Abstract
Two divergent genes encoding fructokinase, Frk1 and Frk2, have been previously shown to be expressed in tomato (Lycopersicon esculentum L.) and have now been further characterized with regard to their spatial expression and the enzymic properties of the encoded proteins. Frk1 and Frk2 mRNA levels were coordinately induced by exogenous sugar, indicating that both belong to the growing class of sugar-regulated genes. However, in situ hybridization indicated that Frk1 and Frk2 were expressed in a spatially distinct manner, with Frk2 mRNA primarily localized in cells of the fruit pericarp, which store starch, and Frk1 mRNA distributed ubiquitously in pericarp tissue. To evaluate the biochemical characteristics of the products of the Frk1 and Frk2 genes, each cDNA was expressed in a mutant yeast (Saccharomyces cerevisiae) line defective in hexose phosphorylation and unable to grow on glucose or fructose (Fru). Both Frk1 and Frk2 proteins expressed in yeast conferred the ability to grow on Fru and exhibited fructokinase activity in vitro. Although both Frk1 and Frk2 both utilized Fru as a substrate, only Frk2 activity was inhibited at high Fru concentrations. These results indicate that Frk2 can be distinguished from Frk1 by its sensitivity to substrate inhibition and by its temporal and spatial pattern of expression, which suggests that it plays a primary role in plant cells specialized for starch storage.
Suc translocated from leaves to sink tissue may be stored directly or metabolized by Suc synthase and/or invertase to provide hexose and hexose phosphate for storage or metabolism. In both Suc synthase- and invertase-mediated metabolic pathways, Fru is formed as a metabolic product and must be phosphorylated for further metabolism. Two enzymes, hexokinase (EC 2.7.1.1) and fructokinase (EC 2.7.1.4), are able to phosphorylate Fru in plants. Hexokinase can effectively utilize several hexoses, including Fru and Glc, whereas fructokinase specifically phosphorylates Fru. Fructokinase is likely to be of primary importance in phosphorylation of Fru in plants because the affinity of fructokinase for Fru is much higher than that of hexokinase (Renz and Stitt, 1993).
Sink tissues such as potato (Solanum tuberosum L.) tubers and tomato (Lycopersicon esculentum L.) fruit have been useful to study the mechanism of Suc import and metabolism. During the early stages of tomato fruit development, it has been proposed that Suc is imported symplastically and metabolized primarily by Suc synthase (Wang et al., 1994; Ruan and Patrick, 1995). Suc synthase activity is correlated, both temporally and spatially, with starch synthesis in developing tomato fruit (Wang et al., 1994). Suc synthase mRNA is localized in vascular tissue and in the tissue surrounding seeds, suggesting that this enzyme plays a role in sugar import and in providing carbohydrates to developing seeds in young tomato fruit. In potato two isoforms of Suc synthase have been shown to be expressed differentially, and one of them is associated with sink function (Fu and Park, 1995). In the Suc-synthase-mediated pathway of Suc assimilation, effective phosphorylation of Fru appears to be necessary to maintain C flow to starch synthesis and respiration, since Suc-synthase activity is inhibited by free Fru (Wolosiuk and Pontis, 1974; Schaffer and Petreikov, 1997b). Thus, the relationship between Suc synthase and fructokinase may be of critical importance in the sink metabolism of Suc.
Fructokinase has been purified and characterized from several plants, and most studies suggest the presence of at least two fructokinase isoforms. For example, fructokinase isoforms can be separated by ion-exchange chromatography from potato (Gardner et al., 1992; Renz and Stitt, 1993), spinach (Schnarrenberger, 1990), barley (Baysdorfer et al., 1989), avocado (Copeland and Tanner, 1988), and maize (Doehlert, 1989), and in some cases the isoforms have been shown to differ in inhibition by Fru and/or in their specificity for nucleotide triphosphates. Recently, two fructokinases were purified from young, green tomato fruit. In this case, the isoforms separated by ion-exchange chromatography exhibited almost identical kinetic characteristics, and it was not clear whether the isoforms represented the products of distinct fructokinase genes (Martinez-Barajas and Randall, 1996). The cDNAs encoding two divergent fructokinases (Frk1 and Frk2) in tomato have been isolated and shown to be differentially regulated (Kanayama et al., 1997; Martinez-Barajas et al., 1997).
