Abstract
Renewed interest in chlamydia vaccination has revealed the need for a greater understanding of the seroprevalence of chlamydial infection in US populations. We used a Chlamydia trachomatis elementary body (EB)–based enzyme-linked immunosorbent assay to define the characteristics of the humoral immune response and to determine seroprevalence. Two groups were analyzed: one consisting of patients with current, laboratory confirmed, genital chlamydial infection (n = 98) and one group of individuals whose chlamydia infection history was unknown (n = 367). C. trachomatis seropositivity was detected in 90% of the infected group and in 31% of the chlamydia-unknown group. IgG1 and IgG3 comprised the predominant anti-Chlamydia serum antibody responses. Serum IgA1 responses were variably positive, and individuals were rarely positive for anti-chlamydia IgG2, IgG4 or IgA2. The magnitude of the IgG1 and IgG3 responses was greatest in female and African American individuals and was sustained for at least 6 months. Antibody responses were not serovar restricted or confounded by Chlamydia pneumoniae cross-reactivity.
Chlamydia trachomatis (Ct) infection is the most commonly reported sexually transmitted bacterial infection in the United States, with >1.3 million cases reported in 2010 [1]. However, because many infections go undetected or unreported, the actual incidence of Ct infection in the United States is estimated to be 2.8 million cases annually [2]. Genital Ct infection is readily diagnosed by a nucleic acid amplification test (NAAT) and is effectively treated with antibiotics. However, because most Ct infections are asymptomatic and many infections go undiagnosed, screening programs have become an essential component of chlamydia prevention strategies. Without effective antibiotic therapy, asymptomatic infections may persist for long periods and lead to serious complications and/or further transmission to sex partners [3]. Although chlamydia screening programs have been shown to be effective in reducing pelvic inflammatory disease incidence [4], they currently reach less than half of the women for whom screening is recommended (routine screening of males is not currently recommended), and Ct infection remains highly prevalent [1, 5]. Thus, although chlamydia screening and treatment strategies reduce the incidence of reproductive sequelae, other prevention strategies, such as vaccination, are needed to control Ct infection.
Evaluation of Ct vaccines will require a more thorough understanding of the natural history of infection, the immune responses elicited after infection, and the seroprevalence of the target population. The proportion of the US population who has had Ct infection is not known, nor can it be inferred from available screening and case report data. Characterizing the Ct-specific antibody class- and subclass-specific responses elicited after infection will provide information needed to determine seroprevalence and to estimate both target and eligible populations for inclusion in vaccine trials. In addition, a central role for antibody in protective immunity has emerged from studies using the murine genital chlamydial infection model [6–8], which provides an impetus for further investigations to characterize antibody responses elicited after human Ct infection. In this study, the specificity and usefulness of an elementary body–based enzyme linked immunosorbent assay (EB ELISA) for characterizing Ct-specific Ig class and subclass responses in individuals with laboratory-confirmed genital Ct infection was demonstrated. The performance of the EB ELISA was compared with a commercial Ct ELISA and was used to measure the seroprevalence among healthy females in Birmingham, Alabama.
MATERIALS AND METHODS
Study Populations
Two groups of individuals evaluated used in this study. Group 1 consisted of 98 patients with laboratory-confirmed genital Ct infection. These patients had been recently screened for sexually transmitted diseases (STDs) and were subsequently returning to the Jefferson County Department of Health (JCDH) STD clinic in Birmingham, Alabama, for treatment of a positive genital Ct nucleic acid amplification test (NAAT; Gen-Probe Aptima Combo 2 Assay; Gen-Probe). At the time of their return, serum samples were collected as part of a chlamydia natural history study. For those patients, a history of Ct infection was sought through self-report and review of the clinic record for prior positive Ct test results. A subgroup of group 1, consisting of 32 patients, also had serum samples collected at a 6-month follow-up visit, allowing for evaluation of changes in serological responses over that period. Group 2, the seroprevalence study group, consisted of 367 adult females (age, 18–30 years) from the Birmingham, Alabama community whose prior and current Ct infection status was unknown and who had serum samples collected at the time of screening for a phase III genital herpes vaccine trial [9]. Demographic, clinical, and behavioral data were collected from group 1 patients, but only demographic data were collected for group 2 individuals (Table 1). Approval for this study was obtained from the Institutional Review Boards of the University of Alabama at Birmingham and the University of Arkansas for Medical Sciences.
Table 1.
Characteristics of Study Populations
| Group 1 | Group 2 | |
|---|---|---|
| Characteristic | Current Genital Ct Infectiona | Ct Infection Status Unknownb |
| Group size (n) | 98 | 367 |
| Median age (range) | 23 (17–54) | 21 (18–30) |
| Sex | ||
| Female | 86 (88%) | 367 (100%) |
| Male | 12 (12%) | 0 (0%) |
| Race | ||
| African American | 80 (82%) | 142 (39%) |
| White | 18 (18%) | 219 (60%) |
| Hispanic | 0 (0%) | 1 (0.3%) |
| Asian | 0 (0%) | 5 (1.4%) |
| Median sex partners 6 months | 1 (1–15) | NAc |
| Prior chlamydia | 45 (46%) | NA |
| Urethritisd | 5 (42%) | NA |
| Cervicitise | 19 (22%) | NA |
| Hormonal contraceptive usee | 28 (33%) | NA |
a Patients with current genital Ct infection as diagnosed by a Ct NAAT.
b The Ct infection status of patients in this group is unknown.
c NA = data not available.
d Data for males only.
e Data for females only.
