Abstract
Fibroblasts from normal human subjects and from a patient who had osteogenesis imperfecta were incubated with [3H]mannose, and types I and III procollagens were isolated from the culture medium. The type I procollagen from the patient's fibroblasts contained 2-3 time more [3H]mannose than the type I procollagen from the normal fibroblasts. In contrast, there was no difference in the [3H]mannose content of the type III procollagen simultaneously synthesized and secreted by the same cells. Isolation of a collagenase-resistant peptide fragment from the type I procollagen showed that the excess mannose was located in the COOH-terminal propeptide of the protein. Radioimmunoassays of the medium and the cell layer showed that the type I procollagen synthesized by the patient's fibroblasts was secreted into the medium more slowly than the type I procollagen synthesized by normal fibroblasts. These results appear to provide evidence for an alteration in the structure of procollagen in osteogenesis imperfecta.
Full text
PDF
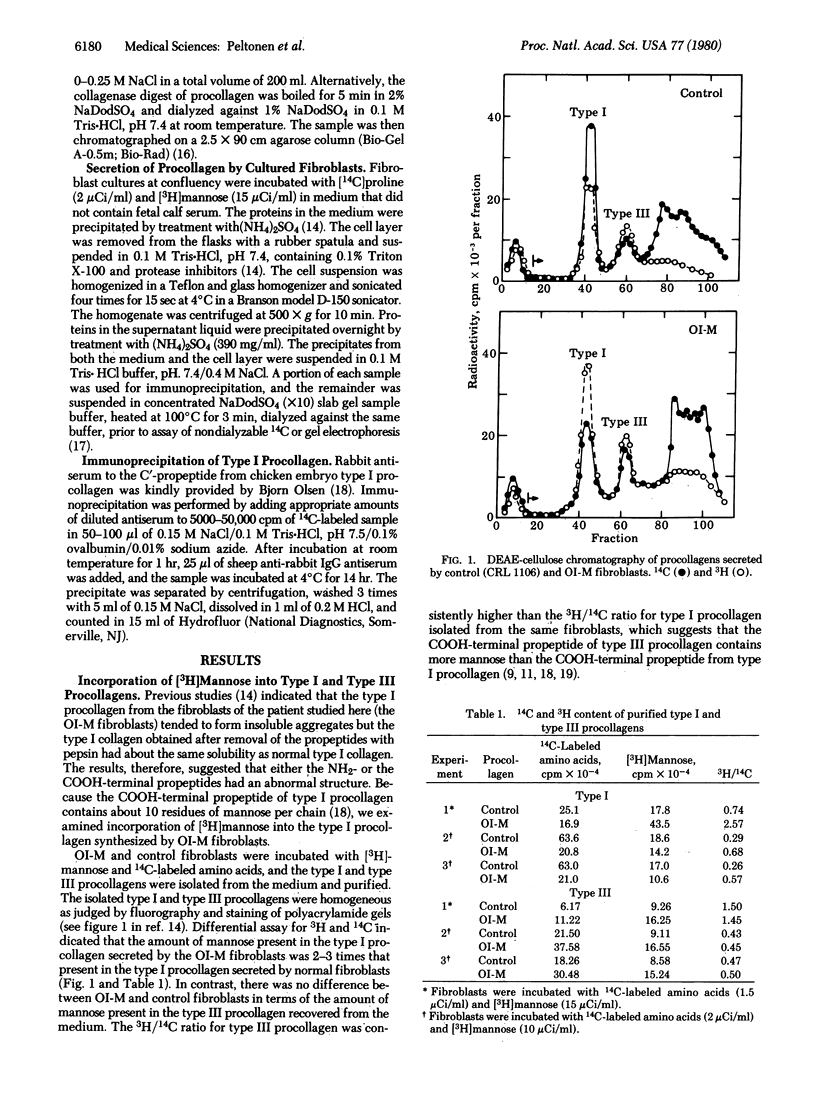
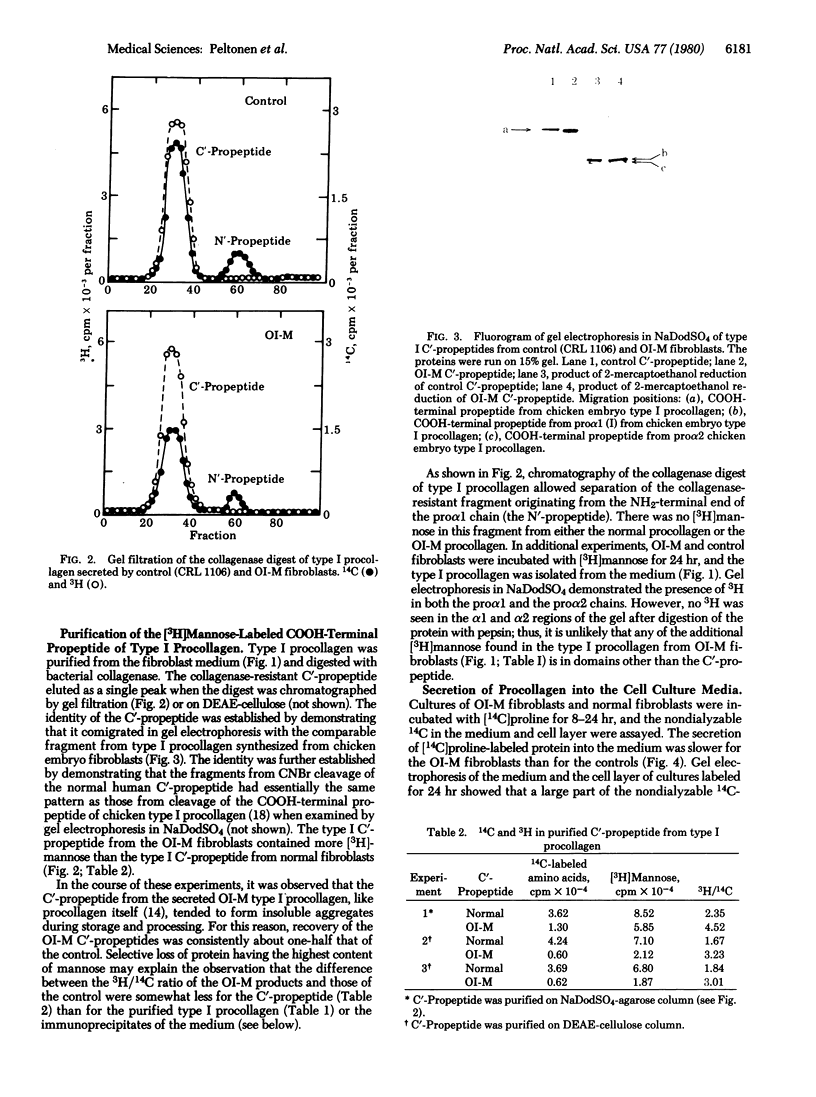
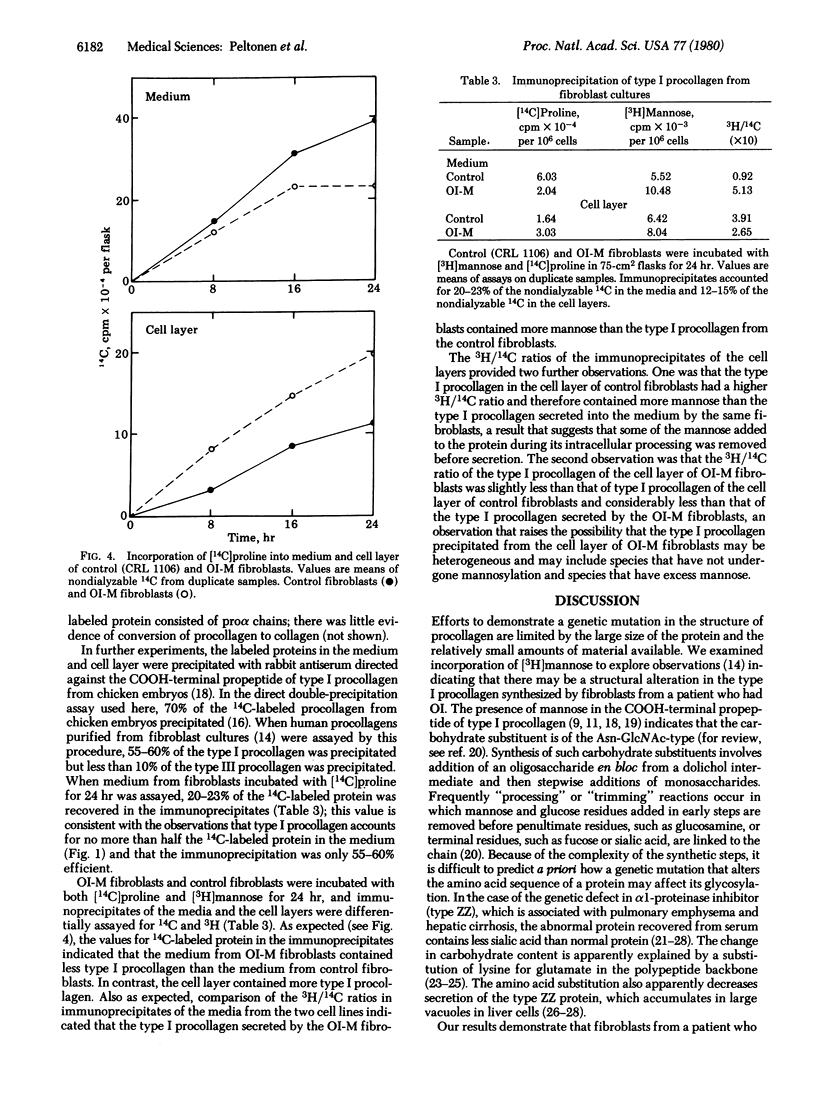

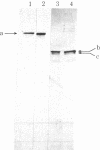
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abe S., Steinmann B. U., Wahl L. M., Martin G. R. High cell density alters the ratio of type III to I collagen synthesis by fibroblasts. Nature. 1979 May 31;279(5712):442–444. doi: 10.1038/279442a0. [DOI] [PubMed] [Google Scholar]
- Bell O. F., Carrell R. W. Basis of the defect in alpha-1-antitrypsin deficiency. Nature. 1973 Jun 15;243(5407):410–411. doi: 10.1038/243410a0. [DOI] [PubMed] [Google Scholar]
