Abstract
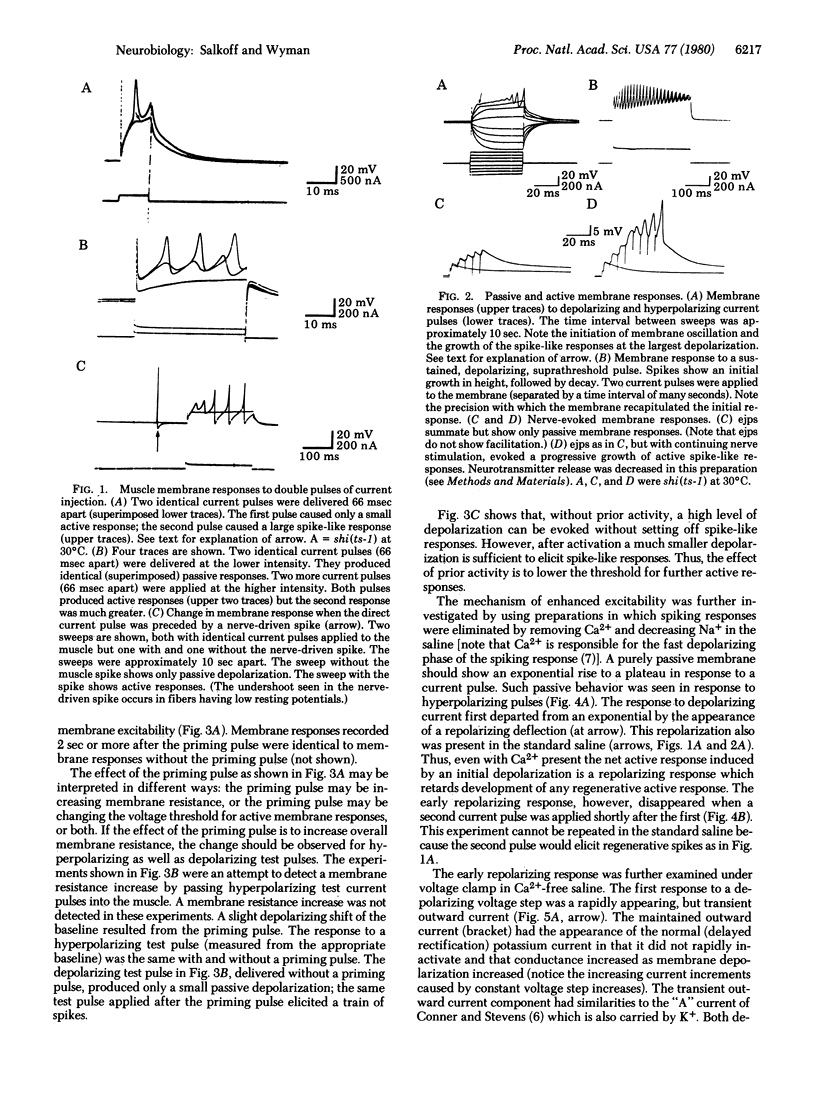
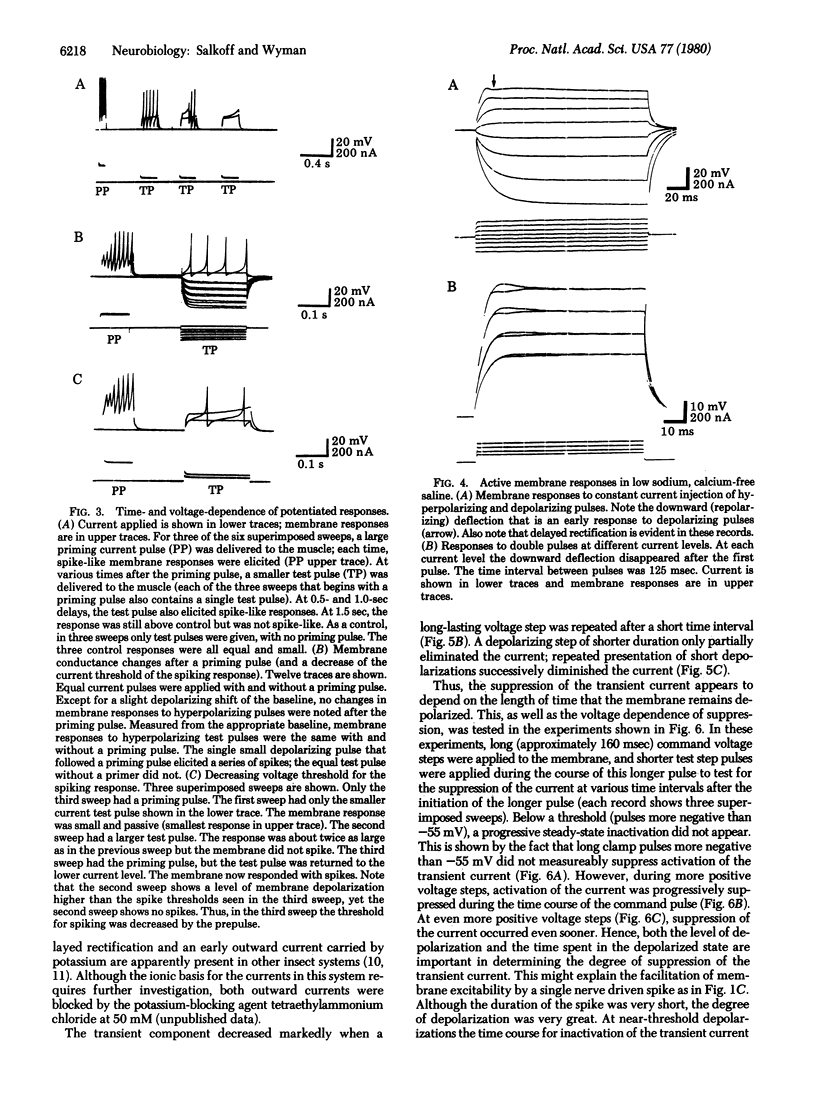
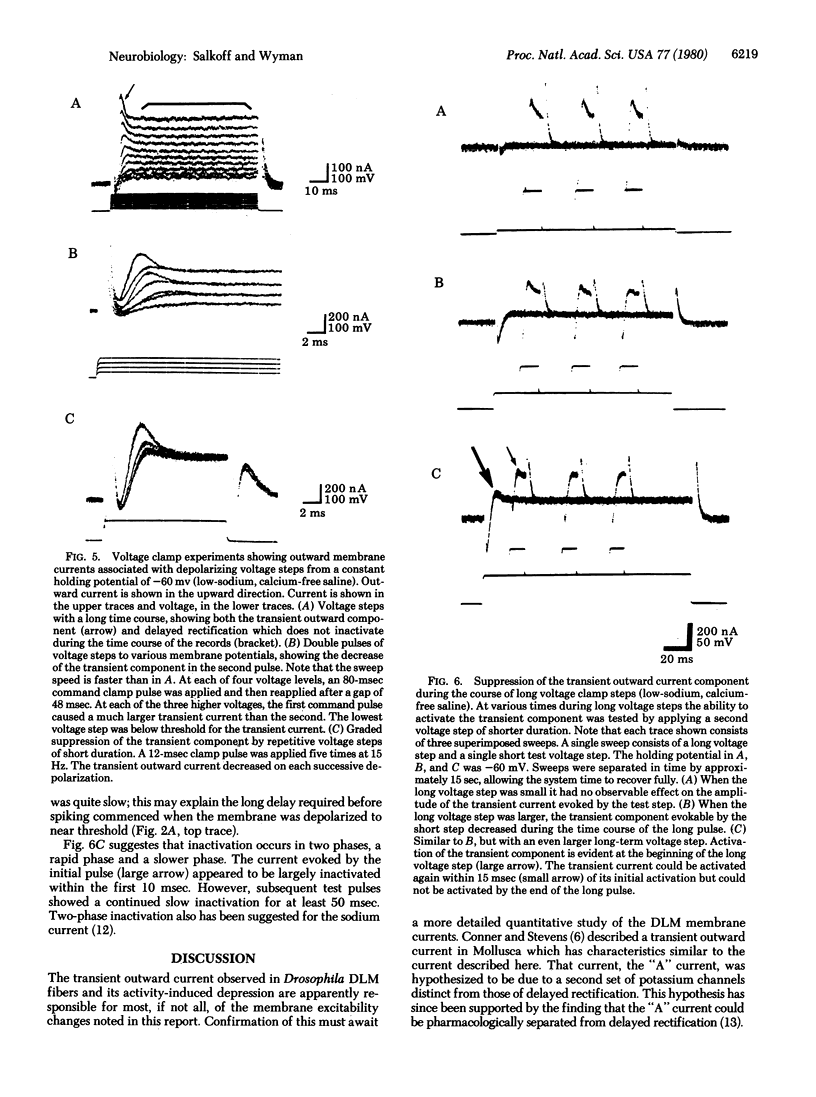
Prior electrical activity in the indirect flight muscles of Drosophila facilitates membrane excitability. The mechanism of facilitation involves the inactivation of an early, fast, transient outward current by prior membrane depolarizaton. In the facilitated state the calcium-dependent spike-like response has a decreased current and voltage threshold. The facilitated state persists for 1.5 sec after a membrane active response. A single nerve-driven spike is sufficient to facilitate membrane excitability.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aldrich R. W., Jr, Getting P. A., Thompson S. H. Mechanism of frequency-dependent broadening of molluscan neurone soma spikes. J Physiol. 1979 Jun;291:531–544. doi: 10.1113/jphysiol.1979.sp012829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne J. H., Shapiro E., Dieringer N., Koester J. Biophysical mechanisms contributing to inking behavior in Aplysia. J Neurophysiol. 1979 Sep;42(5):1233–1250. doi: 10.1152/jn.1979.42.5.1233. [DOI] [PubMed] [Google Scholar]