Preparation of ELISA Antigen
Elementary bodies (EBs) of Ct serovars D/UW-3, F/IC-Cal-13, and J/UW-36, representing serovars from each of the 3 Ct serogroups (B, intermediate, and C serogroups, respectively), and Chlamydia pneumoniae (Cp) strain AR39 were grown in cell culture, density gradient purified [10], and used as antigen in the EB-ELISA. Purified EB preparations were fixed overnight at 4°C in 10 mM phosphate-buffered saline (PBS) containing 0.2% formalin. After fixation, EBs were washed once with PBS and resuspended in PBS with 0.02% formalin. For Ct ELISA antigen, equal volumes of each formalin-fixed serovar (2 mg/mL) were combined to make a 2 mg/mL mixture of serovars D, F, and J. Formalin-fixed Cp EBs were prepared similarly. Ninety-six–well Immunlon 2 HB U-bottoms plates (Thermo Scientific) were coated with 2 µg EB/well (100 µL of 20 µg EB/mL) for use in the ELISA
EB-ELISA
Antibody responses to Ct and Cp were assessed using an EB ELISA as described previously [11]. Anti-Ct or Cp immunoglobulin (Ig) class- and subclass-specific responses were detected using the following alkaline phosphatase–labeled anti-human Ig monoclonal antibodies and Sigma Fast p-nitrophenyl phosphate substrate (1 mg/mL; Sigma): anti-IgM (clone SA-DA4; Southern Biotech), anti-IgG (clone JDC-10; Southern Biotech), anti-IgG1 (a pool of clones 4E3, Southern Biotech; and HP6069; Cal Biochem), anti-IgG2 (clone 31-7-4; Southern Biotech), anti-IgG3 (clone HP6050; Southern Biotech), IgG4 (clone HP6025; Southern Biotech), anti-IgA1 (clone B3506B4; Southern Biotech), and anti-IgA2 (clone A9604D2; Southern Biotech). The optical density of reactions was measured at 405 nm (OD405) using a Biotek µQuant microplate spectrophotometer and analyzed using Gen5 data collection and analysis software (Biotek Instruments).
Preliminary studies demonstrated that accurate measurement of IgG and IgM class-specific Ct responses was not possible because of high EB-ELISA background reactions produced when evaluating titrations of known negative control serum samples. These high background values greatly diminished the sensitivity of the analyses, requiring substantial dilution (1:250–1:1000) of serum to reduce background responses to an acceptable value. However, background responses using monoclonal Ig subclass-specific detection reagents were low, and on the basis of titration of negative control serum samples, we chose to use serum samples diluted 1:32 for this study. Preliminary evaluation of serum samples from the current genital Ct infection group also revealed that anti-Ct IgG2, IgG4, and IgA2 responses were infrequently positive, and the magnitude of those responses was low; therefore, we do not include evaluation of those responses.
To establish optical density cutoff values for positive serologic responses, we first evaluated serum samples, diluted 1:32, from 8 low-risk adult volunteers who had no history of chlamydia. The mean OD405 responses from those subjects + 3 standard deviations (SD) was used to establish an interim value for a positive serologic response. We then identified 60 seronegative individuals (30 white and 30 African American) from group 2 and recalculated the cutoff for positive responses. Cutoff OD405 values for positive IgG1, IgG3, and IgA1 anti-Ct responses were ≥0.4, ≥0.2, and ≥0.3, respectively. These cutoff values represent 3.45 SD [12] above the mean OD405 response of serum samples from the 60 seronegative individuals. The cutoff OD value for a positive anti-Cp IgG1 response was calculated similarly and determined to be ≥0.5. The reported serologic responses of all participants represent the mean of triplicate determinations of 1:32 diluted serum for each Ig subclass response.
Commercial Ct Enzyme Immunoassay
Serum samples were also evaluated using the major outer membrane protein (OmpA) peptide-based C. trachomatis–IgG and IgA ELISA plus Medac assay kits (commercial immunoassay; Medac) according to the manufacturer's instructions.
Statistical Analysis
The Mann–Whitney U test was used for comparing EB ELISA OD readings by sex, race, or prior Ct infection. Differences in EB ELISA OD readings from visit 1 to visit 2 were analyzed using the Wilcoxon signed rank test. Differences in seropositivity assessed by EB ELISA, compared with the commercial immunoassay, were analyzed using McNemar's test.
RESULTS
Ct Seropositivity and Immunoglobulin Subclass-Specific Responses Among Individuals with Current Genital Ct Infection
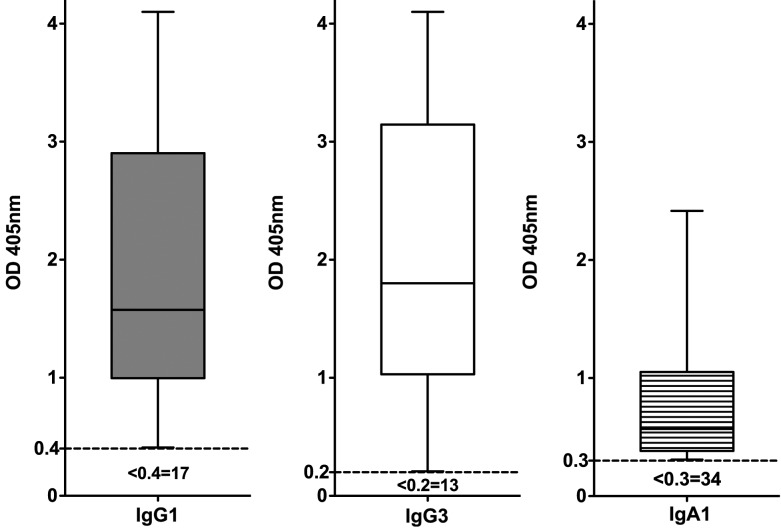
The baseline characteristics of 98 patients with current genital Ct infection (group 1) are detailed in Table 1. Of those patients, 88 (90%) tested seropositive for ≥1 Ig subclass-specific response by Ct EB ELISA. Of the 88 seropositive patients, 81 (92%) were IgG1 positive (median OD, 1.58; range, 0.409–4.1), 85 (97%) were IgG3 positive (median OD, 1.8; range, 0.208–4.1), and 64 (73%) were IgA positive (median OD, 0.57; range, 0.309–2.416) (Figure 1). IgG1 and IgG3 Ct EB ELISAs detected all seropositive patients; adding IgA testing did not identify additional seropositive patients.
