared with wild-type (WT) mice [4]. It has been suggested that PGE2 protects the mitochondrial inner membrane of M. tuberculosis–infected macrophages, preventing their necrosis, in a manner that involves the EP2 receptor [4]. In this way, it is thought that PGE2 favors apoptosis of infected macrophages and promotes cross-presentation of mycobacterial antigens by DCs to prime T-cell responses.
Although it is widely recognized that PGE2 influences the activity of Tregs and effector T cells, few studies have evaluated the direct effects of PGE2 on T cells during the course of M. tuberculosis infection. Studies by Garg et al have shown that human Tregs expand in response to M. tuberculosis infection in a manner that is dependent on PGE2. However, these studies involved ex vivo treatment of isolated CD4+ T cells from healthy tuberculin-positive subjects with M. tuberculosis antigens [10]. Thus, the effects of PGE2 receptor signaling in T cells on the development of protective immune responses during M. tuberculosis infection remain to be explored.
Here, we report that M. tuberculosis infection upregulates messenger RNA (mRNA) expression of EP2. We found that EP2-deficient (EP2−/−) mice exhibit increased susceptibility to M. tuberculosis infection, with a concomitant increase in CD4+CD25+Foxp3+ Tregs. Thus, our findings demonstrate that the EP2 receptor plays an important role in the outcome of M. tuberculosis infection by altering the production of immunosuppressive cytokines and Tregs. Our findings also identify EP2 as a possible new small-molecule target for immunomodulation during M. tuberculosis infection.
METHODS
Ethics Statement
All animal experiments were performed in accordance with guidelines developed and approved by the Institutional Animal Ethics Committee meeting held on 22 November 2007 at the International Centre for Genetic Engineering and Biotechnology (ICGEB), New Delhi, India (approval ID: ICGEB/IAEC/IMM-13/2007) and by Department of Biotechnology guidelines, Government of India.
Mice
C57BL/6 mice (aged 6–8 weeks) were initially purchased from the Jackson Laboratories. Prostaglandin receptor subtype EP2−/− mice, originally generated by Breyer and colleagues [11] were obtained from the Jackson Laboratories. All animals were subsequently bred and maintained in our specific pathogen-free animal facility of the ICGEB. Infected mice were housed under barrier conditions in a biosafety level 3 laboratory.
Antibodies and Reagents
Fluorophore-tagged antibodies against mouse CD4, CD25, Foxp3, interleukin 17A (IL-17A), interleukin 4 (IL-4), and isotype controls were purchased from BD Biosciences. Luminex multiplex cytokine detection kits were purchased from Bio-Rad or Millipore. CD4+ T-cell–specific microbeads, magnetic-activated cell sorting (MACS) columns, and nylon-mesh filters were obtained from Miltenyi Biotech. Fine chemicals, primers, and antibiotics were procured from Sigma-Aldrich. Tritiated thymidine was purchased from PerkinElmer.
Mycobacterium tuberculosis Aerosol Infection and Colony-Forming Unit Estimation
Mycobacterium tuberculosis strain H37Rv (American Type Culture Collection 27294) was grown in Middlebrook 7H9 medium (Difco) supplemented with 0.1% Tween 80 (Sigma-Aldrich), 0.2% glycerol, and 10% v/v albumin, dextrose, and catalase (Difco) as recommended by the manufacturer. Bacterial cultures were grown up to midlog phase (optical density 600, approximately 0.6). Aliquots of bacterial cultures were prepared in 20% glycerol and Middlebrook 7H9 medium and preserved at −80°C. Colony-forming units (CFUs) per milliliter of the stocks were determined by plating on Middlebrook 7H11 plates supplemented with 10% oleic acid, albumin, dextrose, and catalase (Difco). These stocks were used for all subsequent aerosol infections in mice, as per the protocol described elsewhere [12].
T-Cell Proliferation Assay
Splenocytes were isolated from uninfected and infected mice, and red blood cells present in the single-cell suspension of splenocytes were lysed with hypotnic red blood cell lysis buffer. Total splenocytes were used for mixed lymphocyte reactions in the presence of mycobacterial complete soluble antigen. Cells were plated at 0.5 × 106 cells/well in a 96-well plate, cultured for 72 hours, and then pulsed with tritiated thymidine (1 μCi/well) for the last 14 hours of incubation before measuring incorporation of tritiated thymidine using cell harvester and liquid scintillation counter (Wallac Trilux, Perkin–Elmer).
Detection of Cytokines
Splenocytes from infected and uninfected mice were stimulated in the presence of complete soluble antigen at different concentrations and cultured for 48 hours. Culture supernatants were used to detect T-cell cytokines in a Luminex microbead-based multiplexed assay (Qiagen Luminex Liquichip) using commercially available kits according to manufacturer's protocol (BioPlex, Bio-Rad).
Flow Cytometry: Surface and Intracellular Staining
Splenocytes were isolated from uninfected and infected mice. Red blood cells present in the single-cell suspension of splenocytes were lysed with hypotonic red blood cell lysis buffer. Cells were counted and 1 × 106 cells were stained with fluorescently conjugated monoclonal antibodies for surface markers.
Intracellular staining of splenocytes was done using the fluorescently labeled antibodies for anti-IL-4 (clone:11B11), anti-IL-17 (clone: 17B7)-phycoerythrin (PE), and Foxp3 (clone: FJK-16) as described elsewhere [12]. Fluorescence intensity was measured by flow cytometry (FACS CantoTM II, BD Biosciences) and data analysis was performed by FlowJo (Tree Star).