A number of genes encoding enzymes involved in carbohydrate metabolism, including invertase, Suc synthase, Suc-P synthase, amylase, starch phosphorylase, adenosine 5′-diphospho Glc pyrophosphatase, and starch synthase, have been shown to be induced by sugar levels, and it has been proposed that their expression in sink tissues may be modulated by carbohydrate status (Muller-Rober et al., 1990; Visser et al., 1991; Koch et al., 1992; Quick and Schaffer, 1996). In maize Xu et al. (1996) showed differential patterns of expression of Suc synthase and invertase genes in the presence of Glc, and classified genes as sugar enhanced, sugar repressed, or starvation tolerant. Because fructokinase may function cooperatively with enzymes involved in starch biosynthesis, it is possible that fructokinase gene expression may also be responsive to carbohydrate status (Schaffer and Petreikov, 1997a). Fructokinase has been the only gene encoding an enzyme required for Suc-to-starch conversion that had not been demonstrated to be sugar responsive (Quick and Schaffer, 1996).
Here we describe experiments that assess the sugar regulation of fructokinase gene expression in tomato and provide enzymic characterization of two divergent fructokinase gene products from tomato. The results suggest that the two divergent tomato fructokinases may play distinct roles in sink metabolism.
MATERIALS AND METHODS
Yeast Transformation
Yeast (Saccharomyces cerevisiae) transformation and culture were as described in Dai et al. (1997). The yeast strain used was DFY632-MATa, ura3–52, hxk1::LEU2, hxk2::LEU2, glk1::LEU2, lys1–1, leu2–1 (Walsh et al., 1991). Yeast cells were grown on yeast extract-peptone-galactose medium, consisting of 1% yeast extract (Difco, Detroit, MI), 2% Bacto Peptone (Difco), and 110 mm (2%) Gal (Sherman et al., 1986). Selective media for URA auxotrophic growth (−URA, +sugar) contained 0.5% (NH4)2SO4, 0.17% yeast N2 base without amino acids (Difco), 0.2% casamino acids (Difco), 0.004% adenine (Sigma), 0.008% Trp (Sigma), and 110 mm of either Gal, Fru, or Glc.
The yeast shuttle vector pFL61, containing the URA3 gene as a selective marker and the constitutive phosphoglycerate kinase promoter and terminator (Minet et al., 1992), was used for transformation. A full-length Frk1 or Frk2 cDNA was cloned downstream of the phosphoglylcerate kinase promoter in pFL61 to yield pFL61-Frk1 and pFL61-Frk2. Yeast transformations were carried out by growing DFY632 cells in yeast extract-peptone-galactose liquid medium to mid-logarithmic phase, treating the cells with lithium acetate according to the method of Ito et al. (1983), and selecting for transformants on −URA, +Gal plates.
Protein Extraction and Fructokinase Activity
Protein extraction from yeast were carried out as described in Dai et al. (1997). DFY632 yeast cells transformed with either pFL61, pFL61-Frk2, or pFL61-Frk1 were grown in 40 mL of −URA, +Gal liquid medium for 72 h to approximately 5 × 107 cells mL−1. Cells were centrifuged for 5 min at 6,000 rpm, washed twice with water, and resuspended in 0.5 mL of water. Two-hundred-fifty milliliters of the cells was extracted twice with 500 mL of extraction buffer (50 mm Hepes pH 7.5, 1 mm EDTA, and 1 mm PMSF) by vortexing with 250 mL of glass beads. Following vortexing for 90 s, the mixture was centrifuged for 5 min at 12,000g at 4°C, and the supernatant was brought to 80% (NH4)2SO4 saturation. After centrifugation at 12,000g at 4°C, the pellet was resuspended in 0.5 mL of washing buffer (50 mm Hepes, pH 7.5, 1 mm EDTA, and 1 mm DTT), desalted on a G-25 Sephadex column, and used as the crude enzyme extract for subsequent enzymatic analysis.
Fructokinase activity was measured by an enzyme-linked assay, according to a modification of Huber and Akazawa (1985). Assays contained, in a total volume of 1 mL, 30 mm Hepes-NaOH (pH 7.5), 1 mm MgCl2, 0.6 mm EDTA, 9 mm KCl, 1 mm NAD, 1 mm ATP, 2 units of NAD-dependent Glc-6-P dehydrogenase, and 2 units of phosphoglucoisomerase. The reaction was initiated with Fru at concentrations ranging from 0.1 to 10 mm for Frk2 and to 50 mm for Frk1. Reactions were carried out at 37°C and A340 was monitored continuously.
