Abstract
The human hydroxycarboxylic acid receptor 2 (HCA2), also known as GPR109A and HM74a, was first identified as a niacin receptor and has recently received significant attention because of its potential to clinically modify plasma lipids in a favorable manner. Our recent studies have demonstrated that the niacin-induced internalization of HCA2 receptors is regulated by G protein-coupled receptor kinase (GRK) 2 and arrestin3 and that internalized receptors rapidly recycle back to the cell surface. The investigation presented here used a combination of amino acid deletion and site-directed mutagenesis to identify structural and functional domains within the HCA2 C terminus and explore their potential roles in receptor phosphorylation, desensitization, and internalization. We first constructed four mutants with deletions of 10 to 15 amino acids each that were distinct from truncated mutants. We successfully identified different domains responsible for receptor export, constitutive activity, desensitization, phosphorylation, and internalization. We also generated a comprehensive series of alanine substitution mutants, replacing conserved serine and threonine residues in the C terminus with alanine residues to pinpoint the key residues that are essential for GRK2-mediated phosphorylation and arrestin3 association. Moreover, we found that a sequence from residues 329 to 343 in the C-terminal tail of HCA2 plays a crucial role in keeping HCA2 in an inactive conformation. These data demonstrate the importance of distinct domains within the C terminus of HCA2 for receptor cell surface expression, desensitization, and internalization and phosphorylation and stabilization of an inactive receptor conformation.
Introduction
The high-affinity human G protein-coupled receptor for niacin (nicotinic acid), hydroxycarboxylic acid receptor 2 (HCA2), also known as GPR109A or HM74a, has drawn significant attention (Soga et al., 2003; Tunaru et al., 2003; Wise et al., 2003). HCA2 receptor activation not only lowers total plasma cholesterol, free fatty acid, and triglyceride levels but also significantly increases high-density lipoprotein cholesterol levels and thus offers a clinical treatment for dyslipidemia and atherosclerosis (Mack et al., 1993; Taylor et al., 2004). In 2003, the human orphan G protein-coupled receptor, HM74a, was identified as a niacin receptor (Soga et al., 2003; Tunaru et al., 2003; Wise et al., 2003). Two years later, the ketone body, 3-hydroxybutyrate, was found to be an endogenous ligand for HCA2 (Taggart et al., 2005). HCA2 is predominantly expressed in adipose tissue. Upon binding to niacin, HCA2 is coupled to the Gi protein and lowers cAMP levels in adipocytes through inhibition of adenylyl cyclase, resulting in decreased hormone-sensitive lipase activity and reduced hydrolysis of triglycerides to free fatty acids (Soga et al., 2003; Tunaru et al., 2003; Wise et al., 2003). Several studies have indicated that niacin-induced flushing is also mediated by HCA2 through the release of prostaglandin D2 and prostaglandin E2 (Benyó et al., 2005; Meyers et al., 2007). In addition, new nonflushing drugs with low toxicity based on the pharmacological effects of niacin that act via HCA2 are in development to treat dyslipidemia and prevent cardiovascular disease (Kamanna and Kashyap, 2007). Richman et al. (2007) reported that agonists that fail to significantly activate ERK1/2 phosphorylation and do not cause rapid receptor internalization do not lead to a flushing response. Therefore, an understanding of the regulation of HCA2 signaling and trafficking is important for the potential development of pharmacotherapeutic agents with antilipolytic activity that are also nonflushing.
Our previous studies have demonstrated that the membrane-bound Gβγ subunit is dissociated from Gi upon activation of HCA2 by niacin. This is followed by recruitment of GRK2 to catalyze phosphorylation of the activated receptors. GRK2-mediated phosphorylation promotes the binding of arrestin3, targeting receptors to clathrin-coated pits and causing their internalization (Li et al., 2010). Using arrestin2/3-specific siRNAs and an internalization-deficient HCA2 mutant, we found that arrestin2 and arrestin3 are not involved in HCA2-mediated ERK1/2 activation (Li et al., 2011). In addition, mutagenesis and modeling studies have shown that the arginine residue at position 111 in transmembrane helix (TM) 3, together with Phe180 (in extracellular loop 2) and Phe276 and Tyr284 (both present in TM7) are responsible for HCA2 binding with niacin (Tunaru et al., 2005). Structural studies of different members of the GPCR family have demonstrated that the C-terminal tails of the rhodopsin-like GPCRs are functionally important for facilitating association of the receptor with various cellular proteins and thereby regulating receptor desensitization, endocytosis, and intracellular fate (Ferguson, 2001; Moore et al., 2007). However, for the HCA2 receptor, the role of the C-terminal tail in modulating agonist-mediated desensitization and internalization and the underlying mechanisms governing these processes remain unknown.