Quantitative Real-Time Polymerase Chain Reaction
CD4+ T cells were isolated from uninfected and infected mice by positive selection using MACS. Total RNA isolation was performed using RNAeasy isolation kit (Qiagen) according to manufacturer's instructions. After DNAse treatment, complementary DNA (cDNA) was synthesized using iScript cDNA synthesis kit (Bio-Rad). Real-time polymerase chain reaction (PCR) analysis was performed using Bio-Rad CFX96 real-time thermal cycler and IQBio-Rad SYBR green master mix (Bio-Rad) for Gata-3, Foxp3, and Tgf-β expression, respectively. Relative expression level of mRNAs was normalized to that of internal control Gapdh by using 2-ΔΔCt cycle threshold method. Primer sequences used were as follows: Gata-3 (forward): 5′-TTATCAAGCCCAAGCGAAGG-3′ and Gata-3 (reverse): 5′-CATTAGCGTTCCTCCTCCAGAG-3′; Foxp3 (forward): 5′-GGCCCTTCTCCAGGACAGA-3′ and Foxp3 (reverse): 5′-GCTGATCATGGCTGGGTTGT-3′; Tgf-β (forward): 5′-ACCATGCCAACTTCTGTCTG-3′ and Tgf-β (reverse): 5′-CGGGTTGTGTTGGTTGTAGA-3′; and Gapdh (forward): 5′-TCTGACCACAGTGAGGAATGTCCAC-3′ and Gapdh (reverse): 5′-TTGATGGCAACAATCTCCAC-3′.
Immunohistochemistry
Lungs were infused and fixed in 10% paraformaldehyde in phosphate-buffered saline, paraffin embedded, cut, and stained with hematoxylin and eosin according to standard protocols. Lung tissue sections were stained for immunohistochemistry analysis of Tgf-β. Paraffin-embedded blocks were cut in sections of 5–7 μm. The sections were prepared for immunohistochemistry as described elsewhere [13]. Primary antibody for mouse Tgf-β (Santa Cruz Biotechnology) was used at 1:100 dilution.
Statistical Analysis
Student unpaired t test was performed to compare the statistical significance between various groups. P values were considered significant if P ≤ .05. Standard deviation of the mean or standard error of the mean is shown in the figures. GraphPad Prism 5.0 software was used for statistical analyses.
RESULTS
Expression of EP Receptors During M. tuberculosis Infection
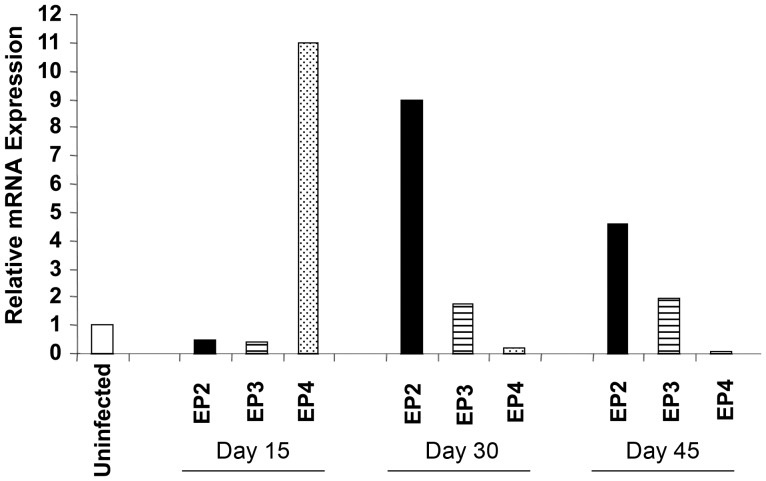
To explore the role of EP receptors during M. tuberculosis infection, we first evaluated the mRNA expression levels of the 4 EP receptors during disease progression. Wild-type C57BL/6 mice were infected with a low dose of M. tuberculosis (110 bacilli), and expression of EP receptors in CD4+ T cells isolated from the spleen was evaluated by real-time PCR at different time points after infection. We found that expression of EP2 was significantly upregulated after infection (Figure 1). EP1 was weakly expressed, if any, in the absence or presence of infection (data not shown), and EP3 expression was not significantly altered by infection. Surprisingly, EP4 was highly expressed 15 days after infection, but expression declined thereafter. However, its regulation did not correlate with microbial burden because negligible CFUs were detected in the spleen 15 days after infection (Figure 2). In contrast, EP2 mRNA expression was consistently upregulated later during infection, at days 30 and 45 (Figure 1).
Figure 1.
Regulation of EP2 messenger RNA (mRNA) expression in CD4+ T cells by Mycobacterium tuberculosis and its role in tuberculosis pathogenesis in mice. Mice were challenged with a low dose of M. tuberculosis by the aerosol route (110 bacilli). CD4+ T cells were purified from splenocytes of uninfected and infected C57BL/6 (wild-type) mice, and EP receptor (EP2, EP3, EP4) expression levels were measured in CD4+ T cells at the indicated time points after infection by real-time polymerase chain reaction. Data are representative of 2 independent experiments (3 mice per experiment).
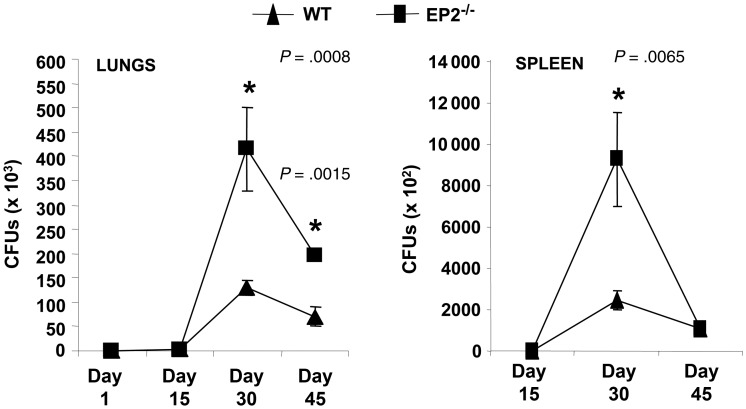
Figure 2.