- Clark C. C. The distribution and initial characterization of oligosaccharide units on the COOH-terminal propeptide extensions of the pro-alpha 1 and pro-alpha 2 chains of type I procollagen. J Biol Chem. 1979 Nov 10;254(21):10798–10802. [PubMed] [Google Scholar]
- Cox D. W. Letter: Defect in alpha1-antitrypsin deficiency. Lancet. 1973 Oct 13;2(7833):844–845. doi: 10.1016/s0140-6736(73)90884-2. [DOI] [PubMed] [Google Scholar]
- Fujii K., Tanzer M. L. Osteogenesis imperfecta: biochemical studies of bone collagen. Clin Orthop Relat Res. 1977 May;(124):271–277. [PubMed] [Google Scholar]
- Gay S., Martin G. R., Muller P. K., Timpl R., Kuhn K. Simultaneous synthesis of types I and III collagen by fibroblasts in culture. Proc Natl Acad Sci U S A. 1976 Nov;73(11):4037–4040. doi: 10.1073/pnas.73.11.4037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon H. W., Dixon J., Rogers J. C., Mittman C., Lieberman J. Alpha 1 -antitrypsin (A1AT) accumulation in livers of emphysematous patients with A1AT deficiency. Hum Pathol. 1972 Sep;3(3):361–370. doi: 10.1016/s0046-8177(72)80037-6. [DOI] [PubMed] [Google Scholar]
- Hance A. J., Crystal R. G. Rigid control of synthesis of collagen types I and III by cells in culture. Nature. 1977 Jul 14;268(5616):152–154. doi: 10.1038/268152a0. [DOI] [PubMed] [Google Scholar]
- Jeppsson J. O. Amino acid substitution Glu leads to Lys alpha1-antitrypsin PiZ. FEBS Lett. 1976 Jun 1;65(2):195–197. doi: 10.1016/0014-5793(76)80478-4. [DOI] [PubMed] [Google Scholar]
- Jeppsson J. O., Larsson C., Eriksson S. Characterization of alpha1-antitrypsin in the inclusion bodies from the liver in alpha 1-antitrypsin deficiency. N Engl J Med. 1975 Sep 18;293(12):576–579. doi: 10.1056/NEJM197509182931203. [DOI] [PubMed] [Google Scholar]