In this study, we attempted to delineate the mechanisms regulating HCA2 receptor desensitization and internalization. We also attempted to identify structural motifs within the C-terminal HCA2 domains that are responsible for downstream signaling pathway activation and direct interactions between the HCA2 receptor and the intracellular sorting machinery. We demonstrated for the first time that several distinct domains within the C-terminal tail of HCA2 are responsible for the regulation of HCA2 receptor export, phosphorylation, desensitization, and internalization.
Materials and Methods
Materials.
Lipofectamine 2000 and G418 were purchased from Invitrogen (Carlsbad, CA). Cell culture media and fetal bovine serum were obtained from HyClone (Beijing, China). The pCMV-FLAG, pCDNA3.1, and pEGFP-N1 vectors were purchased from Sigma-Aldrich (St. Louis, MO), Invitrogen, and Clontech (Mountain View, CA), respectively. pDsRed2-ER was a gift from Dr. Tao Xu (Institute of Biophysics, Academy of Sciences, Beijing, China). 1,1′-Dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate (DiI) was purchased from Beyotime (Haimen, China). Monoclonal anti-FLAG M2 antibody, monoclonal anti-FLAG M2-FITC antibody, and horseradish peroxidase (HRP)-conjugated anti-mouse IgG were obtained from Sigma-Aldrich. Anti-phospho-ERK1/2 (Thr202/Tyr204), ERK1/2 antibodies, and HRP-conjugated anti-rabbit IgG were obtained from Cell Signaling Technology (Danvers, MA).
Cell Culture and Transfection.
HEK-293 cells were maintained in Dulbecco's modified Eagle's medium supplemented with 10% heat-inactivated fetal bovine serum and 4 mM l-glutamine. Plasmid constructs were transfected or cotransfected into HEK-293 cells using Lipofectamine 2000 according to the manufacturer's instructions. All cells were incubated at 37°C in a humidified chamber with 5% CO2 and 95% air.
Molecular Cloning and Plasmid Construction.
For fluorescence detection experiments, the cDNA of the HCA2 receptor was C-terminally fused to the cDNA of enhanced green fluorescent protein (EGFP). To perform enzyme-linked immunosorbent assays (ELISA) for quantification of receptor expression and internalization, the cDNA of the HCA2 receptor was inserted between the HindIII and EcoRI sites of the pCMV-FLAG vector as described previously (Li et al., 2010). Mutations and truncations were introduced by overlap extension polymerase chain reaction. All constructs were sequenced to verify sequences and orientations. The arrestin3-EGFP was generated as described previously (Li et al., 2011).
Measurement of Cell Surface and Total Cellular Receptor Levels by ELISA.
To assess levels of cell surface receptors, cells transiently transfected with either wild-type (WT) or mutant FLAG-HCA2 cDNA constructs were fixed with 3.7% formaldehyde in Tris-buffered saline (TBS) for 5 min, washed three times with TBS, and then blocked for 1 h with 1% BSA/TBS. Cells were then incubated at room temperature with anti-FLAG M2 antibody (1:1000) diluted in TBS (1% BSA) for 1 h, probed with HRP-labeled secondary antibody, and detected by chemiluminescence using HRP substrate (Sigma-Aldrich). Development was stopped by removing 0.1 ml of the substrate to a 96-well microtiter plate containing 0.1 ml of 1% SDS. Plates were read at 405 nm in a microplate reader (Bio-Rad Laboratories, Hercules, CA) using Microplate Manager software. The HRP background absorbance from cells transfected with untagged receptor cDNA was subtracted from the total absorbance to determine final absorbance values. To measure total cellular receptor levels, cells were permeabilized with methanol for 20 min at −20°C after fixation and before incubation with the anti-FLAG (M2) antibody.
Quantitative Measurement of Cell Surface Receptor Internalization.
Cell surface receptor internalization was quantitatively assessed by ELISA as described previously (Li et al., 2010).
Measurement of Receptor Localization and Internalization by Confocal Imaging.