Figure 1.
Seropositivity and immunoglobulin subclass-specific responses in patients with current genital Ct infection. Serum samples from 98 patients with current genital Ct infection (group 1) were analyzed using EB ELISA. Data for positive serum samples only are presented as box-and-whiskers graphs of OD405 values. The box extends from the 25th percentile to the 75th percentile, with the median response indicated by the horizontal line within the box. Whiskers represent the range of responses from minimum to maximum for positive serum samples. The broken line (---) indicates the cutoff OD value for a positive response. The number of patients who tested negative for a given response is indicated beneath the cutoff line. IgG1 (shaded boxes), IgG3 (open boxes), and IgA1 (lined boxes) responses are indicated on the graph.
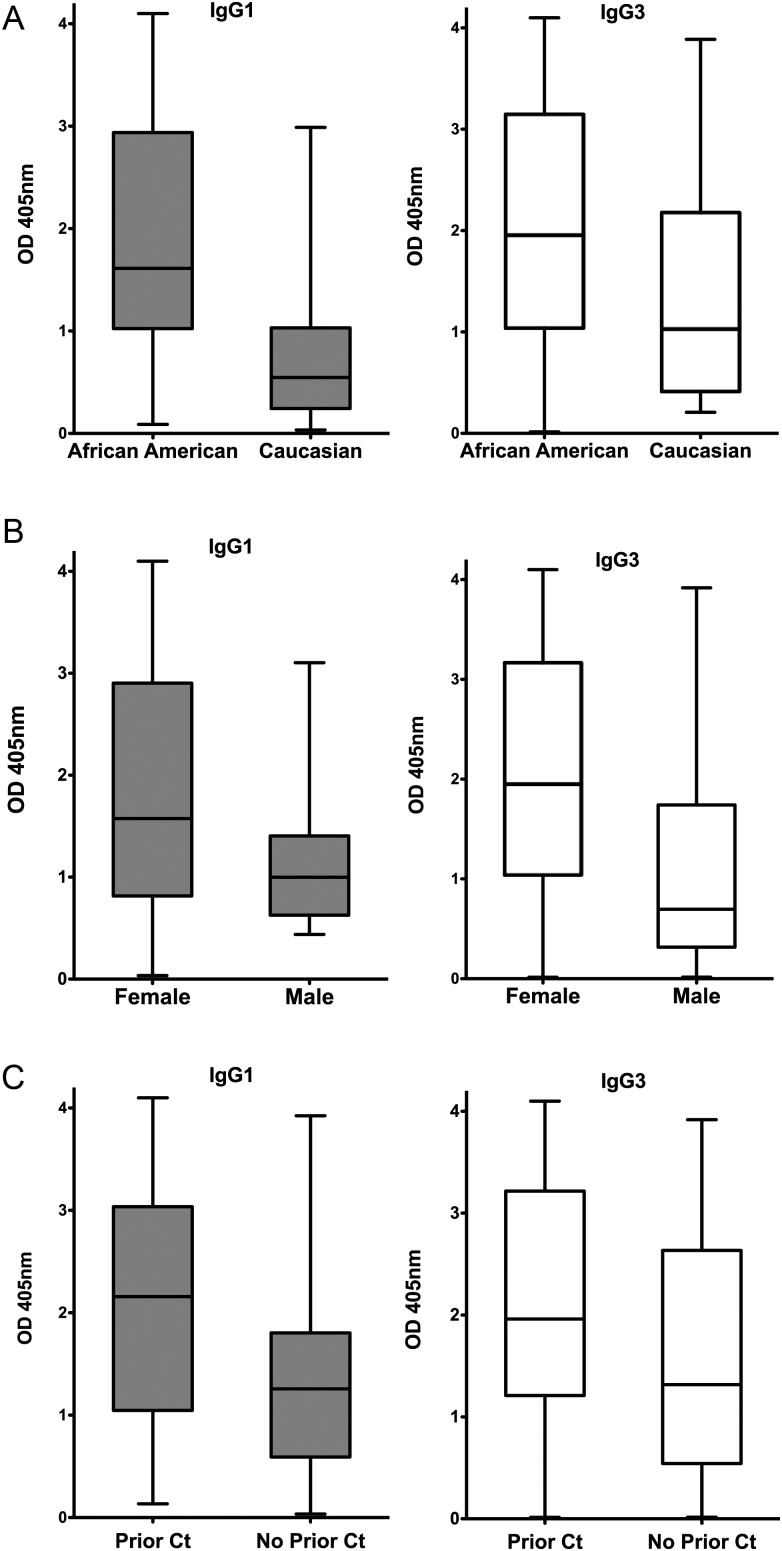
Among the Ct-seropositive patients, median IgG1 and IgG3 Ct EB ELISA responses were significantly higher in African American than in white patients (IgG1: median OD, 1.61 vs 0.55 [P < .001]; IgG3: median OD, 1.96 vs 1.03 [P = .048]) (Figure 2A). Females had a more robust IgG3 response compared with males (median OD, 1.95 vs 0.7; P = .018), and the IgG1 response trended higher in females than in males but did not reach statistical significance (median OD, 1.58 vs 1.00; P = .082) (Figure 2B). Patients with a history of Ct infection (either by self-reporting or by documentation through medical record review for positive test results) had higher IgG1 (median OD, 2.16 vs 1.26; P = .013) and IgG3 responses (1.96 vs 1.32; P = .029) than did those reporting no history of infection (Figure 2C), and anti-Ct IgG3 responses were significantly higher in females with a cervicitis diagnosis (2.90 vs 1.72; P = .018). Antibody responses did not significantly differ by age or hormonal contraceptive use.