EP2−/− mice have increased bacterial burden following Mycobacterium tuberculosis infection. EP2−/− and C57BL/6 (wild-type) mice were challenged with M. tuberculosis by the aerosol route (low-dose, 110 bacilli) and colony-forming units (CFUs) were determined in lungs and spleen of infected mice at the indicated time points after infection. Data shown here are cumulative of 3 experiments, representing mean ± standard deviation values of 8–10 mice per group. Each CFU count was carried out in triplicate (3–5 mice per experiment).
Role of EP2 in Tuberculosis Pathogenesis
EP2 has been shown to play a role in many immunological functions, such as antigen-specific T-cell generation and T-cell polarization, favoring Th2 cell differentiation [14, 15]. It has been well documented that M. tuberculosis polarizes Th2-cell responses in susceptible hosts as a survival strategy [16]. Therefore, we next examined whether EP2 plays a role in tuberculosis pathogenesis. We infected EP2−/− mice with a low-dose of M. tuberculosis through the aerosol route and determined bacterial load in lungs and spleen at different time points. We found only a few CFUs present in the spleen and lungs of WT and EP2−/− mice at 15 days after infection (Figure 2). In contrast, we detected dramatically increased amounts of CFUs in spleen and lungs of EP2−/− mice compared with WT mice at 30 days after infection (Figure 2). The CFU levels in the lungs but not the spleen of EP2−/− mice remained increased at day 45. These data indicated that EP2−/− mice have a relative increased susceptibility to M. tuberculosis infection compared with WT animals.
EP2−/− Animals Exhibit Hyperactive Immune Responses to M. tuberculosis Antigens
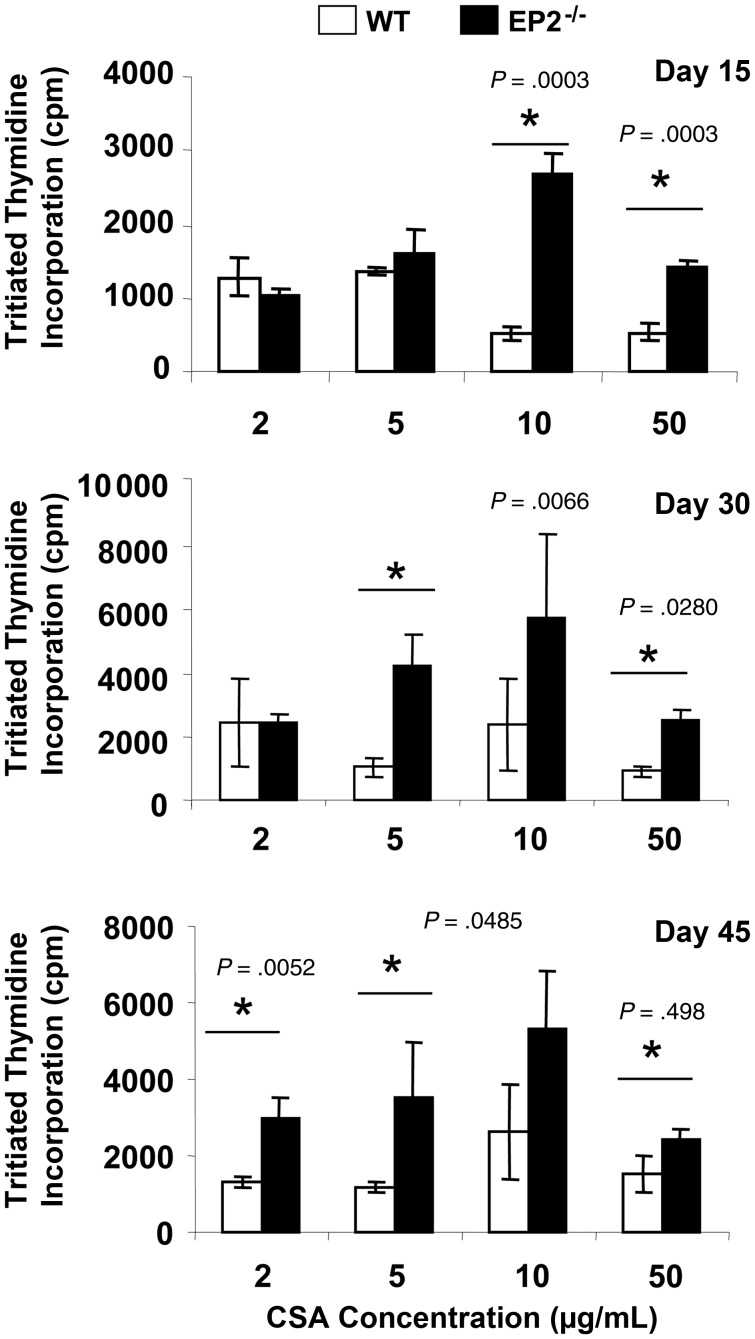
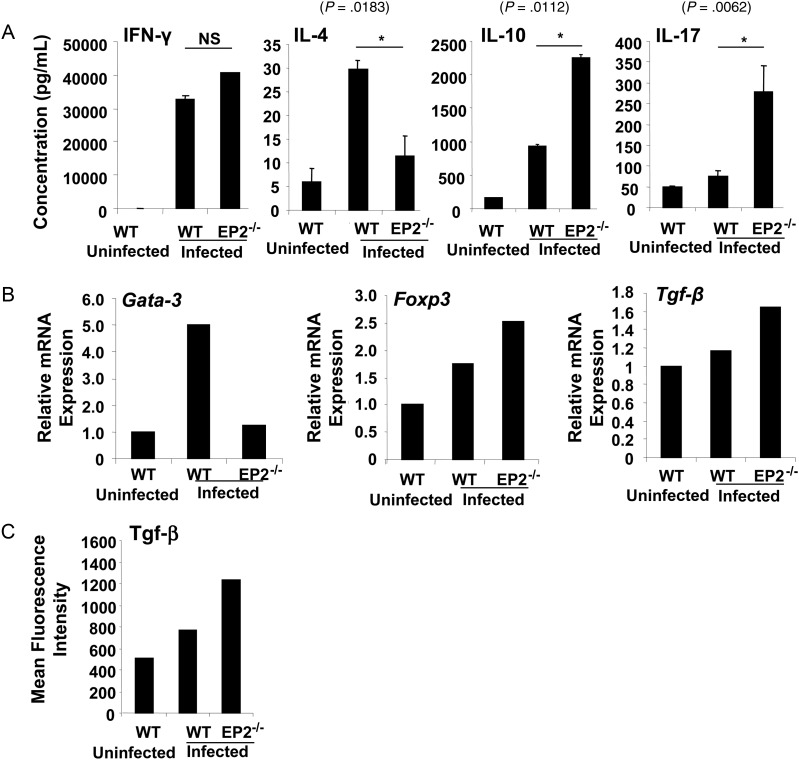
Because EP2−/− animals exhibited increased susceptibility to M. tuberculosis infection, we next examined whether these mice are impaired in mounting antigen-specific proliferative T-cell responses. To test this hypothesis, we harvested splenocytes from infected WT or EP2−/− mice and rechallenged these cells ex vivo with M. tuberculosis complete soluble antigen. Proliferation was measured by tritiated thymidine incorporation. We observed that splenocytes from EP2−/− mice proliferated at much higher levels than splenocytes from WT animals (Figure 3). This observation indicated that EP2−/− animals do not exhibit impaired lymphoproliferative activities. We next determined if