To measure receptor localization, HEK-293 cells were transiently transfected with WT or mutant HCA2-EGFP with and without pDsRed2-ER. On the following day, cells were seeded in covered, glass-bottomed six-well plates and then fixed with 3% paraformaldehyde for 15 min. For the internalization assay, HEK-293 cells stably expressing WT or mutant HCA2-EGFP were treated with 200 μM niacin for 1 h. After removal of the agonist, cells were fixed with 3% paraformaldehyde for 15 min. Confocal images were taken on a Zeiss LSM 510 microscope with an attached Axiovert 200 microscope and LSM5 computer system.
Immunofluorescence Microscopy.
HEK-293 cells were transfected with WT or mutant FLAG-HCA2. Then cells were incubated with anti-FLAG-FITC antibody (1:250 dilution) for 1 h at 4°C in DMEM supplemented with 1% BSA. Cells were washed twice with PBS and then were incubated in media alone (vehicle) or with 200 μM niacin for 30 min at 37°C. Cells were then fixed with 3% paraformaldehyde-PBS for 15 min, and receptor-antibody complexes were visualized by confocal microscopy.
cAMP Accumulation.
HEK-293 cells transiently cotransfected with HCA2 or HCA2 mutants and pCRE-Luc were grown to 90 to 95% confluence in DMEM without fetal bovine serum. Cells were then stimulated with 10 μM forskolin alone or 10 μM forskolin with different concentrations of niacin and incubated for 4 h at 37°C. Luciferase activity was detected using a firefly luciferase kit (Promega, Madison, WI). For the receptor desensitization assay, HEK-293 cells transiently transfected with HCA2 or HCA2 mutants were pretreated with water (control) or 200 μM niacin for 1 h, washed three times with ice-cold PBS, and stimulated with 10 μM forskolin and 200 μM niacin for 20 min. The reaction was stopped by a quick wash with ice-cold PBS followed by the addition of cell lysis buffer. The cAMP concentration was assessed using a commercially available cAMP detection kit (R&D Systems, Minneapolis, MN).
Synthesis of siRNAs and siRNA Transfection.
All arrestins and clathrin siRNAs were chemically synthesized by Dharmacon RNA Technologies (Lafayette, CO). The transfection protocols for arrestin and clathrin siRNAs were reported previously (Li et al., 2010). Forty-eight hours after transfection, cells were split for the indicated assay on the following day.
Immunoblotting and Immunoprecipitation Assay.
Phosphorylated ERK immunoblotting using the antibody anti-phospho-ERK1/2 (diluted 1:2000; Cell Signaling Technology) was performed as described previously (Zhou et al., 2012). Total ERK1/2 was detected with anti-ERK1/2 (diluted 1:2000; Cell Signaling Technology). Clathrin was detected using mouse monoclonal antibody anti-clathrin HC (TD.1, diluted 1:2000; Santa Cruz Biotechnology, Inc., Santa, Cruz, CA). For immunoprecipitation, cells were treated with serum-free media with or without niacin. Cells were washed once with PBS at 4°C, harvested by gentle scraping, pelleted, and resuspended in glycerol lysis buffer including complete protease inhibitor. Lysates were normalized for equal protein concentrations and immunoprecipitated with conjugated M2 beads (Sigma-Aldrich). Immunoprecipitation reactions were incubated at 4°C for 12 h, washed three times with glycerol lysis buffer, and resuspended in SDS running buffer. Samples were subjected to SDS-PAGE analysis and Western blotting with anti-clathrin HC (TD.1) antibody. The blots were stripped and reprobed using mouse monoclonal anti-FLAG antibody (1:1000) as a control for protein loading.
Arrestin Translocation Assay.
The fluorescence detection experiment of arrestin translocation was performed as described previously (Li et al., 2011). The method of quantitative arrestin3 membrane translocation was described previously (Li et al., 2010).
Analysis of Receptor Phosphorylation by Gel Shift Analysis.
Plasmid constructs of Flag-tagged HCA2 receptor wild-type or STS/A mutant were transiently transfected into HEK-293 cells and stably selected using G418. Cells grown in six-well plates were incubated in Dulbecco's modified Eagle's medium for 24 h at 37°C to reach ∼90% confluence. Cells were stimulated with water (control) or 200 μM niacin for 10 min, washed twice with ice-cold PBS and then with 0.3 ml of ice-cold lysis buffer containing 150 mM NaCl, 20 mM Hepes, pH 7.2, 5 mM EDTA, and 1% Triton X-100, and one complete EDTA-free protease inhibitor cocktail tablet (Roche, Mannheim, Germany) was added. The plates were rocked for 30 min at 4°C, and supernatants were collected by centrifugation at 12,000g for 20 min at 4°C. The samples were subjected to electrophoresis on a 10% polyacrylamide gel for 2 h at 120 V and then transferred to nitrocellulose. After blocking with 5% milk, blots were probed for HCA2 receptor with 1:500 anti-Flag mouse antibody (M2 antibody; Sigma-Aldrich), HRP-conjugated anti-mouse secondary antibody, and chemiluminescent detection.