Figure 2.
Effect of race, sex, and prior Ct infection on IgG1 and IgG3 Ct EB ELISA responses. The IgG1 and IgG3 CT EB ELISA responses of the 88 seropositive patients from group 1 (patients with current genital Ct infection) were compared to assess differences in responses between (A) African American vs white patients (IgG1: median OD, 1.61 vs 0.55 [P < .001]; IgG3: median OD, 1.96 vs 1.03 [P = .048]); (B) female vs male patients (IgG1: median OD, 1.58 vs 1.00 [P = .082]; IgG3: median OD, 1.95 vs 0.7 [P = .018]); and C, subjects with vs without prior Ct infection (as assessed by self-report or clinic record review); IgG1: median OD, 2.16 vs 1.26 [P = .013]; IgG3: median OD, 1.96 vs 1.32 [P = .029]). Data are presented as described in Figure 1 legend.
Seroprevalence was also assessed using a commercial immunoassay (Medac). Seventy-two (73%) of 98 patients with current genital Ct infection tested seropositive using the commercial immunoassay, compared with 90% seropositive by our Ct EB ELISA. Of the 72 patients seropositive by the commercial immunoassay, 71 (99%) were positive by Ct EB ELISA. A number of female patients (11 [14%] of 78) who were Ct NAAT positive and Ct EB ELISA positive tested negative by the commercial assay, and the Ct EB ELISA missed 1 patient (1.3%) who was positive by the commercial immunoassay. Five (46%) of 11 males with current genital Ct infection tested seronegative by commercial immunoassay, but all (11) tested seropositive by Ct EB ELISA. Thus, more patients with current Ct infection tested seropositive by Ct EB ELISA than by the commercial immunoassay.
Ten patients with current genital Ct infection tested seronegative by EB ELISA. To further evaluate their seronegativity and to demonstrate that the seronegative responses did not result from under-representation of Ct serovars in the antigen mixture, we analyzed serum samples with use of additional serovar mixtures. We tested the serum samples from genital Ct–positive patients from whom we had performed Ct ompA genotyping to identify the infecting serovar. No statistically significant differences were found in antibody responses when serum samples were tested by EB ELISA using either pooled (serovars D, F, and J) or infection-matched serovar EB antigen, even when the infecting serovar was not included in the pooled EB antigen preparation. Furthermore, the 10 genital Ct–positive patients who were seronegative by Ct ELISA using pooled serovar D, F, and J EBs as antigen also tested seronegative with other serovar mixtures. Collectively, seronegative responses do not appear to result from failure of Ct ELISA to detect positive antibody responses for patients with infecting serovars not represented in the EB antigen mixture.
Ct Seropositivity at a 6-Month Follow-up Visit
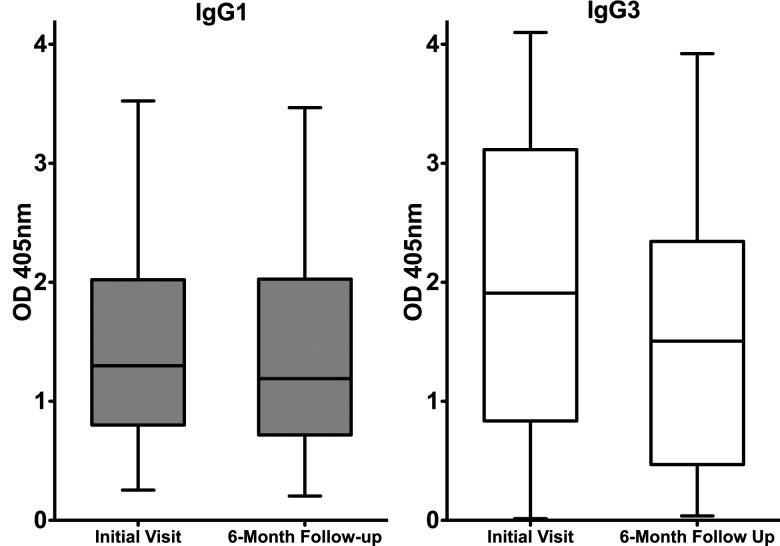
A subset of 32 patients from the group of 98 patients with current genital Ct infection (group 1) had serum samples collected during a 6-month follow-up visit (median follow-up, 168 days; range, 106–245 days), at which time a repeat Ct NAAT was performed; all patients received treatment at their initial (baseline) visit, and 4 (13%) were Ct positive by NAAT at the follow-up visit. Testing serum samples from the follow-up visit enabled us to evaluate changes in Ct serum antibody responses over time and the relationship of those responses to recurrent genital Ct infection. Ct EB ELISA IgG1, IgG3, and IgA responses were compared among serum samples collected at the initial and 6-month follow-up visits. Twenty-eight (88%) were seropositive for IgG1 and IgG3 at their initial visit, with 18 of the 28 also testing positive for Ct IgA. All 28 patients who were IgG1 and IgG3 seropositive at initial visit remained seropositive at follow-up, and only 11 (61%) of 18 IgA-positive patients remained Ct IgA positive at follow-up. The median Ct EB ELISA IgG1 response was stable between initial and follow-up visits (median OD, 1.30 vs 1.20; P = .67), but the magnitude of the median IgG3 response diminished slightly during that period (median OD, 1.90 vs 1.50; P = .015) (Figure 3). Four patients were seronegative by Ct EB ELISA and Medac at both baseline and follow-up visits, despite having a positive baseline genital Ct NAAT result. Although the IgG1 and IgG3 antibody responses to Ct appears to be durable, it was not possible to know whether the relative stable serologic response over the 6-month period was attributable specifically to the durability of the antibody response from the initial infection or whether re-exposure/re-infection during the follow-up period served to maintain the antibody response. However, we observed no upward trend in the magnitude of the antibody responses in the 4 patients who were Ct positive at the 6-month follow-up.