Sugar Induction of Fructokinase mRNA Accumulation
Fully expanded cotyledons of tomato (Lycopersicon esculentum) seedlings were incubated in various sugar solutions (100 mm), and RNA was extracted from them as described by Mito et al. (1996) for gel-blot analysis. RNA gel-blot analysis was carried out as described by Kanayama et al. (1997) using total RNA obtained.
In Situ RNA Hybridization
The in situ hybridization was performed essentially as described by Van de Wiel et al. (1990). Tissue of tomato fruit of approximately 10 mm in diameter, harvested from greenhouse-grown plants, was fixed with 4% (w/v) paraformaldehyde and 0.25% (v/v) glutaraldehyde in 0.01 m sodium phosphate buffer, pH 7.2, at room temperature, dehydrated, and embedded in paraplast. Sections, 8 μm, attached to poly-1-Lys-coated slides were deparaffinized with xylene and rehydrated. They were subsequently pretreated with 1 μg mL−1 proteinase K in 100 mm Tris-HCl, pH 7.5, containing 50 mm EDTA at 37°C for 30 min, and with 0.1 m triethanolamine, pH 8.0, at room temperature for 10 min, following the addition of acetic anhydride to a final concentration of 0.25% (v/v). They were then dehydrated and dried under a vacuum until hybridization. The Frk1 35S-labeled sense and antisense RNA probes were synthesized with T7 and SP6 RNA polymerase from a SpeI- or EcoRV-linearized pCRII vector (Invitrogen, Carlsbad, CA) containing a partial Frk1 cDNA, as described in Kanayama et al. (1997). The Frk2 sense and antisense RNA probes were synthesized with T3 and T7 RNA polymerase from the XhoI- or SmaI-linearized pBluescript SK vector containing 911 nucleotides of the 5′ end of Frk2 cDNA, as described in Kanayama et al. (1997). The probes were partially degraded to 150 nucleotides by heating at 60°C in 0.2 m Na2CO3/0.2 m NaHCO3.
Sections were hybridized with RNA probes in 50% (v/v) formamide, 300 mm NaCl, 10 mm Tris-HCl, pH 7.5, 1 mm EDTA, 0.02% (w/v) Ficoll, 0.02% (w/v) PVP, 0.02% (w/v) BSA, 10% (w/v) dextran sulfate, 60 mm DTT, and 0.15 mg mL−1 yeast tRNA at 42°C for 16 h. After washing three times in 4× SSC and 5 mm DTT at room temperature, slides were treated with 50 μg mL−1 RNase A in 500 mm NaCl, 10 mm Tris-HCl, pH 7.5, 1 mm EDTA at 37°C for 30 min, and washed four times in the same buffer with 5 mm DTT at 37°C for 20 min. After a low-stringency wash in 2× SSC with 1 mm DTT at room temperature, the final wash consisted of 0.1× SSC with 1 mm DTT at 37°C. Slides were dehydrated in graded ethanol (each with 300 mm ammonium acetate) and 100% ethanol. After vacuum drying, slides were coated with NTB2 nuclear emulsion (Kodak), diluted 1:1 with 600 mm ammonium acetate, and exposed for 21 d for Frk1 or for 10 d for Frk2 at 4°C. They were developed in D19 developer (Kodak) for 5 min at 15°C and fixed (Kodak). Sections were stained with 2 g of KI and 1 g of I2 in 300 mL of water for 5 min and then in 0.02% (w/v) toluidine blue for 5 min.
RESULTS
Induction of Fructokinase mRNA Accumulation by Sugar
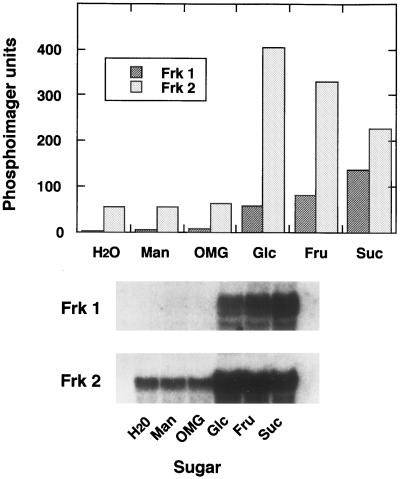
To assess the regulation of expression of the Frk1 and Frk2 genes, mRNA corresponding to each gene was assayed in cotyledons incubated in buffer or in the presence of various sugars. Both Frk1 and Frk2 mRNA abundance increased by the exogenous application of Glc, Fru, or Suc, although the abundance of Frk1 mRNA was consistently lower than that of Frk2 (Fig. 1). Frk1 mRNA levels were essentially undetectable in the absence of exogenous sugar treatment, whereas basal levels of Frk2 mRNA were readily detected (Fig. 1). Treatment with mannitol or 3-o-methylglucose did not influence the abundance of Frk1 and Frk2 mRNA, indicating that the sugar regulation of fructokinase gene expression is not due to the osmotic effects of sugar treatment, but is most likely related to cellular C metabolite status.