EP2−/− animals induce biased T-cell responses following M. tuberculosis infection. To delineate the nature of antigen-specific responses generated in EP2−/− mice, we analyzed the cytokines in splenocyte culture supernatants. Surprisingly, there were no significant differences in the production of IFN-γ, whereas IL-4 was significantly reduced in the cultures from EP2−/− mice (Figure 4A). Interestingly, we noted that IL-17 (P = .0062) and interleukin 10 (IL-10) (P = .0112) were significantly increased in the cultures from EP2−/− mice (Figure 4A). These data suggested the possibility that infected EP2−/− animals might have increased levels of T helper 17 (Th17) cells and Tregs.
Figure 3.
Splenocytes from EP2−/− mice exhibit increased antigen-specific proliferation in response to mycobacterial complete soluble antigen (CSA). Splenocytes were isolated from uninfected and Mycobacterium tuberculosis H37Rv–infected EP2−/− and C57BL/6 (wild-type) mice and stimulated with different concentrations of CSA. Proliferation was analyzed after 72 hours using tritiated thymidine. Data shown here is representative of 3 independent experiments with 3 mice in each group and represents the mean ± standard deviation values.
Figure 4.
EP2-deficient mice induce regulatory T cells upon infection with Mycobacterium tuberculosis. A, T helper 1 (IFN-γ), T helper 2 (IL-4 and IL-10), and T helper 17 (Th17) cytokine responses were assayed in the culture supernatants of splenocytes derived from uninfected and infected C57BL/6 (wild-type [WT]) and EP2−/− mice at 48 hours after activation with M. tuberculosis H37Rv protein lysate (complete soluble antigen [CSA], 20 μg/mL). Data shown here are representative of 3 independent experiments, with 3 mice per experiment. Abbreviations: IFN-γ, interferon γ; IL-4, interleukin 4; IL-10, interleukin 10; IL-17, interleukin 17; NS, not significant. B, EP2−/− mice show increased T regulatory cell–specific transcription factor (Foxp3) and cytokine (Tgf-β) expression. CD4+ T cells derived from uninfected and infected C57BL/6 (WT) and EP2−/− mice were analyzed for expression levels of Gata-3, Foxp3, and Tgf-β messenger RNA by real-time polymerase chain reaction. Data are representative of 2 experiments, with 3 mice per group. C, Infected EP2−/− mice show increased Tgf-β secretion in the culture supernatants of splenocytes rechallenged ex vivo with M. tuberculosis CSA. Data are representative of 2 experiments, with 3 mice per group.