Results
Expression and Functional Characterization of C-Terminal Deletion Mutants of Human HCA2.
Multiple domains and motifs within the C-terminal tail of GPCRs have been implicated in the regulation of receptor expression, dimerization, internalization, trafficking, and signaling (Pankevych et al., 2003; Levoye et al., 2006; Delhaye et al., 2007; Labasque et al., 2008; McCulloch et al., 2008). We constructed an expression vector containing the HCA2 receptor fused with EGFP at the carboxyl terminus for direct visualization of HCA2 receptor membrane localization and trafficking. To identify the functional domains within HCA2 that regulate receptor localization, internalization, desensitization, and trafficking, we constructed a series of C-terminal deletion mutants of human HCA2 (Fig. 1a). We first examined the functional signaling of HCA2-EGFP by assaying cAMP accumulation. As shown in Supplemental Fig. 1, treatment of HEK-293 cells that transiently transfected HCA2-EGFP with niacin inhibited forskolin-stimulated cAMP production comparably to that in cells expressing FLAG-HCA2. These results demonstrate that a fusion of the C terminus of HCA2 receptors to EGFP functions similarly to the untagged HCA2 receptor.
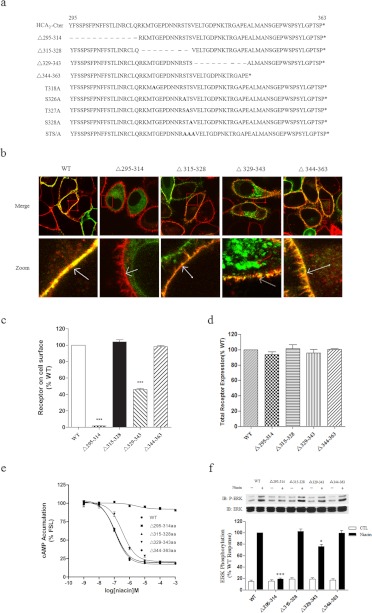
Fig. 1.
Expression and functional characterization of C-terminal deletion mutants of human HCA2. a, sequence comparison of the COOH domains of the wild-type and mutant HCA2 constructs. A series of mutants were made in the carboxyl terminus of HCA2 using overlap polymerase chain reaction as described under Materials and Methods. The deleted amino acids are indicated with a dash (–). b, HEK-293 cells stably expressing WT or mutant HCA2-EGFP (green) were stained with DiI (5 μM, red), fixed, and examined by confocal microscopy. c and d, HEK-293 cells transiently transfected with WT or mutant FLAG-HCA2 were used to assess cell surface receptor levels and total cell receptor levels by ELISA (see Materials and Methods). e, cAMP accumulation in HEK-293 cells transiently cotransfected with HCA2 or HCA2 mutants and pCRE-Luc was determined in response to treatment with forskolin and niacin. f, activation of ERK1/2 in HEK-293 cells stably expressing HCA2 or HCA2 mutants by a challenge with 200 μM niacin for 5 min. Error bars represent the S.E.M. for four replicates. Data were analyzed using Student's t test (*, p < 0.05; ***, p < 0.001). All data shown are representative of at least three independent experiments. IB, immunoblot; CTL, control.
Confocal microscopy revealed that HCA2 and the Δ315–328 and Δ344–363 mutants were expressed and colocalized with the membrane marker DiI with minimal intracellular accumulation. In contrast, there was nearly no expression of the Δ295–314 construct, and only half of the expressed Δ329–343 construct colocalized with DiI (Fig. 1b). The same result was also observed by ELISA analysis (Fig. 1c). The decrease in cell surface expression was not simply attributable to deficient cellular expression of the mutant receptor proteins, because similar total cellular levels of HCA2 wild-type protein and all mutant proteins with C-terminal deletions were detected by ELISA analysis after cell permeabilization (Fig. 1d). These data suggest that the C-terminal tail of HCA2 is important for the correct localization of the HCA2 receptor in the plasma membrane.