Figure 1.
Total RNA was isolated following the incubation in water or in 100 mm mannitol (Man), 3-o-methylglucose (OMG), Glc, Fru, or Suc for 15 h. After quantifying abundance of mRNA with a phosphor imager, the x-ray film was exposed for 12 and 2 d, respectively, for Frk1 and Frk2.
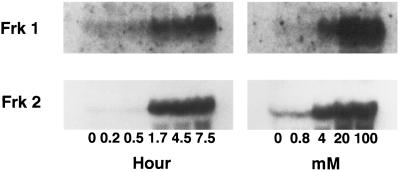
The time dependence of Frk1 and Frk2 mRNA accumulation in response to exogenous sugars was similar for both genes (Fig. 2). Frk1 and Frk2 mRNA increased markedly 1.7 h after the addition of 100 mm Glc, and then increased only slightly over the next 5 to 6 h. Starch accumulation in tissues exposed to Glc was not measured. Similarly, the concentration dependence of Frk1 and Frk2 mRNA accumulation was similar, with the expression of both genes significantly enhanced by 4 mm Glc and not repressed by up to 100 mm Glc, a concentration that suppressed maize invertase and Suc synthase levels (Koch et al., 1992; Xu et al., 1996). Based on these results, we conclude that both Frk1 and Frk2 gene expression is sugar regulated in cotyledon tissue and that this regulation appears to be coordinate for both genes.
Figure 2.
Total RNA was isolated from tomato cotyledons incubated with 100 mm Glc for varying periods or with varying concentrations of Glc for 15 h. The x-ray film was exposed for 12 and 2 d, respectively, for Frk1 and Frk2.
In Situ Localization of Frk1 and Frk2 Transcripts in Fruit Tissue
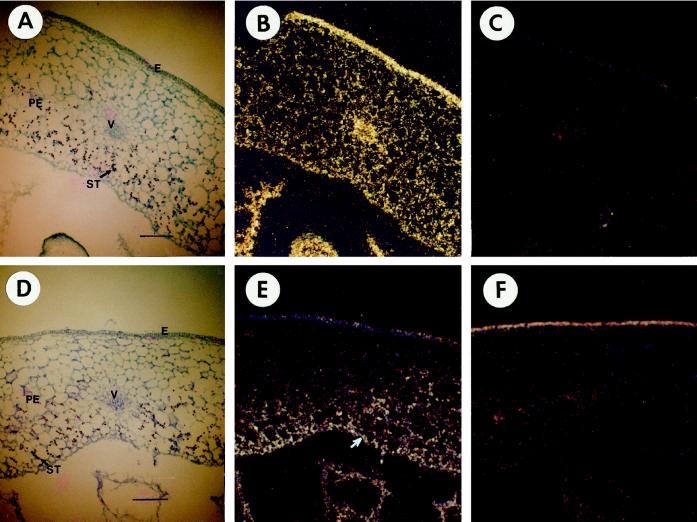
Because Frk1 and Frk2 appeared to be coordinately regulated by sugar, we also examined whether they showed the same spatial pattern of expression in developing fruit tissue. Young, green fruit of approximately 10 mm in diameter were harvested and used for in situ hybridization with both Frk1 and Frk2 hybridization probes. The localization of starch and mRNA for Suc synthase has already been reported at this stage of tomato fruit development (Wang et al., 1994). Because Frk2 mRNA is approximately 20-fold more abundant than Frk1 mRNA, sections to be hybridized with the Frk2 probe were exposed for 10 d and the sections hybridized with the Frk1 probes were exposed for 21 d. Starch granules at this stage of fruit development were also localized by staining with KI/I2 and were found to be localized in the inner cell layers of the pericarp wall (Fig. 3, A and D). It is interesting that Frk1 mRNA appeared to be distributed ubiquitously in all cells of the pericarp tissue (Fig. 3B), whereas Frk2 mRNA was most abundant in the inner cell layers of the pericarp wall (Fig. 3E). The spatial pattern of Frk2 mRNA accumulation was very similar to the spatial pattern of starch deposition. We previously showed that the developmental pattern of Frk2 mRNA accumulation closely paralleled the temporal pattern of starch accumulation in tomato fruit (Kanayama et al., 1997). Together, these results suggest that Frk2 gene expression may be both temporally and spatially coupled with starch accumulation, although the results do not rule out the possibility that the less-abundant Frk1 expression may contribute to the total fructokinase activity and to starch accumulation in these tissues.