Figure 3.
Comparison of IgG1 and IgG3 Ct EB ELISA responses at the initial and 6-month follow-up visits. Data represent OD405 values of serum samples from 28 patients with current genital Ct infection who were seropositive at baseline and who had serum samples collected at a 6-month follow-up visit. Data are presented as described in Figure 1 legend. No statistically significant difference was found in the magnitude of the median IgG1 response of serum samples collected at the time of the initial visit and at 6 months after infection diagnosis and treatment (IgG1: median OD, 1.30 vs 1.20; P = .67), but the median IgG3 response decreased slightly during that period (IgG3: median OD, 1.90 vs 1.50; P = .015).
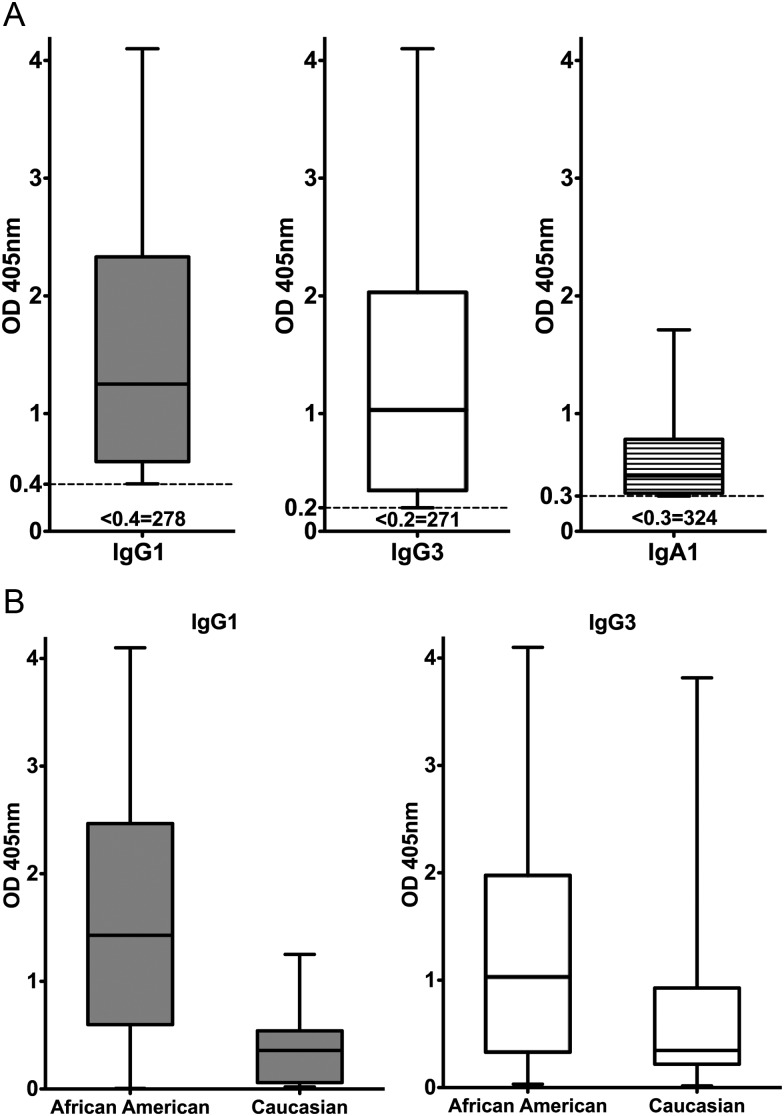
Ct Seroprevalence and Immunoglobulin Subclass-Specific Responses in Patients with Unknown Ct Infection Status
Characteristics of the 367 patients whose prior and current Ct infection status was unknown are provided in Table 1. One hundred thirteen (31%) of 367 patients tested Ct seropositive by EB ELISA. Of the 113 seropositive patients, 89 (79%) were IgG1 positive (median OD reading, 1.25; range, 0.404–4.1), 96 (85%) were IgG3 positive (median OD, 1.03; range, 0.203–4.1), and 43 (38%) were IgA positive (median OD, 0.471; range, 0.30–1.71) (Figure 4A). Thus, the frequency of Ig class and subclass Ct-specific antibody responses in this group was similar to that seen in patients with current genital Ct infection (group 1). The IgG1 and IgG3 EB ELISA captured all but 2 (2%) of the Ct EB-ELISA–seropositive patients, who were only seropositive by IgA. Of 112 seropositive patients who were either African American or white (the single Asian subject is not included), median IgG1 and IgG3 responses were significantly higher among African American patients than among white patients (IgG1: median OD, 1.43 vs 0.36 [P <.001]; IgG3: median OD, 1.03 vs 0.35 [P = .011]) (Figure 4B), a finding comparable to that for patients with current genital Ct infection (Figure 2A).
Figure 4.
Ct seropositivity and immunoglobulin subclass-specific responses in patients with unknown chlamydia infection status (group 2). The study population consisted of 367 adult females whose prior and current Ct infection status was unknown. Data are presented as described in Figure 1 legend. (A) the IgG1, IgG3, and IgA1 Ct EB ELISA responses of seropositive patients. The number of patients testing seronegative for each specific Ig subclass is indicated below the positive OD cutoff value. (B) comparison of IgG1 and IgG3 responses of seropositive group 2 African American vs white patients (total of 112 IgG1 or IgG3 seropositive patients; IgG1: median OD, 1.43 vs 0.36 [P < .001]; IgG3: median OD. 1.03 vs 0.35 [P = .011]).