We next examined whether signaling of the HCA2 C-terminal deletion mutants was functional by assaying CRE-driven luciferase activity and ERK1/2 activation. As shown in Fig. 1, e and f, the Δ315–328 and Δ343–363 mutants functioned identically to the intact HCA2 receptor. However, the Δ329–343 mutant showed decreased activity in the inhibition of forskolin-induced luciferase activity, whereas the Δ295–314 mutant exhibited complete loss in agonist-induced inhibition of forskolin-stimulated CRE-driven luciferase activity (Fig. 1e). Identical results were observed for ERK1/2 activation (Fig. 1f). Taken together, these data suggest that the C-terminal tail of HCA2 plays an important role in the regulation of receptor cell surface localization but not in the modulation of receptor expression and signaling.
The C-Terminal Domain between Residues Tyr295 and Gln314 Plays an Essential Role in the Regulation of HCA2 Export from the Endoplasmic Reticulum to the Cell Surface.
To determine whether the stably expressed Δ295–314 mutant undergoes internalization upon agonist binding, HEK-293 cells stably transfected with HCA2-EGFP or Δ295–314-EGFP were incubated with 200 μM niacin for 40 min at 37°C and examined by confocal microscopy. As shown in Fig. 2a, wild-type HCA2 receptors were internalized from the cell surface into the cytoplasm with a punctate distribution upon activation by niacin, whereas the Δ295–314 mutant was still localized primarily in the cytoplasm.
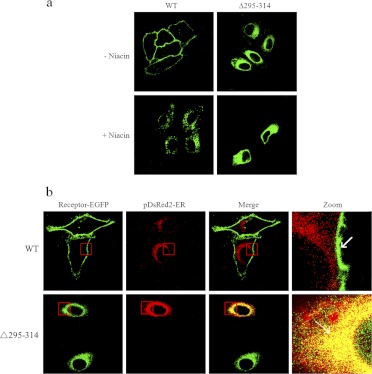
Fig. 2.
The C-terminal domain between residues Y295 and Q314 plays an essential role in the regulation of HCA2 export from the endoplasmic reticulum to the cell surface. a, HEK-293 cells stably expressing EGFP-tagged wild type HCA2 (HCA2-EGFP) or Δ295–314 (Δ295–314-EGFP) were stimulated with 200 μM niacin for 40 min. b, HEK-293 cells were transiently cotransfected with HCA2-EGFP or Δ295–314-EGFP (green) and with pDsRed2-ER (red). After 24 h, cells were fixed and examined by confocal microscopy as described under Materials and Methods. All images are representative of at least three independent experiments.
We proceeded to further investigate the intracellular compartments into which the Δ295–314 mutant was retained. The wild-type and Δ295–314 mutant were coexpressed with the endoplasmic reticulum (ER) marker DsRed2-ER, and their respective colocalization was analyzed by confocal microscopy. As anticipated, the wild type showed no colocalization with DsRed2-ER. In contrast, the Δ295–314 mutant was extensively colocalized with DsRed2-ER (Fig. 2b). These results are consistent with previous studies showing that the F(X)6LL motif in the C termini of GPCRs is essential for the export of receptors from the ER to the cell surface (Duvernay et al., 2004, 2009) and suggesting that the F(X)6LI motif may function as a signal dictating the ER export of HCA2.
The C-Terminal Ser/Thr cluster of 326STS328 Is Involved in the Regulation of Arrestin Recruitment, Receptor Internalization and Phosphorylation, and Signaling Desensitization of HCA2.