EP2−/− Animals Induce Increased Th17 and Treg Cell Responses to M. tuberculosis
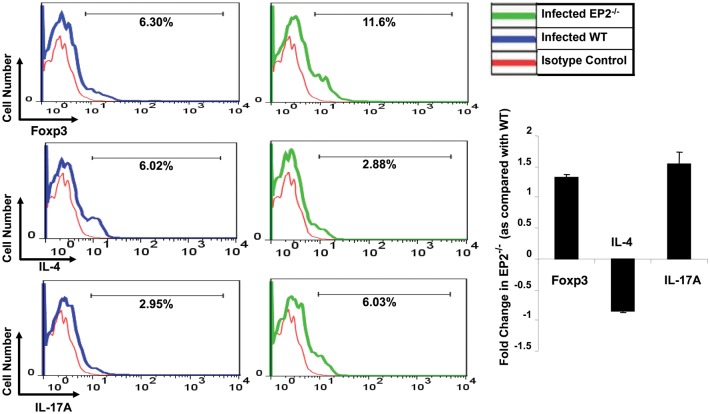
From the preceding data, it was clear that IFN-γ levels in splenocyte cultures from M. tuberculosis–infected EP2−/− and WT animals were similar, whereas IL-17 and IL-10 levels were dramatically increased in cultures from EP2−/− animals. Because IL-10 is predominantly produced by Th2 cells and Tregs, and because we detected little IL-4 in the cultures, we considered it most likely that Tregs were the relevant source of IL-10. Thus, to obtain insight into the cellular source of IL-10, we examined the mRNA expression levels of Gata-3 and Foxp3, which are the lineage-specific transcription factors of Th2 cells and Tregs, respectively. Consistent with the cytokine data, expression of Gata-3 was reduced in EP2−/− mice, whereas expression of Foxp3 was increased, as compared with WT mice (Figure 4B). This observation suggested that there were increased numbers of Tregs in EP2−/− animals. Tregs also produce Tgf-β, which is often considered to be the most relevant cytokine produced by these cells. Therefore, we next determined the expression of Tgf-β. As expected, we observed significantly increased expression levels of Tgf-β in EP2−/− animals (Figure 4B). We also analyzed Tgf-β in the culture supernatants of splenocytes rechallenged ex vivo with M. tuberculosis complete soluble antigen. The Tgf-β levels were higher in cultures from EP2−/− animals than from WT animals (Figure 4C). To further confirm these data, we next examined Tregs in EP2−/− mice by fluorescence-activated cell sorting (FACS). Splenocytes were isolated from WT and EP2−/− mice at 30 days after infection and stained with fluorescently labeled antibodies for CD4 and CD25 surface markers and the intracellular transcription factor Foxp3. We also evaluated cells for intracellular expression of IL-4, the prototypical Th2 cytokine. EP2−/− mice exhibited a 2-fold increase in the levels of CD4+CD25+Foxp3+ Tregs, as compared with WT mice, whereas these animals had lower levels of IL-4–producing cells (Figure 5). Collectively, these data suggested that infected EP2−/− mice produce increased levels of Tregs. We also evaluated IL-17–producing T helper cells in EP2−/− and WT mice by intracellular staining. T helper cells from EP2−/− mice showed increased levels of IL-17, as compared with WT mice (Figure 5).
Figure 5.
Infected EP2−/− mice produce increased levels of CD4+CD25+Foxp3+ T regulatory cells (Tregs), increased levels of interleukin 17A (IL-17A)–producing cells, and reduced levels of interleukin 4 (IL-4)–producing cells. Splenocytes were isolated from uninfected and infected C57BL/6 (wild-type [WT]) and EP2−/− mice (30 days after infection) and were stained with fluorescently labeled antibodies for CD4 and CD25 (for Tregs) surface markers, and intracellular staining was performed for the Foxp3 transcription factor and IL-17A and IL-4 cytokines. Data shown here are representative of 3 independent experiments, with 3–5 mice per experiment. Data are expressed as fold change in expression levels (± standard deviation).
Histological Analysis of Granulomatous Lesions
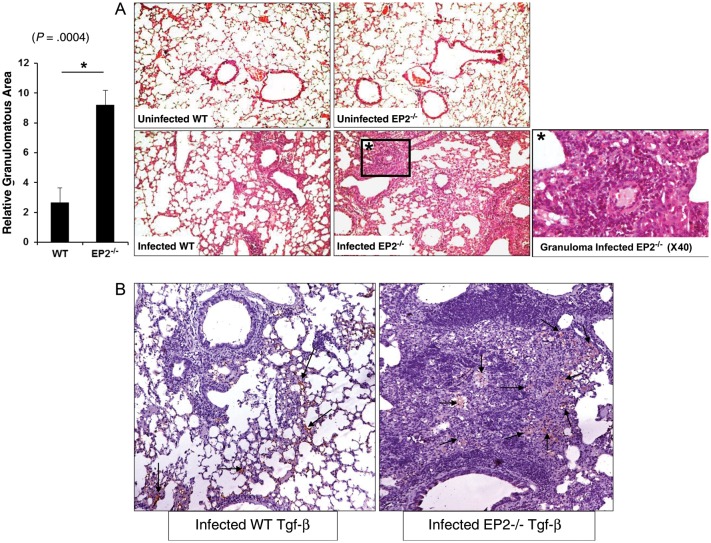
To investigate lung pathology of the infected animals, we examined the histological sections of uninfected and infected EP2−/− and WT mice. Lung sections were stained with hematoxylin and eosin and analyzed by light microscopy. In agreement with the CFU data, we observed higher numbers of granuloma-like structures in EP2−/− mice (Figure 6A) than in WT mice. We also determined Tgf-β expression in the lung by immunohistochemistry. Consistent with the cytokine data for splenocytes, we found that EP2−/− animals produced dramatically increased levels of Tgf-β in the lung (Figure 6B).
Figure 6.
Differences in granulomatous responses to Mycobacterium tuberculosis infection in EP2−/− and C57BL/6 (wild-type [WT]) mice. A, Granuloma formation in EP2−/− and WT mice after low-dose M. tuberculosis aerosol infection was analyzed 30 days after infection. Sections of formalin-fixed and paraffin-embedded lung tissue of uninfected and infected WT and EP2−/− mice were used to visualize hematoxylin and eosin staining. Data are presented as relative granulomatous area image compared per fixed field of view for the entire lung and represent the mean ± standard error of the mean values. One representative image of 3 independent experiments is shown. Original magnification, ×10, unless otherwise noted. B, Immunohistochemical staining of cytokine Tgf-β in infected WT and EP2−/− mice 30 days after infection. Photographs are representative images from lung tissue sections from each group of mice. The staining for Tgf-β in these photographs is depicted in red color and marked by arrows. Original magnification, ×10.