In contrast to the 31% seropositivity by Ct EB ELISA, 14% (53 of 367 patients) were seropositive by the commercial immunoassay. Of the 113 patients seropositive by Ct EB ELISA, 46 (41%) were positive by commercial immunoassay. Forty-six (87%) of 53 commercial immunoassay–seropositive patients were positive by Ct EB ELISA. Overall seropositivity rates were higher by Ct EB ELISA than by the commercial immunoassay (31% vs 14%; P < .001).
The Relationship of Cp Seropositivity to Ct Seropositivity
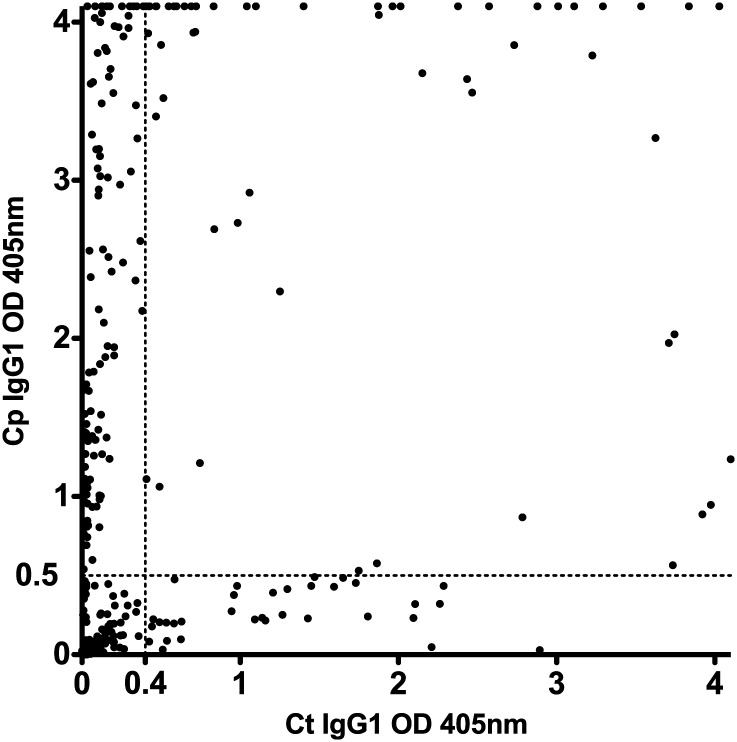
Because Ct seropositivity could potentially be confounded by cross-reacting antibodies produced in response to a current or past Cp infection, we sought to determine specificity of the EB ELISA. Serum samples from all 367 patients with unknown Ct infection status (group 2) were tested using a Cp EB ELISA and compared with their Ct EB ELISA results. Both IgG1 and IgG3 responses were analyzed, but IgG1 Cp EB ELISA responses were more consistently positive than were IgG3 responses (eg, some patients with IgG1 responses had low or absent IgG3 responses, but no patients had IgG3 responses without IgG1 responses); thus, only IgG1 responses are shown (Figure 5). Patients fell into 4 groups based on their seroreactivity: Ct-negative Cp-negative (168 patients), Ct-positive Cp-negative (40), Ct-positive Cp-positive (73), and Ct-negative Cp-positive (86). The high proportion of Ct-seronegative Cp-seropositive patients with marked antibody responses to Cp (OD > 2.5) in the absence of a positive Ct serologic response (OD < 0.4) was quite remarkable. Those results strongly suggest that a positive Ct serologic result, as determined by Ct EB ELISA, was not attributable to cross-reacting antibodies produced in response to a past or current Cp infection, but rather attributable to past or current Ct infection.
Figure 5.
Relationship of Cp seropositivity to Ct seropositivity. Serum samples from all 367 group 2 patients (Ct infection status unknown) were tested using a Cp EB ELISA, and the results of the IgG1 responses are plotted along with the Ct IgG1 EB ELISA results. Each dot represents a single patient. Ct EB ELISA OD405 values are plotted along the x axis, and the Cp EB-ELISA values are plotted along the y axis. Cutoff values for seropositive Ct (OD405 ≥ 0.4) and Cp (OD405 ≥ 0.5) EB ELISA responses are indicated by the dashed lines (––).
DISCUSSION
Most prior Ct prevalence studies have been performed in non-US populations and have included patients undergoing infertility evaluation, community-based or low STD risk populations, and higher STD risk populations (eg, STD clinics). In general, those studies have reported Ct seroprevalence of 5%–55% in community-based or low STD risk populations [13–17] and sometimes >55% in higher STD risk populations [18, 19]. Using EB ELISA, we demonstrated that the overall Ct seroprevalence among a group of healthy adult females in the Birmingham, Alabama, community was approximately 30%, which is similar to rates reported in comparable populations in some European countries [13, 15]. Considerably lower seroprevalence (14%) was found using a commercial peptide-based assay (Medac), which is in agreement with previous reports using assays based on Ct peptides or single recombinant Ct proteins [13, 20, 21]. Overall, EB ELISA was a useful assay for assessing Ct seroprevalence and was not dependent on serovar-specific responses or confounded by concurrent antibody responses to Cp. Therefore, our pilot study establishes that the EB ELISA may be useful for establishing the Ct seroprevalence in other US populations and for defining eligible populations for inclusion in Ct vaccine trials.