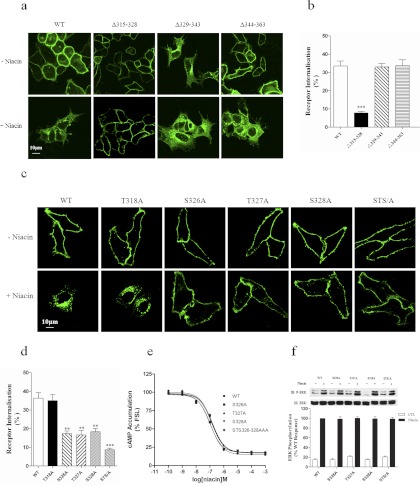
We next used confocal imaging and ELISA analysis to assess the ability of HCA2 and HCA2 mutants with C-terminal deletions to undergo agonist-induced internalization in stably transfected HEK-293 cells. As shown in Fig. 3, a and b, after niacin stimulation (200 μM), both the Δ329–343 and Δ344–363 mutants displayed agonist-induced internalization comparable to that of the wild-type receptor, whereas the Δ315–328 mutant exhibited a significant decrease in niacin-mediated internalization. The domain between residues 315 and 328 seems to play a critical role in HCA2 internalization. These results, together with the potent inhibition of receptor internalization caused by GRK2 knockdown (Li et al., 2010), suggest that the serine and threonine residues within this domain may serve as GRK phosphorylation sites and may be critical for arrestin binding and agonist-induced receptor internalization. To address the potential functional role of the serine and threonine residues within this domain, we constructed site-directed mutants that substituted alanines for the serine and threonine residues within this domain. These mutants were then stably transfected into HEK-293 cells. The replacement of threonine 318 with alanine resulted in a loss of inhibition of niacin-induced internalization compared with that for wild-type HCA2. However, the niacin-induced loss of cell surface receptors in HEK-293 cells expressing the S326A, T327A, or S328A mutants was significantly decreased, and the STS/A mutant exhibited an ∼75% decrease in receptor internalization compared with that for wild-type HCA2 (Fig. 3, c and d). Although the S326A, T327A, S328A, and STS/A mutants were resistant to agonist-induced rapid internalization, there were no differences in CRE-driven luciferase activity or niacin-induced ERK1/2 phosphorylation between these mutants and the wild-type HCA2 (Fig. 3, e and f).
Fig. 3.
The C-terminal Ser/Thr cluster of 326TST328 is involved in the regulation of receptor internalization. HEK-293 cells stably expressing HCA2 or HCA2 mutants were stimulated with 200 μM niacin for 1 h and examined with confocal microscopy (a and c) or ELISA (b and d). e, cAMP accumulation in HEK-293 cells transiently cotransfected with HCA2 or HCA2 mutants and with pCRE-Luc was determined in response to treatment with forskolin and niacin. The dose-dependent inhibition of forskolin-induced cAMP accumulation in cells expressing HCA2 or HCA2 mutants was measured. f, Activation of ERK1/2 after a 5-min challenge with 200 μM niacin was assessed by Western blot in HEK-293 cells stably expressing HCA2 or HCA2 mutants starved in serum-free DMEM media for 2 h as described under Materials and Methods. Error bars represent S.E.M.s for four replicates. Data were analyzed using Student's t test (**, p < 0.01, *** p < 0.001). All data shown are representative of at least three independent experiments. FSL, forskolin; IB, immunoblot; CTL, control.
Our previous studies have demonstrated that niacin-activated HCA2 is phosphorylated by GRK2 followed by binding of arrestin3 at the C-terminal tail, which targets HCA2 receptors to clathrin-coated pits and causes their internalization in HEK-293-HCA2 cells (Li et al., 2010). To further investigate the interaction of these HCA2 mutants with arrestin3, we cotransfected HCA2 wild-type, Δ315–328, STS/A, and Δ329–343 stably expressing HEK-293 cells with arrestin3-EGFP and visualized the translocation of the latter after agonist-mediated receptor activation. In the absence of niacin, arrestin3 was localized primarily in the cytoplasm in both the wild-type and mutant cells (Fig. 4a). Stimulation with niacin resulted in robust recruitment of arrestin3-GFP to the cell membrane in the wild-type HCA2-expressing cells (Fig. 4a), but no redistribution of arrestin3-GFP was observed upon agonist activation in the Δ315–328, STS/A, and Δ329–343 mutant-expressing cells.
Fig. 4.
The C-terminal Ser/Thr cluster of 326TST328 is involved in regulation of arrestin3 recruitment and desensitization of HCA2. a, HEK-293 cells stably expressing HCA2 or HCA2 mutants were transiently transfected with arrestin3-EGFP. Then cells were treated with 200 μM niacin for 8 min, and confocal images were taken on a Zeiss LSM 510 microscope. b, HEK-293 cells transfected with Flag-HCA2 or STS/A mutant were stimulated with 200 μM niacin for 10 min. An equal volume of whole-cell lysate was separated by 10% SDS-polyacrylamide gel electrophoresis, and receptor phosphorylation was detected by the mobility shift of receptor with anti-Flag antibody as described under Materials and Methods. c, HEK-293 cells stably expressing HCA2, Δ315–328, or STS/A mutant were pretreated with water (control) or 200 μM niacin for 1 h, washed three times with ice-cold PBS, and stimulated with 10 μM forskolin and 200 μM niacin for 20 min. The reaction was stopped by a quick wash with ice-cold PBS followed by the addition of cell lysis buffer. The cAMP concentration was assessed using a commercially available kit. Data were analyzed using Student's t test (**, p < 0.01). All images and data are representative of at least three independent experiments.