- Jimenez S. A., Dehm P., Prockop D. J. Further evidence for a transport form of collagen. Its extrusion and extracellular conversion to tropocollagen in embryonic tendon. FEBS Lett. 1971 Oct 1;17(2):245–248. doi: 10.1016/0014-5793(71)80156-4. [DOI] [PubMed] [Google Scholar]
- Müller P. K., Raisch K., Matzen K., Gay S. Presence of type III collagen in bone from a patient with osteogenesis imperfecta. Eur J Pediatr. 1977 Apr 26;125(1):29–37. doi: 10.1007/BF00470603. [DOI] [PubMed] [Google Scholar]
- Olsen B. R., Guzman N. A., Engel J., Condit C., Aase S. Purification and characterization of a peptide from the carboxy-terminal region of chick tendon procollagen type I. Biochemistry. 1977 Jun 28;16(13):3030–3036. doi: 10.1021/bi00632a034. [DOI] [PubMed] [Google Scholar]
- Peltonen L., Palotie A., Hayashi T., Prockop D. J. Thermal stability of type I and type III procollagens from normal human fibroblasts and from a patient with osteogenesis imperfecta. Proc Natl Acad Sci U S A. 1980 Jan;77(1):162–166. doi: 10.1073/pnas.77.1.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penttinen R. P., Lichtenstein J. R., Martin G. R., McKusick V. A. Abnormal collagen metabolism in cultured cells in osteogenesis imperfecta. Proc Natl Acad Sci U S A. 1975 Feb;72(2):586–589. doi: 10.1073/pnas.72.2.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prockop D. J., Kivirikko K. I., Tuderman L., Guzman N. A. The biosynthesis of collagen and its disorders (second of two parts). N Engl J Med. 1979 Jul 12;301(2):77–85. doi: 10.1056/NEJM197907123010204. [DOI] [PubMed] [Google Scholar]
- Shochat D., Staples S., Hargrove K., Kozel J. S., Chan S. K. Primary structure of human alpha1-protease inhibitor. The complete amino acid sequence of cyanogen bromide fragment II. J Biol Chem. 1978 Aug 25;253(16):5630–5634. [PubMed] [Google Scholar]