Four patients from the current genital Ct infection group (those with a positive genital Ct NAAT at baseline; group 1) tested seronegative at both baseline and follow-up visits. Although persons with a primary Ct infection might test seronegative at baseline, seroconversion in a few weeks would be expected. Several possible explanations could account for these negative serologic results: reduced infection duration and antigenic burden resulting from early detection and therapy, inadequate dose of chlamydiae to cause productive infection, early infection IgM response (we do not report IgM responses), or undiagnosed immunosuppressive disease. Confirmed Ct infection in the absence of detectable antibody appears to be rare but has been reported previously [22].
There is limited information regarding Ig class and subclass anti-Ct responses after genital Ct infection. We demonstrated that IgG1 and IgG3 comprise the predominant serum anti-Ct antibody response, with IgA detected at a lower frequency and not contributing to overall determination of Ct seropositivity. Furthermore, anti-Ct IgG1 and IgG3 seropositivity was retained over a 6-month period, whereas only 60% of patients retained IgA seropositivity. The magnitude of Ig subclass anti-Ct antibody responses differed by sex and race, with higher responses in females than in males and in African American patients than in white patients. To our knowledge, such associations have not been reported previously for Ct serology, but sex and race influences on antibody responses have been reported for smallpox and HIV vaccine trials [23–25]. It is possible that multiple prior genital Ct infection episodes influence the magnitude of antibody responses, but verifiable information on the number of prior Ct infections was unavailable for most patients tested. Of interest, however, the variation in magnitude of Ct serologic responses by race was consistent across both the current genital Ct infection group and the chlamydia-unknown group, and sex and race differences were not observed in the Cp serological analysis. Therefore, differences in the magnitude of the serologic response by sex and race likely represent differences in their immune response to infection, rather than intrinsic limitations of the EB ELISA. Of note, however, a limitation of our current study is the low number of males (12) tested. Therefore, although observed differences in serologic response between sexes is quite interesting, additional studies testing larger groups of male patients will be needed to confirm the observation.
A functional role for antibody in immunity to genital chlamydial infection has been established unequivocally for the murine model of infection [7, 8], and that response has some similarities to that produced after human infection. Although human and mouse subtypes of IgG are not direct homologues, functional similarities do exist [26, 27], and comparing the responses may provide insight into the possible role of antibody in immunity to human infection. Similar to murine infection, antibody responses generated in response to human genital infection tend to remain elevated for months [11], and the responses are composed of antibody subtypes with similar functions. For example, the predominant anti-chlamydia IgG antibody responses in mice are subtypes IgG2a (IgG2c in C57BL/6 mice) and IgG2b [11, 28], and IgG1 and IgG3 subtypes predominate after human infection (the current study). Mouse IgG2a and IgG2b and human IgG1 and IgG3 preferentially bind protein antigens, activate complement, and function as opsonins. Mouse IgG3 and human IgG2 anti-chlamydia responses are more variably positive and of lower magnitude and tend to predominantly recognize polysaccharide antigens. Anti-chlamydia IgA responses, in both mice and humans, are of shorter duration are more variable, compared with IgG responses.
We demonstrated that EB ELISA is a specific and sensitive assay for evaluating Ct seroprevalence and is useful in characterizing class- and subclass-specific Ct antibody responses. Moreover, because functionally similar class and subclass antibody responses are elicited after human and murine genital chlamydial infection, future studies aimed at defining the mechanism of antibody-mediated protection in the mouse and identifying the antigens recognized by human and mouse immune serum may provide additional insight into the role of antibody in protective immunity to human Ct infection.
Notes
Financial support. This work was supported by a grant from Sanofi Pasteur (to R.P.M., W.M.G.), the National Institutes of Health (K23-AI069505 to W.M.G.), and the Arkansas Biosciences Institute (to R.P.M.).
Potential conflicts of interest. W.M.G. and R.P.M. have received research grant funding from Sanofi Pasteur. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Centers for Disease Control and Prevention. Atlanta, US: Department of Health and Human Services; 2011. Sexually Transmitted Disease Surveillance 2010. [Google Scholar]
- 2.Centers for Disease Control and Prevention. CDC Grand Rounds: Chlamydia prevention: challenges and strategies for reducing disease burden and sequelae. MMWR. 2011;60:370–3. [PubMed] [Google Scholar]
- 3.Geisler WM. Duration of untreated uncomplicated genital Chlamydia trachomatis infection and factors associated with chlamydia resolution: a review of human studies. J Infect Dis. 2010;201:S104–13. doi: 10.1086/652402. [DOI] [PubMed] [Google Scholar]
- 4.Scholes D, Stergachis A, Heidrich FE, Andrilla H, Holmes KK, Stamm WE. Prevention of pelvic inflammatory disease by screening for cervical chlamydia infection. NEJM. 1996;334:1362–6. doi: 10.1056/NEJM199605233342103. [DOI] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. Chlamydia screening among sexually active young female enrollees of health plans – United States, 2000–2007. MMWR. 2009;58:362–5. [PubMed] [Google Scholar]