Figure 3.
Cross-sections of fruit approximately 10 mm in diameter were hybridized with the Frk1 probe in B and C, and with the Frk2 probe in E and F. Antisense probes were used in B and E, and sense (control) probes were used in C and F. Hybridization signals are visible as white dots in dark-field microscopy of the sections. The arrows in E indicate the concentrated signals of Frk2 transcripts. Bright-field microscopy of the sections hybridized with the antisense or sense probe are shown in A and D. The dyes used in staining these sections were toluidine blue and KI/I2. Bars = 200 μm; PE, pericarp; E, epidermis; V, vascular tissue; and ST, starch granule.
Characterization of Frk1 and Frk2 Products Expressed in Yeast Cells
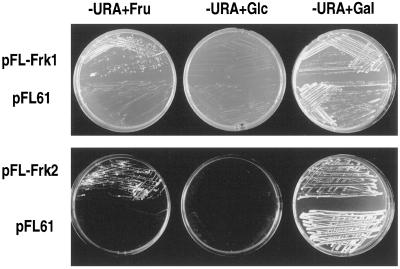
To assess the biochemical characteristics of the Frk1 and Frk2 isoforms of fructokinase, each cDNA was cloned into a yeast expression vector, pFL61, and expressed in DFY632, a yeast triple mutant that is unable to phosphorylate either Glc or Fru. We previously demonstrated that DFY632 cells expressing the tomato Frk1 cDNA were able to grow on Fru but not on Glc, indicating that Frk1 encodes a genuine fructokinase with a high specificity for Fru (data in top panel of Fig. 4 republished from Kanayama et al. [1997]). DFY632 cells expressing the tomato Frk2 cDNA (pFL-Frk2) are also able to grow on Fru, but not Glc, confirming that tomato Frk2, similar to Frk1, also encodes a genuine fructokinase (Fig. 4).
Figure 4.
The Frk1 and Frk2 cDNAs were subcloned into pFL61 and expressed in yeast cells. Yeast cells transformed with pFL61 were used as a control. Fru, Glc, or Gal (110 mm each) were added to selective media (−URA) for URA auxothrophic strains. The top panel illustrating the ability of pFL-Frk-1 to complement the yeast mutant was previously published (Kanayama et al., 1997) and is shown here for direct comparison with similar results using pFL-Frk2.
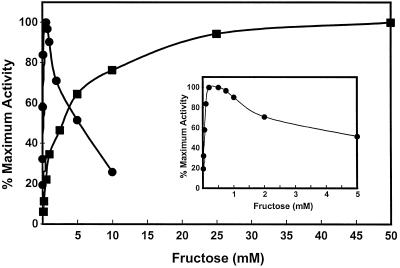
Fructokinase activity in crude protein extracts from both yeast lines expressing Frk1 and Frk2 cDNAs was measured at various concentrations of Fru (Fig. 5). The activity of both Frk1 and Frk2 obeyed Michaelis-Menten kinetics at substrate concentrations below 0.2 mm. However, Frk2 activity was inhibited by concentrations of Fru higher than 0.5 mm, whereas Frk1 activity was not inhibited by concentrations of Fru up to 50 mm. In addition, the affinity of Frk2 for Fru was much higher than that for Frk1, with the respective Km values for Fru of 1.3 mm (Frk1) and 0.054 mm (Frk2) calculated from Lineweaver-Burke plots.
Figure 5.
Relative activity was based on the maximal activity observed for each enzyme (166 nmol mg−1 protein min−1 for Frk1 at 50 mm Fru and 15 nmol mg−1 protein min−1 for Frk2 at 0.2 mm Fru). Inset shows an expanded axis for Frk2 activity over the low concentration range. Mutant yeast cells transformed with the plasmid vector alone did not show any Fru phosphorylation activity.