DISCUSSION
It is well known that PGE2 is an immunomodulatory molecule produced by infected macrophages [3]. PGE2 has four receptors with several different effects on antigen-presenting, cell-mediated effector T-cell functions and homeostasis [3]. However, the role played by individual PGE2 receptors on the modulation of T-cell responses has not been well documented. Our data show that EP2 receptor mRNA expression is selectively modulated in CD4+ T cells during M. tuberculosis infection. EP2 receptor mRNA expression was highly upregulated at 30 days after infection and correlated with the bacterial burden in lungs and spleen. However, the expression profile of the other 3 EP receptors did not show such a pattern, with EP1 receptor levels being almost undetectable. Because PGE2 is a ubiquitous mediator of inflammation that affects the systemic response in a receptor-dependent manner, this pattern of expression of the EP2 receptor during M. tuberculosis infection of mice appeared relevant to the activity of PGE2. Prior studies have shown that EP2 modulates adaptive immune responses by influencing DC function and antigen-specific T-cell generation and by promoting Th2-cell responses [3]. Because Th2 cells inhibit T helper 1 (Th1) cells, the effect of EP2 would be expected to suppress host-protective immune responses. In this context, it has been well documented that virulent M. tuberculosis strains induce exuberant Th2-type immune responses [17–19]. Furthermore, animals unable to mount Th2 responses, such as IL-4−/− or Stat-6−/− mice, are partially resistant to the development of tuberculosis [20]. We found that EP2 receptor–deficient mice exhibit increased susceptibility to M. tuberculosis infection, as shown by an increased bacterial load in the lungs and spleen of these animals as compared with WT mice.
Our recent findings have shown that EP2-mediated signaling specifically inhibits activation-induced cell death in Th2 cells [7]. Consistent with these studies, we were unable to detect differences in IFN-γ production, whereas IL-4 production by M. tuberculosis antigen-specific CD4+ T cells was decreased in EP2−/− mice. Such a Th1 vs Th2 cytokine profile would be expected to render EP2−/− animals less susceptible to bacterial infection than WT mice. Indeed, previous studies have shown enhanced clearance of several gram-negative [21] and gram-positive [22] bacteria from the lungs of EP2−/− mice. It was, therefore, surprising to observe that these animals had increased susceptibility to M. tuberculosis infection. A second surprising result of our studies was that, despite such a reduction in Th2 cytokine responses, splenocytes from EP2−/− mice exhibited enhanced proliferative responses against M. tuberculosis antigens. This finding is consistent with previous reports showing that EP2 receptors primarily inhibit mixed lymphocyte reactions through direct effects on T cells [23]. Our analysis of cytokines and Th-cell subsets further indicated that infected EP2−/− mice developed enhanced Th17 and Treg responses. In this context, it is important to note that Tregs inhibit the activation and proliferation of antigen-specific T cells in a Tgf-β–dependent manner. However, Tgf-β is unable to inhibit Th17-cells and instead augments Th17-cell differentiation [24, 25]. Thus, the immune response against M. tuberculosis appears to be regulated by PGE2-EP2 axis in a manner that is distinct from the response generated against most other bacterial organisms. This might be attributed to the chronicity of the M. tuberculosis infection, in which Tregs play an important role.
The roles of Th17 cells and IL-17 in the pathogenesis of tuberculosis have been extensively debated. Although studies have shown a protective role of IL-17 during M. tuberculosis infection, Th17 cells and Th17-derived cytokines also mediate inflammation and tissue damage in autoimmune diseases and infections [26]. During M. tuberculosis infection of genetically susceptible animals, IL-17 promotes granuloma formation as well as neutrophil accumulation and tissue damage [26]. Neutrophils provide a favorable environment for bacterial replication because they are the predominant cell type infected in tuberculosis patients [27]. Tgf-β has been shown to be involved in the development and/or consequences of granuloma formation during M. tuberculosis infection [28]. Hence, the increased secretion of IL-17 and Tgf-β by CD4+ T cells in EP2−/− mice as compared with WT mice might contribute to the increased lung pathology in these animals. However, additional studies are required to delineate the precise mechanism by which EP2 regulates IL-17 and Tgf-β production during M. tuberculosis infection.
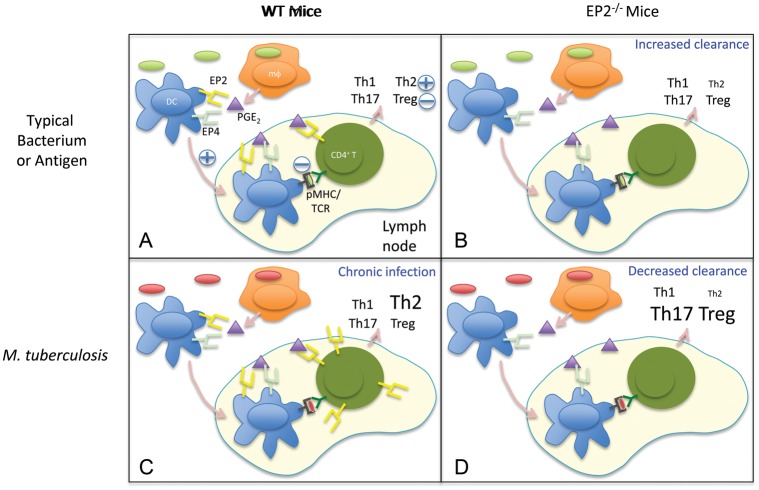
Based on published studies [4, 5, 7–9, 16, 22, 29] and the findings reported here, we propose the following role of PGE2 and EP2 during infection. Upon exposure to antigen or infection by typical bacteria, macrophages produce PGE2, which engages with EP2 and EP4 on DCs and EP2 on T cells to keep the immune response in balance. Thus, for most bacteria, EP2 deficiency results in increased resistance to infection. In the case of tuberculosis, the microorganisms upregulate EP2 expression on CD4+ T cells, and engagement of this receptor with PGE2 enhances IL-4–producing cells, which facilitates chronic infection. Consequently, infection of EP2−/− animals with M. tuberculosis results in reduced levels of antigen-specific Th2 cells in the face of unaltered levels of Th1 cells and increased levels of Tregs and Th17 cells. As a result of these alterations in Th-cell responses, disease progression in EP2-deficient animals is enhanced (Figure 7). We speculate that the effects of EP2 expression on other microorganisms that cause a chronic infection might be similar to those described here for M. tuberculosis infection.