- 6.Farris CM, Morrison SG, Morrison RP. CD4+ T cells and antibody are required for optimal major outer membrane protein vaccine-induced immunity to Chlamydia muridarum genital infection. Infect Immun. 2010;78:4374–83. doi: 10.1128/IAI.00622-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morrison SG, Morrison RP. A predominant role for antibody in acquired immunity to chlamydial genital tract reinfection. J Immunol. 2005;175:7536–42. doi: 10.4049/jimmunol.175.11.7536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morrison SG, Su H, Caldwell HD, Morrison RP. Immunity to murine Chlamydia trachomatis genital tract reinfection involves B cells and CD4+ T cells but not CD8+ T cells. Infect Immun. 2000;68:6979–87. doi: 10.1128/iai.68.12.6979-6987.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Belshe RB, Leone PA, Bernstein DI, et al. Herpevac trial for women: efficacy results of a trial of a herpes simplex vaccine. NEJM. 2012;366:34–43. doi: 10.1056/NEJMoa1103151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Caldwell HD, Kromhout J, Schachter J. Purification and partial characterization of the major outer membrane protein of Chlamydia trachomatis. Infect Immun. 1981;31:1161–76. doi: 10.1128/iai.31.3.1161-1176.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morrison RP, Feilzer K, Tumas DB. Gene knockout mice establish a primary protective role for major histocompatibility complex class II-restricted responses in Chlamydia trachomatis genital tract infection. Infect Immun. 1995;63:4661–8. doi: 10.1128/iai.63.12.4661-4668.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frey A, Canzio JD, Zurakowski D. A statistically defined endpoint titer determination method for immunoassays. J Immunol Methods. 1998;221:35–41. doi: 10.1016/s0022-1759(98)00170-7. [DOI] [PubMed] [Google Scholar]
- 13.Bax CJ, Mutsaers JA, Jansen CL, Trimbos JB, Dorr PJ, Oostvogel PM. Comparison of serologic assays for detection of Chlamydia trachomatis antibodies in different groups of obstetrical and gynecological patients. Clin Diag Lab Immunol. 2003;10:174–6. doi: 10.1128/CDLI.10.1.174-176.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gorander S, Lagergard T, Romanik M, Viscidi RP, Martirosian G, Liljeqvist JA. Seroprevalences of herpes simplex virus type 2, five oncogenic human papillomaviruses, and Chlamydia trachomatis in Katowice, Poland. Clin Vaccine Immunol. 2008;15:675–80. doi: 10.1128/CVI.00260-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jonsson M, Karlsson R, Persson K, et al. The influence of sexual and social factors on the risk of Chlamydia trachomatis infections: a population-based serologic study. Sex Trans Dis. 1995;22:355–63. doi: 10.1097/00007435-199511000-00007. [DOI] [PubMed] [Google Scholar]
- 16.Lyytikainen E, Kaasila M, Koskela P, et al. Chlamydia trachomatis seroprevalence atlas of Finland 1983–2003. Sex Transm Infect. 2008;84:19–22. doi: 10.1136/sti.2007.027409. [DOI] [PubMed] [Google Scholar]
- 17.Verkooyen RP, Peeters MF, van Rijsoort-Vos JH, van der Meijden WI, Mouton JW. Sensitivity and specificity of three new commercially available Chlamydia trachomatis tests. Internatl J STD AIDS. 2002;13(Suppl 2):23–25. doi: 10.1258/095646202762226119. [DOI] [PubMed] [Google Scholar]
- 18.Caterino-de-Araujo A, de-los-Santos Fortuna E. Seropositivity to Chlamydia trachomatis in prostitutes: relationship to other sexually transmitted diseases (STDs) Braz J Med Biolog Res. 1990;23:697–700. [PubMed] [Google Scholar]
- 19.Paroli E, Franco E, Mele A, et al. Seroprevalence of anti-Chlamydia trachomatis IgG in outpatients attending a sexually transmitted disease clinic in Italy. Eur J Epidemiol. 1990;6:329–31. doi: 10.1007/BF00150443. [DOI] [PubMed] [Google Scholar]
- 20.Morre SA, Munk C, Persson K, et al. Comparison of three commercially available peptide-based immunoglobulin G (IgG) and IgA assays to microimmunofluorescence assay for detection of Chlamydia trachomatis antibodies. J Clin Microbiol. 2002;40:584–7. doi: 10.1128/JCM.40.2.584-587.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wills GS, Horner PJ, Reynolds R, et al. Pgp3 antibody enzyme-linked immunosorbent assay, a sensitive and specific assay for seroepidemiological analysis of Chlamydia trachomatis infection. Clin Vaccine Immunol. 2009;16:835–43. doi: 10.1128/CVI.00021-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang S-P, Grayston JT. Human serology in Chlamydia trachomatis infection with microimmunofluorescence. J Infect Dis. 1974;130:388–97. doi: 10.1093/infdis/130.4.388. [DOI] [PubMed] [Google Scholar]
- 23.Gilbert P, Wang M, Wrin T, et al. Magnitude and breadth of a nonprotective neutralizing antibody response in an efficacy trial of a candidate HIV-1 gp120 vaccine. J Infect Dis. 2010;202:595–605. doi: 10.1086/654816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kennedy RB, Ovsyannikova IG, Pankratz VS, et al. Gender effects on humoral immune responses to smallpox vaccine. Vaccine. 2009;27:3319–23. doi: 10.1016/j.vaccine.2009.01.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Montefiori DC, Metch B, McElrath MJ, Self S, Weinhold KJ, Corey L. HIV vaccine trials network. Demographic factors that influence the neutralizing antibody response in recipients of HIV-1 gp120 vaccines. J Infect Dis. 2004;190:1962–9. doi: 10.1086/425518. [DOI] [PubMed] [Google Scholar]
- 26.Clark MR. IgG effector mechanisms. Chem Immunol. 1997;65:88–110. [PubMed] [Google Scholar]
- 27.Mestas J, Hughes CCW. Of mice and not men: differences between mouse and human immunology. J Immunol. 2004;172:2731–8. doi: 10.4049/jimmunol.172.5.2731. [DOI] [PubMed] [Google Scholar]
- 28.Morrison SG, Farris CM, Sturdevant GL, Whitmire WM, Morrison RP. Murine Chlamydia trachomatis genital infection is unaltered by depletion of CD4+ T cells and diminished adaptive immunity. J Infect Dis. 2011;203:1120–8. doi: 10.1093/infdis/jiq176. [DOI] [PMC free article] [PubMed] [Google Scholar]