Thus, the STS/A mutant exhibited a significant decrease in niacin-mediated internalization and arrestin3 translocation. These results, together with the potent inhibition of receptor internalization caused by GRK2 knockdown (Li et al., 2010), suggest that the serine and threonine residues within this domain may serve as GRK2 phosphorylation sites. To address this hypothesis, we transfected HEK-293 cells with either HCA2 wild-type or STS/A mutant receptor and analyzed receptor phosphorylation by gel shift analysis. As shown in Fig. 4b, in the absence of niacin, both wild-type and STS/A mutant HCA2 receptors migrate as a major band of ∼43 kDa. Treatment of cells expressing the wild-type receptor with 200 μM niacin resulted in a significant mobility shift of the receptor with a few slower migrating bands being observed. In striking contrast, this agonist-promoted shift was not observed in cells expressing the STS/A mutant HCA2 receptor. We attribute this mobility shift to agonist-promoted phosphorylation of the receptor, which is lost in the STS/A mutant.
Next, we further evaluated the agonist-induced desensitization of these HCA2 mutants. Cells were pretreated with vehicle (control) or with 200 μM niacin for 1 h, washed, and incubated with 200 μM niacin and 10 μM forskolin for 20 min. cAMP accumulation was then determined using a commercially available kit. Compared with wild-type HCA2, the Δ315–328 and STS/A mutants were impaired in agonist-induced rapid receptor desensitization (Fig. 4c). Taken together, our findings strongly suggest that the serine/threonine cluster of residues from 326 to 328 plays an essential role in receptor internalization and phosphorylation, desensitization, and association with arrestin3.
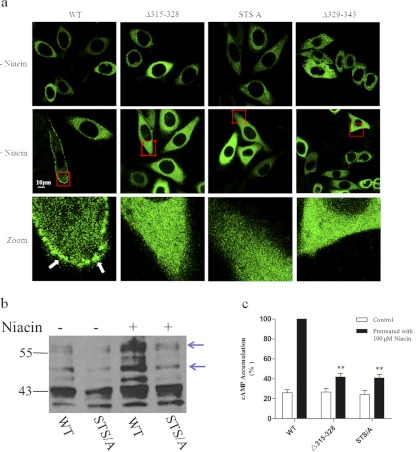
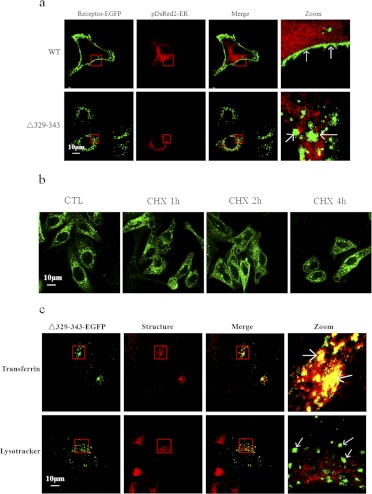
Deletion of the C-Terminal Domain from Val329 to Glu343 Leads to Constitutive Activation of HCA2.
As shown above, the data obtained from confocal microscopy and ELISA analysis showed that only half of the expressed Δ329–343 mutant protein localized to the plasma membrane. To determine the intracellular compartments to which the intracellular Δ329–343 mutant was localized, the Δ329–343 mutant was coexpressed with the ER marker DsRed2-ER, and their colocalization was analyzed. Of interest, the Δ329–343 mutant receptor did not significantly colocalize with DsRed2-ER in the cytoplasm (Fig. 5a). To evaluate the primary source of intracellular Δ329–343 receptors, the distribution of Δ329–343-EGFP was monitored after treatment (1, 2, and 4 h) with cycloheximide (100 μg/ml), an inhibitor of protein synthesis. This treatment failed to induce a significant reduction of intracellular Δ329–343 mutant (Fig. 5b). In addition, we analyzed the labeling of early endosomes and lysosomes in cycloheximide-pretreated Δ329–343-expressing cells. As shown in Fig. 5c, intracellular Δ329–343 mutants do not colocalize significantly with degradative pathways (lysosomes). However, a substantial proportion of intracellular Δ329–343 did colocalize with transferrin-positive early endosomes. These data suggest that the majority of intracellular Δ329–343 mutant protein localizes to early and recycling endosomes and originates from constitutive endocytosis, not from the accumulation of newly synthesized proteins.
Fig. 5.