Figure 7.
Proposed model for the role of EP2 in PGE2-mediated activities during T-cell responses against bacterial infection. A, In wild-type (WT) mice exposed to typical bacteria or antigens, PGE2 secreted by macrophages and dendritic cells (DCs) acts mainly on EP2 and EP4 receptors to modulate DC migration and antigen presentation to naive CD4+ T cells in the peripheral lymph nodes and on EP2 receptors expressed by CD4+ T cells to modulate the generation of T-cell responses. Effects of PGE2 are indicated by + and − symbols. Font sizes of distinct T helper (Th) subsets represent an estimation of their prevalence. pMHC/TCR, peptide-MHC/T cell receptor. B, Following infection with typical bacteria, EP2-deficient mice show increased bacterial clearance and reduced Th2 type responses. C, In chronic bacterial infections such as tuberculosis, induction of EP2 on CD4+ T cells results in increased Th2 responses and bacterial persistence. D, Despite a reduction in Th2 responses, EP2-deficient mice show reduced bacterial clearance of Mycobacterium tuberculosis organisms. This is likely due to an increase in Th17 and T regulatory cell (Treg) responses, which leads to increased bacterial persistence in granulomas and enhanced bacterial burden.
In summary, our data have shown that absence of the PGE2 receptor EP2 increases susceptibility to M. tuberculosis infection. Although reduced Th2 responses are generated in the absence of the EP2 receptor, increased levels of immunosuppressive cytokines, such as IL-10, produced by Tregs lead to a reduction in protective immune responses in these animals. This effect is reinforced by increased levels of the proinflammatory cytokine IL-17 produced by antigen-specific CD4+ T cells in EP2-deficient mice. Thus, the EP2 receptor mediates host resistance during M. tuberculosis infection and might serve as a novel target for immunotherapy.
Notes
Acknowledgements. Mycobacterium tuberculosis strain H37Rv was a kind gift from Prof David Sherman (Seattle Institute of Biomedical Research, Seattle, Washington).
Financial support. This work was supported by the Wellcome Trust–Department of Biotechnology (DBT) alliance (WT01/GD/09/339), and the DBT, Government of India (DBT01/GD/08/284, DBT02/GD/08/300, and DBT03/GD/08/306). V. K. is the recipient of a fellowship (CSIR-JRF (NET) 09/512(0101)/2007-EMR-I) from the Council for Scientific and Industrial Research, Government of India.
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Reference
- 1.World Health Organization. WHO global tuberculosis control report 2010. Summary. Cent Eur J Public Health. 2010;18:237. [PubMed] [Google Scholar]
- 2.Urdahl KB, Shafiani S, Ernst JD. Initiation and regulation of T-cell responses in tuberculosis. Mucosal Immunol. 2011;4:288–93. doi: 10.1038/mi.2011.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harizi H, Grosset C, Gualde N. Prostaglandin E2 modulates dendritic cell function via EP2 and EP4 receptor subtypes. J Leukoc Biol. 2003;73:756–63. doi: 10.1189/jlb.1002483. [DOI] [PubMed] [Google Scholar]
- 4.Chen M, Divangahi M, Gan H, et al. Lipid mediators in innate immunity against tuberculosis: opposing roles of PGE2 and LXA4 in the induction of macrophage death. J Exp Med. 2008;205:2791–801. doi: 10.1084/jem.20080767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rangel Moreno J, Estrada Garcia I, De La Luz Garcia Hernandez M, et al. The role of prostaglandin E2 in the immunopathogenesis of experimental pulmonary tuberculosis. Immunology. 2002;106:257–66. doi: 10.1046/j.1365-2567.2002.01403.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weinlich R, Bortoluci KR, Chehab CF, et al. TLR4/MYD88-dependent, LPS-induced synthesis of PGE2 by macrophages or dendritic cells prevents anti-CD3-mediated CD95L upregulation in T cells. Cell Death Differ. 2008;15:1901–9. doi: 10.1038/cdd.2008.128. [DOI] [PubMed] [Google Scholar]
- 7.Kaul V, Kaer LV, Das G, Das J. Prostanoid receptor EP2 signaling protects T helper 2 cells from Balb/c mice against activation-induced cell death. J Biol Chem. 2012;287:25434–9. doi: 10.1074/jbc.C111.324707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee BP, Juvet SC, Zhang L. Prostaglandin E2 signaling through E prostanoid receptor 2 impairs proliferative response of double negative regulatory T cells. Int Immunopharmacol. 2009;9:534–9. doi: 10.1016/j.intimp.2009.01.023. [DOI] [PubMed] [Google Scholar]
