Abstract
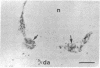
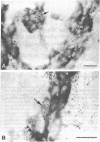
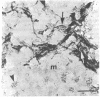
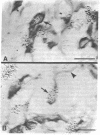
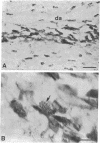
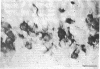
We sought to determine whether the precursors of catecholamine-containing neurons in the developing peripheral and central nervous systems of chickens and rats express the biosynthetic enzymes tyrosine hydroxylase [THase; tyrosine 3-monooxygenase; L-tyrosine, tetrahydropteridine: oxygen oxidoreductase (3-hydroxylating), EC 1.14.16.2] or dopamine beta-hydroxylase [DBHase; 3,4-dihydroxyphenylethylamine, ascorbate:oxygen oxidoreductase (beta-hydroxylating), EC 1.14.17.1], prior to the time they withdraw from the cell cycle. Chicken embryos (stages 26-27) were injected with [3H-thymidine and 4 hr later were prepared for the simultaneous demonstration of radioautographically labeled nuclei in immunoreactive THase cells. The brains and sympathetic chains of rat fetuses (days E12-E14), exposed for 2 hr to [3H]thymidine, were treated similarly except that peripheral tissues were stained with a specific antibody to DBHase as well as anti-THase. In the peripheral nervous system of both chicken and rat, nuclei of THase-containing cells were radioautographically labeled. DBHase-containing cells in the peripheral nervous system of rats were also labeled and thus are noradrenergic. THase was localized in cells of the brain of the same rat fetuses beginning on day E12 (no THase was detected on day E11 or E11.5) in the mantle layer of the ventral mesencephalic and rostrolateral rhombocephalic cellular groups; however. THase-containing cells in the central nervous system did not incorporate [3H]thymidine. We conclude that, during development, the adrenergic neuronal precursors of the peripheral nervous system but not of the central, have the capacity to synthesize catecholamines before they withdraw from the cell cycle. Differences in the maturation of peripheral and central neurons may be related to differences in their embryological origin.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altman J. Autoradiographic and histological studies of postnatal neurogenesis. 3. Dating the time of production and onset of differentiation of cerebellar microneurons in rats. J Comp Neurol. 1969 Jul;136(3):269–293. doi: 10.1002/cne.901360303. [DOI] [PubMed] [Google Scholar]
- Cochard P., Goldstein M., Black I. B. Initial development of the noradrenergic phenotype in autonomic neuroblasts of the rat embryo in vivo. Dev Biol. 1979 Jul;71(1):100–114. doi: 10.1016/0012-1606(79)90085-x. [DOI] [PubMed] [Google Scholar]
- ENEMAR A., FALCK B. OBSERVATIONS ON THE APPEARANCE OF NOREPINEPHRINE IN THE SYMPATHETIC NERVOUS SYSTEM OF THE CHICK EMBRYO. Dev Biol. 1965 Apr;11:268–283. doi: 10.1016/0012-1606(65)90060-6. [DOI] [PubMed] [Google Scholar]
- Eccles J. C. Neurogenesis and morphogenesis in the cerebellar cortex. Proc Natl Acad Sci U S A. 1970 Jun;66(2):294–301. doi: 10.1073/pnas.66.2.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FUJITA S. ANALYSIS OF NEURON DIFFERENTIATION IN THE CENTRAL NERVOUS SYSTEM BY TRITIATED THYMIDINE AUTORADIOGRAPHY. J Comp Neurol. 1964 Jun;122:311–327. doi: 10.1002/cne.901220303. [DOI] [PubMed] [Google Scholar]
- Hanaway J., McConnell J. A., Netsky M. G. Histogenesis of the substantia nigra, ventral tegmental area of Tsai and interpeduncular nucleus: an autoradiographic study of the mesencephalon in the rat. J Comp Neurol. 1971 May;142(1):59–73. doi: 10.1002/cne.901420105. [DOI] [PubMed] [Google Scholar]
- Hendry I. A. Cell division in the developing sympathetic nervous system. J Neurocytol. 1977 Jun;6(3):299–309. doi: 10.1007/BF01175193. [DOI] [PubMed] [Google Scholar]
