Abstract
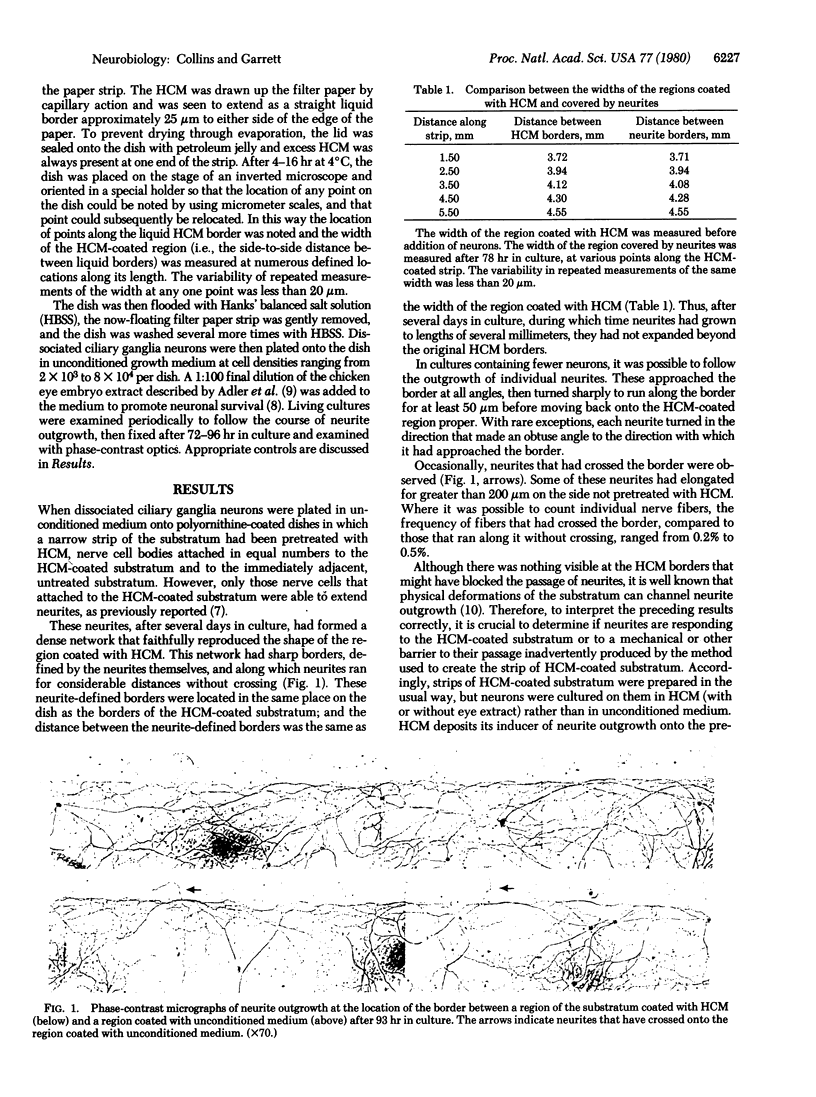
Dissociated parasympathetic neurons rapidly initiate neurite outgrowth when exposed to culture medium previously conditioned by the growth of embryonic heart cells. The inducer of neurite outgrowth in the conditioned medium is a substratum-conditioning factor; that is, it does not act in a soluble form, but acts only when bound to the nerve cell culture substratum. When a sharp border is created between a region of the substratum coated with this factor and a region coated with unconditioned medium, neurites fail to cross this border; rather, they change their direction of outgrowth so as to remain on the conditioned substratum. Thus, long after the initiation of outgrowthhas been induced, elongating neurites continue to respond to the substratum-conditioning factor in a manner that allows their outgrowth to be channeled along a pathway of this neurotropic substratum-associated material.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler R., Landa K. B., Manthorpe M., Varon S. Cholinergic neuronotrophic factors: intraocular distribution of trophic activity for ciliary neurons. Science. 1979 Jun 29;204(4400):1434–1436. doi: 10.1126/science.451576. [DOI] [PubMed] [Google Scholar]
- Collins F. Axon initiation by ciliary neurons in culture. Dev Biol. 1978 Jul;65(1):50–57. doi: 10.1016/0012-1606(78)90178-1. [DOI] [PubMed] [Google Scholar]
- Collins F. Induction of neurite outgrowth by a conditioned-medium factor bound to the culture substratum. Proc Natl Acad Sci U S A. 1978 Oct;75(10):5210–5213. doi: 10.1073/pnas.75.10.5210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins F. Neurite outgrowth induced by the substrate associated material from nonneuronal cells. Dev Biol. 1980 Sep;79(1):247–252. doi: 10.1016/0012-1606(80)90089-5. [DOI] [PubMed] [Google Scholar]
- Grinnell F. Cellular adhesiveness and extracellular substrata. Int Rev Cytol. 1978;53:65–144. doi: 10.1016/s0074-7696(08)62241-x. [DOI] [PubMed] [Google Scholar]
- Helfand S. L., Smith G. A., Wessells N. K. Survival and development in culture of dissociated parasympathetic neurons from ciliary ganglia. Dev Biol. 1976 Jun;50(2):541–547. doi: 10.1016/0012-1606(76)90174-3. [DOI] [PubMed] [Google Scholar]
- Letourneau P. C. Cell-to-substratum adhesion and guidance of axonal elongation. Dev Biol. 1975 May;44(1):92–101. doi: 10.1016/0012-1606(75)90379-6. [DOI] [PubMed] [Google Scholar]
- Letourneau P. C. Possible roles for cell-to-substratum adhesion in neuronal morphogenesis. Dev Biol. 1975 May;44(1):77–91. doi: 10.1016/0012-1606(75)90378-4. [DOI] [PubMed] [Google Scholar]
- Ludueña M. A. Nerve cell differentiation in vitro. Dev Biol. 1973 Aug;33(2):268–284. doi: 10.1016/0012-1606(73)90137-1. [DOI] [PubMed] [Google Scholar]
- Patrick J., Heinemann S., Schubert D. Biology of cultured nerve and muscle. Annu Rev Neurosci. 1978;1:417–443. doi: 10.1146/annurev.ne.01.030178.002221. [DOI] [PubMed] [Google Scholar]
- Schubert D., LaCorbiere M., Whitlock C., Stallcup W. Alterations in the surface properties of cells responsive to nerve growth factor. Nature. 1978 Jun 29;273(5665):718–723. doi: 10.1038/273718a0. [DOI] [PubMed] [Google Scholar]