DISCUSSION
Isoforms of fructokinase previously have been separated by ion-exchange chromatography and characterized from a number of plant tissues, including tomato fruit (Martinez-Barajas and Randall, 1996). It is likely that the FKI and FKII described by Martinez-Barajas and Randall (1996) are both products of the Frk2 gene because both FKI and FKII were shown to be inhibited by Fru, similar to Frk2. The calculated molecular mass of Frk2 is 34.8 kD (Kanayama et al., 1997), which is close to the size of FKI and FKII (35 kD) (Martinez-Barajas and Randall, 1996, 1997). The differences in pI reported for FKI and FKII could be due to posttranslational modification of the Frk2 polypeptide, because it appears that Frk2 is encoded by a single gene in tomato (Kanayama et al., 1997; Martinez-Barajas and Randall, 1997).
Potato fructokinase can be separated into three isoforms by anion-exchange chromatography (Renz et al., 1993). The first two peaks of activity (FK1 and FK2) are high in sink tissues (tubers) but very low in leaves. In contrast, the third peak of activity (FK3) is similar in sink (tuber) and source (leaf) tissues and FK3 is not inhibited by Fru, as are the other isoforms (Gardner et al., 1992). The tomato Frk1 gene is similar to potato FK3 in that it is expressed in all organs (Kanayama et al., 1997) and Frk1 activity is not inhibited by Fru. Thus, FK3 in potato may correspond to Frk1 in tomato, and together they may represent a class of ubiquitous fructokinases distributed in both sink and source organs, which are not inhibited by high concentrations of Fru.
Invertase and Suc synthase, which metabolize Suc in the first step of C assimilation by sink tissues, are sugar-modulated genes (Koch et al., 1992; Xu et al., 1996). All of the genes encoding enzymes involved in the conversion of Suc to starch, except fructokinase, have been shown to be regulated by sugar levels (Quick and Schaffer, 1996). Here we have shown that expression of genes encoding fructokinase is also enhanced by the addition of exogenous sugar. The mRNAs encoding invertase, Suc synthase, and fructokinase can all be significantly increased by 4 to 10 mm sugar and are almost maximal at 20 to 30 mm sugar. These similar responses to sugar at the level of gene expression may serve to coordinate the metabolism of imported sugars and to initiate efficient Suc utilization in sink tissues. Some storage proteins and starch-related genes are also sugar modulated. However, these genes appear to be less sensitive to sugar than to fructokinases, which were induced within 1.7 h and showed maximum mRNA levels at 20 mm sugar. In contrast, patatin required 2 d and 300 to 400 mm sugar to induce maximal mRNA levels (Wenzler et al., 1989), and starch synthase and sporamin required approximately 200 mm sugar and at least 6 h to significantly induce mRNA levels (Nakamura et al., 1991; Visser et al., 1991). Our results suggest that enzymes involved in primary metabolism in sink tissue, such as fructokinase, are induced by comparatively low sugar concentrations and over shorter time periods than are genes related to storage-product biosynthesis. Because the sugar concentration in tomato fruit apoplast is more than 20 mm (Damon et al., 1988; Schaffer and Petreikov, 1997b), fructokinase gene expression may be maximally induced in tomato fruit tissues, and both Frk1 and Frk2 mRNAs are detectable at almost all the stages of fruit development (Kanayama et al., 1997). However, developmental expression patterns are quite different for Frk1 and Frk2 genes, suggesting that they are regulated during fruit development by other factor(s) in addition to sugar.
Frk1 mRNA is distributed ubiquitously in tomato pericarp cells and is present in fruit at a relatively constant level at all stages of fruit development (Kanayama et al., 1997). These results suggest that Frk1 may play a primary role in carbohydrate metabolism in all plant cells, essentially acting as a housekeeping enzyme supplying glycolysis with F6P. Frk2 mRNA is present at a very low abundance in leaves and increases to very high levels at the early stage of fruit development, a period of starch deposition (Kanayama et al., 1997). In situ hybridization showed that Frk2 mRNA was also localized in cells of inner pericarp, where starch storage is predominant. These data suggest that Frk2 may play a particularly important role in starch synthesis in tomato fruit. The hypothesis that fructokinase is important in starch synthesis in sink tissue has been proposed in tomato (Kanayama et al., 1997; Schaffer and Petreikov, 1997a) and in potato by Ross et al. (1994). Fructokinase and phosphoglucose isomerase may supply F6P to the starch biosynthetic pathway and may reduce Fru-inhibition of Suc synthase by decreasing cytosolic Fru accumulation. The role of fructokinase in contributing to starch biosynthesis has also been implicated in potato tubers, in which Fru is the preferred substrate, resulting in the low ratio of Fru to Glc (Davies and Oparka, 1985). The differential inhibition of Frk2 relative to Frk1 is also consistent with its coordinate action in starch biosynthesis with Suc synthase, an enzyme that shows a similar pattern of Fru inhibition.