- Joh T. H., Geghman C., Reis D. Immunochemical demonstration of increased accumulation of tyrosine hydroxylase protein in sympathetic ganglia and adrenal medulla elicited by reserpine. Proc Natl Acad Sci U S A. 1973 Oct;70(10):2767–2771. doi: 10.1073/pnas.70.10.2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurecka W., Lassmann H., Hörandner H. The proliferation of adrenal medullary cells in newborn and adult mice. A light and electron microscopic autoradiographic study. Cell Tissue Res. 1978 May 29;189(2):305–312. doi: 10.1007/BF00209279. [DOI] [PubMed] [Google Scholar]
- Kirby M. L., Gilmore S. A. A correlative histofluorescence and light microscopic study of the formation of the sympathetic trunks in chick embryos. Anat Rec. 1976 Nov;186(3):437–449. doi: 10.1002/ar.1091860309. [DOI] [PubMed] [Google Scholar]
- Lauder J. M., Bloom F. E. Ontogeny of monoamine neurons in the locus coeruleus, Raphe nuclei and substantia nigra of the rat. I. Cell differentiation. J Comp Neurol. 1974 Jun 15;155(4):469–481. doi: 10.1002/cne.901550407. [DOI] [PubMed] [Google Scholar]
- Olson L., Seiger A. Early prenatal ontogeny of central monoamine neurons in the rat: fluorescence histochemical observations. Z Anat Entwicklungsgesch. 1972;137(3):301–316. doi: 10.1007/BF00519099. [DOI] [PubMed] [Google Scholar]
- Pickel V. M., Joh T. H., Field P. M., Becker C. G., Reis D. J. Cellular localization of tyrosine hydroxylase by immunohistochemistry. J Histochem Cytochem. 1975 Jan;23(1):1–12. doi: 10.1177/23.1.234988. [DOI] [PubMed] [Google Scholar]
- Pickel V. M., Joh T. H., Reis D. J. Immunohistochemical localization of tyrosine hydroxylase in brain by light and electron microscopy. Brain Res. 1975 Feb 28;85(2):295–300. doi: 10.1016/0006-8993(75)90084-0. [DOI] [PubMed] [Google Scholar]
- Pickel V. M., Joh T. H., Reis D. J. Monoamine-synthesizing enzymes in central dopaminergic, noradrenergic and serotonergic neurons. Immunocytochemical localization by light and electron microscopy. J Histochem Cytochem. 1976 Jul;24(7):792–306. doi: 10.1177/24.7.8567. [DOI] [PubMed] [Google Scholar]
- Ross R. A., Joh T. H., Reis D. J. Reversible changes in the accumulation and activities of tyrosine hydroxylase and dopamine-beta-hydroxylase in neurons of nucleus locus coeruleus during the retrograde reaction. Brain Res. 1975 Jul 4;92(1):57–72. doi: 10.1016/0006-8993(75)90527-2. [DOI] [PubMed] [Google Scholar]
- Rothman T. P., Gershon M. D., Holtzer H. The relationship of cell division to the acquisition of adrenergic characteristics by developing sympathetic ganglion cell precursors. Dev Biol. 1978 Aug;65(2):322–341. doi: 10.1016/0012-1606(78)90030-1. [DOI] [PubMed] [Google Scholar]
- SIDMAN R. L., MIALE I. L., FEDER N. Cell proliferation and migration in the primitive ependymal zone: an autoradiographic study of histogenesis in the nervous system. Exp Neurol. 1959 Oct;1:322–333. doi: 10.1016/0014-4886(59)90024-x. [DOI] [PubMed] [Google Scholar]
- Seiger A., Olson L. Late prenatal ontogeny of central monoamine neurons in the rat: Fluorescence histochemical observations. Z Anat Entwicklungsgesch. 1973 Aug 30;140(3):281–318. doi: 10.1007/BF00525058. [DOI] [PubMed] [Google Scholar]
- Specht L. A., Pickel V. M., Joh T. H., Reis D. J. Immunocytochemical localization of tyrosine hydroxylase in processes within the ventricular zone of prenatal rat brain. Brain Res. 1978 Nov 10;156(2):315–321. doi: 10.1016/0006-8993(78)90511-5. [DOI] [PubMed] [Google Scholar]
- Teitelman G., Baker H., Joh T. H., Reis D. J. Appearance of catecholamine-synthesizing enzymes during development of rat sympathetic nervous system: possible role of tissue environment. Proc Natl Acad Sci U S A. 1979 Jan;76(1):509–513. doi: 10.1073/pnas.76.1.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WESTON J. A. A radioautographic analysis of the migration and localization of trunk neural crest cells in the chick. Dev Biol. 1963 Jun;6:279–310. doi: 10.1016/0012-1606(63)90016-2. [DOI] [PubMed] [Google Scholar]