- 9.Tian M, Schiemann WP. PGE2 receptor EP2 mediates the antagonistic effect of COX-2 on TGF-beta signaling during mammary tumorigenesis. Faseb J. 2010;24:1105–16. doi: 10.1096/fj.09-141341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garg A, Barnes PF, Roy S, et al. Mannose-capped lipoarabinomannan- and prostaglandin E2-dependent expansion of regulatory T cells in human Mycobacterium tuberculosis infection. Eur J Immunol. 2008;38:459–69. doi: 10.1002/eji.200737268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kennedy CR, Zhang Y, Brandon S, et al. Salt-sensitive hypertension and reduced fertility in mice lacking the prostaglandin EP2 receptor. Nat Med. 1999;5:217–20. doi: 10.1038/5583. [DOI] [PubMed] [Google Scholar]
- 12.Chatterjee S, Dwivedi VP, Singh Y, et al. Early secreted antigen ESAT-6 of Mycobacterium tuberculosis promotes protective T helper 17 cell responses in a Toll-like receptor-2-dependent manner. PLoS Pathog. 2011;7:e1002378. doi: 10.1371/journal.ppat.1002378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rosas-Taraco AG, Higgins DM, et al. Local pulmonary immunotherapy with siRNA targeting TGFbeta1 enhances antimicrobial capacity in Mycobacterium tuberculosis infected mice. Tuberculosis (Edinb) 2011;91:98–106. doi: 10.1016/j.tube.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Okano M, Sugata Y, Fujiwara T, et al. E prostanoid 2 (EP2)/EP4-mediated suppression of antigen-specific human T-cell responses by prostaglandin E2. Immunology. 2006;118:343–52. doi: 10.1111/j.1365-2567.2006.02376.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kubo S, Takahashi HK, Takei M, et al. E-prostanoid (EP)2/EP4 receptor-dependent maturation of human monocyte-derived dendritic cells and induction of helper T2 polarization. J Pharmacol Exp Ther. 2004;309:1213–20. doi: 10.1124/jpet.103.062646. [DOI] [PubMed] [Google Scholar]
- 16.Flynn JL, Chan J. Immunology of tuberculosis. Annu Rev Immunol. 2001;19:93–129. doi: 10.1146/annurev.immunol.19.1.93. [DOI] [PubMed] [Google Scholar]
- 17.Rook GA, Dheda K, Zumla A. Immune responses to tuberculosis in developing countries: implications for new vaccines. Nat Rev Immunol. 2005;5:661–7. doi: 10.1038/nri1666. [DOI] [PubMed] [Google Scholar]
- 18.Dlugovitzky D, Bottasso O, Dominino JC, et al. Clinical and serological studies of tuberculosis patients in Argentina receiving immunotherapy with Mycobacterium vaccae (SRL 172) Respir Med. 1999;93:557–62. doi: 10.1016/s0954-6111(99)90155-5. [DOI] [PubMed] [Google Scholar]
- 19.Fenhalls G, Wong A, Bezuidenhout J, van Helden P, Bardin P, Lukey PT. In situ production of gamma interferon, interleukin-4, and tumor necrosis factor alpha mRNA in human lung tuberculous granulomas. Infect Immun. 2000;68:2827–36. doi: 10.1128/iai.68.5.2827-2836.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rook GA, Hernandez-Pando R. The pathogenesis of tuberculosis. Annu Rev Microbiol. 1996;50:259–84. doi: 10.1146/annurev.micro.50.1.259. [DOI] [PubMed] [Google Scholar]
- 21.Sadikot RT, Zeng H, Azim AC, et al. Bacterial clearance of Pseudomonas aeruginosa is enhanced by the inhibition of COX-2. Eur J Immunol. 2007;37:1001–9. doi: 10.1002/eji.200636636. [DOI] [PubMed] [Google Scholar]
- 22.Medeiros AI, Serezani CH, Lee SP, Peters-Golden M. Efferocytosis impairs pulmonary macrophage and lung antibacterial function via PGE2/EP2 signaling. J Exp Med. 2009;206:61–8. doi: 10.1084/jem.20082058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nataraj C, Thomas DW, Tilley SL, et al. Receptors for prostaglandin E(2) that regulate cellular immune responses in the mouse. J Clin Invest. 2001;108:1229–35. doi: 10.1172/JCI13640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bettelli E, Carrier Y, Gao W, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–8. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 25.Mangan PR, Harrington LE, O'Quinn DB, et al. Transforming growth factor-beta induces development of the T(H)17 lineage. Nature. 2006;441:231–4. doi: 10.1038/nature04754. [DOI] [PubMed] [Google Scholar]
- 26.Khader SA, Cooper AM. IL-23 and IL-17 in tuberculosis. Cytokine. 2008;41:79–83. doi: 10.1016/j.cyto.2007.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eum SY, Kong JH, Hong MS, et al. Neutrophils are the predominant infected phagocytic cells in the airways of patients with active pulmonary TB. Chest. 2010;137:122–8. doi: 10.1378/chest.09-0903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aung H, Toossi Z, McKenna SM, et al. Expression of transforming growth factor-beta but not tumor necrosis factor-alpha, interferon-gamma, and interleukin-4 in granulomatous lung lesions in tuberculosis. Tuber Lung Dis. 2000;80:61–7. doi: 10.1054/tuld.2000.0235. [DOI] [PubMed] [Google Scholar]
- 29.Serezani CH, Chung J, Ballinger MN, Moore BB, Aronoff DM, Peters-Golden M. Prostaglandin E2 suppresses bacterial killing in alveolar macrophages by inhibiting NADPH oxidase. Am J Respir Cell Mol Biol. 2007;37:562–70. doi: 10.1165/rcmb.2007-0153OC. [DOI] [PMC free article] [PubMed] [Google Scholar]