The results presented here suggest that Frk1 and Frk2 do not represent redundant enzyme activities in tomato fruit but are likely to be active in vivo in different cells and under different physiological conditions, especially relative to tissue Fru concentration.
Abbreviation:
- URA
uracil
Footnotes
This research was supported by grants from the Binational Agricultural Research and Development Fund (no. US-2451-94) and the University of California BIO-STAR program (no. S96-17).
LITERATURE CITED
- Baysdorfer C, Kremer DF, Sicher RC. Partial purification and characterization of fructokinase activity from barley leaves. J Plant Physiol. 1989;134:156–161. [Google Scholar]
- Copeland L, Tanner GJ. Hexose kinases of avocado. Physiol Plant. 1988;74:531–536. [Google Scholar]
- Dai N, Schaffer A, Petreikov M, Granot D. Potato (Solanum tuberosum L.) fructokinase expressed in yeast exhibits inhibition by fructose of both in vitro enzyme activity and rate of cell proliferation. Plant Sci. 1997;128:191–197. [Google Scholar]
- Damon S, Hewitt J, Nieder M, Bennett AB. Sink metabolism in tomato fruit. II. Phloem unloading and sugar uptake. Plant Physiol. 1988;87:731–736. doi: 10.1104/pp.87.3.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies HV, Oparka KJ. Hexose metabolism in developing tubers of potato (Solanum tuberosum L.) cv Maris Piper. J Plant Physiol. 1985;119:311–316. [Google Scholar]
- Doehlert DC. Separation and characterization of four hexose kinases from developing maize kernels. Plant Physiol. 1989;89:1042–1048. doi: 10.1104/pp.89.4.1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu H, Park WD. Sink- and vascular-associated sucrose synthase functions are encoded by different gene classes in potato. Plant Cell. 1995;7:1369–1385. doi: 10.1105/tpc.7.9.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner A, Davies HV, Burch LR. Purification and properties of fructokinase from developing tubers of potato (Solanum tuberosum L.) Plant Physiol. 1992;100:178–183. doi: 10.1104/pp.100.1.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber SC, Akazawa T. A novel sucrose synthase pathway for sucrose degradation in cultured sycamore cells. Plant Physiol. 1985;81:1008–1013. doi: 10.1104/pp.81.4.1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito H, Fukada Y, Murata K, Kimura A. Transformation of intact yeast cells treated with alkali cations. J Bacteriol. 1983;153:163–168. doi: 10.1128/jb.153.1.163-168.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanayama Y, Dai N, Granot D, Petreikov M, Schaffer A, Bennett AB. Divergent fructokinase genes are differentially expressed in tomato. Plant Physiol. 1997;113:1379–1384. doi: 10.1104/pp.113.4.1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch KE, Nolte KD, Duke ER, McCarty DR, Avigne WT. Sugar levels modulate differential expression of maize sucrose synthase genes. Plant Cell. 1992;4:59–69. doi: 10.1105/tpc.4.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Barajas E, Luethy MH, Randall DD. Molecular cloning and analysis of fructokinase expression in tomato (Lycopersicon esculentum Mill.) Plant Sci. 1997;125:13–20. [Google Scholar]
- Martinez-Barajas E, Randall DD. Purification and characterization of fructokinase from developing tomato (Lycopersicon esculentum Mill.) fruits. Planta. 1996;199:451–458. doi: 10.1007/s004250050357. [DOI] [PubMed] [Google Scholar]
- Minet M, Dufour ME, Lacroute F. Complementation of Saccharomyces cerevisiae auxotrophic mutants by Arabidopsos thaliana cDNAs. Plant J. 1992;2:417–422. doi: 10.1111/j.1365-313x.1992.00417.x. [DOI] [PubMed] [Google Scholar]