- Sillence D. O., Senn A., Danks D. M. Genetic heterogeneity in osteogenesis imperfecta. J Med Genet. 1979 Apr;16(2):101–116. doi: 10.1136/jmg.16.2.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith B. D., Byers P. H., Martin G. R. Production of procollagen by human fibroblasts in culture. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3260–3262. doi: 10.1073/pnas.69.11.3260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmann B. U., Martin G. R., Baum B. I., Crystal R. G. Synthesis and degradation of collagen by skin fibroblasts from controls and from patients with osteogenesis imperfecta. FEBS Lett. 1979 May 15;101(2):269–272. doi: 10.1016/0014-5793(79)81023-6. [DOI] [PubMed] [Google Scholar]
- Sykes B., Francis M. J., Smith R. Altered relation of two collagen types in osteogenesis imperfecta. N Engl J Med. 1977 May 26;296(21):1200–1203. doi: 10.1056/NEJM197705262962104. [DOI] [PubMed] [Google Scholar]
- Trelstad R. L., Rubin D., Gross J. Osteogenesis imperfecta congenita: evidence for a generalized molecular disorder of collagen. Lab Invest. 1977 May;36(5):501–508. [PubMed] [Google Scholar]
- Tuderman L., Kivirikko K. I., Prockop D. J. Partial purification and characterization of a neutral protease which cleaves the N-terminal propeptides from procollagen. Biochemistry. 1978 Jul 25;17(15):2948–2954. doi: 10.1021/bi00608a002. [DOI] [PubMed] [Google Scholar]
- Yoshida A., Lieberman J., Gaidulis L., Ewing C. Molecular abnormality of human alpha1-antitrypsin variant (Pi-ZZ) associated with plasma activity deficiency. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1324–1328. doi: 10.1073/pnas.73.4.1324. [DOI] [PMC free article] [PubMed] [Google Scholar]