- Connor J. A., Stevens C. F. Prediction of repetitive firing behaviour from voltage clamp data on an isolated neurone soma. J Physiol. 1971 Feb;213(1):31–53. doi: 10.1113/jphysiol.1971.sp009366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor J. A., Stevens C. F. Voltage clamp studies of a transient outward membrane current in gastropod neural somata. J Physiol. 1971 Feb;213(1):21–30. doi: 10.1113/jphysiol.1971.sp009365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daut J. Modulation of the excitatory synaptic response by fast transient K+ current in snail neurones. Nat New Biol. 1973 Dec 19;246(155):193–196. doi: 10.1038/newbio246193a0. [DOI] [PubMed] [Google Scholar]
- Dionne V. E., Stevens C. F. Voltage dependence of agonist effectiveness at the frog neuromuscular junction: resolution of a paradox. J Physiol. 1975 Oct;251(2):245–270. doi: 10.1113/jphysiol.1975.sp011090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckert R., Lux H. D. Calcium-dependent depression of a late outward current in snail neurons. Science. 1977 Jul 29;197(4302):472–475. doi: 10.1126/science.17921. [DOI] [PubMed] [Google Scholar]
- Heyer C. B., Lux H. D. Properties of a facilitating calcium current in pace-maker neurones of the snail, Helix pomatia. J Physiol. 1976 Nov;262(2):319–348. doi: 10.1113/jphysiol.1976.sp011598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima S., Kusano K. Behavior of delayed current under voltage clamp in the supramedullary neurons of puffer. J Gen Physiol. 1966 Mar;49(4):613–628. doi: 10.1085/jgp.49.4.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salkoff L., Kelly L. Temperature-induced seizure and frequency-dependent neuromuscular block in a ts mutant of Drosophila. Nature. 1978 May 11;273(5658):156–158. doi: 10.1038/273156a0. [DOI] [PubMed] [Google Scholar]
- Schauf C. L., Davis F. A. Further studies of activation-inactivation coupling in Myxicola axons. Insensitivity to changes in calcium concentration. Biophys J. 1975 Nov;15(11):1111–1116. doi: 10.1016/S0006-3495(75)85887-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson S. H. Three pharmacologically distinct potassium channels in molluscan neurones. J Physiol. 1977 Feb;265(2):465–488. doi: 10.1113/jphysiol.1977.sp011725. [DOI] [PMC free article] [PubMed] [Google Scholar]