- Mito N, Wimmers LE, Bennett AB. Sugar regulates mRNA abundance of H+-ATPase gene family members in tomato. Plant Physiol. 1996;112:1229–1236. doi: 10.1104/pp.112.3.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller-Rober BT, Kobmann J, Hannah LC, Willmitzer L, Sonnewald U. One of two different ADP-glucose pyrophosphorylase genes from potato responds strongly to elevated levels of sucrose. Mol Gen Genet. 1990;224:136–146. doi: 10.1007/BF00259460. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Ohto M, Yoshida N, Nakamura K. Sucrose-induced accumulation of β-amylase occurs concomitant with the accumulation of starch and sporamin in leaf-petiole cuttings of sweet potato. Plant Physiol. 1991;96:902–909. doi: 10.1104/pp.96.3.902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quick PW, Schaffer AA (1996) Sucrose metabolism in sinks and sources. In E Zamski, A Schaffer, eds, Photoassimilate Distribution in Plants and Crops. Marcel Dekker, New York, pp 115–156
- Renz A, Merlo L, Stitt M. Partial purification from potato tubers of three fructokinases and three hexokinases which show differing organ and developmental specificity. Planta. 1993;190:156–165. [Google Scholar]
- Renz A, Stitt M. Substrate specificity and product inhibition of different forms of fructokinases and hexokinases in developing potato tubers. Planta. 1993;190:166–175. [Google Scholar]
- Ross HA, Davies HV, Burch LR, Viola R, McRae D. Developmental changes in carbohydrate content and sucrose degrading enzymes in tuberising stolons of potato (Solanum tuberosum) Physiol Plant. 1994;90:748–756. [Google Scholar]
- Ruan YL, Patrick JW. The cellular pathway of postphloem sugar transport in developing tomato fruit. Planta. 1995;196:434–444. [Google Scholar]
- Schaffer AA, Petreikov M. Sucrose-to-starch metabolism in tomato fruit undergoing transient starch accumulation. Plant Physiol. 1997a;113:739–746. doi: 10.1104/pp.113.3.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffer AA, Petreikov M. Inhibition of fructokinase and sucrose synthase by cytological levels of fructose in young tomato fruit undergoing transient accumulation of starch. Physiol Plant. 1997b;110:800–806. doi: 10.1104/pp.113.3.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnarrenberger C. Characterization and compartmentation, in green leaves, of hexokinases with different specificities for glucose, fructose, and mannose and for nucleoside triphosphates. Planta. 1990;181:249–255. doi: 10.1007/BF02411547. [DOI] [PubMed] [Google Scholar]
- Sherman F, Fink GR, Hicks JB. Methods in Yeast Genetics: Laboratory Manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1986. [Google Scholar]
- Van de Wiel C, Scheres B, Franssen H, Van Lierop MJ, Van Lammeren A, Van Kammen A, Bisseling T. The early nodulin transcript ENOD2 is located in the nodule parenchyma (inner cortex) of pea and soybean root nodules. EMBO J. 1990;9:1–7. doi: 10.1002/j.1460-2075.1990.tb08073.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visser RGF, Stolte A, Jacobsen E. Expression of a chimaeric granule-bound starch synthase-GUS gene in transgenic potato plants. Plant Mol Biol. 1991;17:691–699. doi: 10.1007/BF00037054. [DOI] [PubMed] [Google Scholar]
- Walsh RB, Clifton D, Horak J, Fraenkel DG. Saccharomyces cerevisiae null mutants in glucose phosphorylation: metabolism and invertase expression. Genetics. 1991;128:7–12. doi: 10.1093/genetics/128.3.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Smith AG, Brenner ML. Temporal and spatial expression of pattern of sucrose synthase during tomato fruit development. Plant Physiol. 1994;104:535–540. doi: 10.1104/pp.104.2.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenzler HC, Mignery GA, Fisher LM, Park WD. Anaylsis of a chimeric class-I patatin-GUS gene in transgenic potato plants: high-level expression in tubers and sucrose-inducible expression in cultured leaf and stem explants. Plant Mol Biol. 1989;12:41–50. doi: 10.1007/BF00017446. [DOI] [PubMed] [Google Scholar]
- Wolosiuk RA, Pontis HG. Studies on sucrose synthase. Arch Biochem Biophys. 1974;165:140–145. doi: 10.1016/0003-9861(74)90151-9. [DOI] [PubMed] [Google Scholar]
- Xu J, Avigne WT, McCarty DR, Koch KE. A similar dichotomy of sugar modulation and developmental expression affects both paths of sucrose metabolism: Evidence from a maize invertase gene family. Plant Cell. 1996;8:1209–1220. doi: 10.1105/tpc.8.7.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]