Cytoplasmic localization of the Δ329–343 mutant receptor. a, HEK-293 cells were transiently cotransfected with HCA2-EGFP or Δ329–343-EGFP (green) and pDsRed2-ER (red). After 24 h, cells were fixed and examined by confocal microscopy. b, HEK-293 cells stably expressing Δ329–343-EGFP were treated (1, 2, and 4 h) with cycloheximide (CHX; 25 μM). c, HEK-293 cells stably expressing Δ329–343-EGFP (green) were incubated with either 100 μg/ml Alexa Fluor 546-labeled transferrin (red) or 50 nM LysoTracker DND-99 (red) for 40 min. All images are representative of at least three independent experiments. CTL, control.
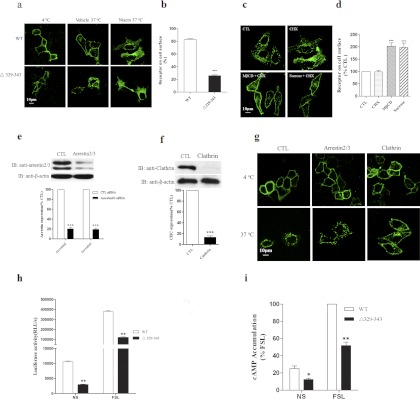
To further assess whether the decreased cell surface expression of the Δ329–343 mutant protein in the absence of agonists is caused by constitutive endocytosis, HEK-293 cells stably expressing either the FLAG-tagged wild-type HCA2 receptor or the FLAG-tagged Δ329–343 mutant (the FLAG-HCA2 receptor or the FLAG-Δ329–343 mutant) were incubated at 4°C with monoclonal anti-FLAG-FITC (M2) antibody (1:250) for 1 h in DMEM with 1% BSA. After washing three times with PBS, cells were incubated in media alone (vehicle) or with 200 μM niacin for 30 min at 37°C. Receptor-antibody complexes were then visualized by confocal microscopy. When endocytosis was inhibited by incubating cells at 4°C, the majority of the immunofluorescent signal from the wild-type and Δ329–343 mutant receptors remained at the cell surface. After a 30-min incubation in media alone at 37°C, the majority of the immunofluorescent signal from the Δ329–343 mutant receptors was detected within the cell, whereas the signal from the wild-type receptors remained predominantly associated with the plasma membrane (Fig. 6a). However, upon stimulation with niacin, both the wild-type and mutant receptors appeared to traffic to a similar perinuclear compartment within the cell (Fig. 6a). No detectable immunofluorescence was observed when cells were transfected with pCMV-FLAG vector alone (data not shown). Similar results were also obtained by ELISA analysis (Fig. 6b).
Fig. 6.
Deletion of the C-terminal domain from Val329 to Glu343 leads to constitutive activation of HCA2. a and b, HEK-293 cells stably expressing Flag-HCA2 or Flag-Δ329–343 were prelabeled with anti-FLAG-FITC (a) or anti-FLAG (b) antibody (1:250 dilution) for 1 h at 4°C. Cells were next incubated in media alone (vehicle) or with 200 μM niacin for 30 min at 37°C. Receptor-antibody complexes were then analyzed by confocal microscopy (a) or ELISA (b). c and d, HEK-293 cells stably expressing Δ329–343-EGFP or FLAG-Δ329–343 were incubated in media alone or with cycloheximide (CHX) or with cycloheximide and MβCD (5 mM) or sucrose (450 mM) for 2 h and then were analyzed by confocal microscopy (c) or ELISA (d). e and f, arrestin and clathrin expression was measured as described under Materials and Methods. g, The role of arrestin2/3 and clathrin in the regulation of Δ329–343 constitutive endocytosis was measured by confocal microscopy. h, HEK-293 cells transiently cotransfected with HCA2 or Δ329–343 mutant and pCRE-Luc were stimulated with media alone or with 10 μM forskolin for 4 h at 37°C. Luciferase activity was detected by a firefly luciferase kit. i, HEK-293 cells stably expressing wild-type HCA2 or Δ329–343 mutant were stimulated with media alone or with 10 μM forskolin for 20 min. The cAMP concentration was assessed with a commercially available kit. Data were analyzed by using Student's t test (*, p < 0.05; **, p < 0.01; ***, p < 0.001). All images and data shown are representative of at least three independent experiments. IB, immunoblot; CTL, control.
If receptors are constitutively endocytosed, a reasonable prediction is that blocking endocytosis without interfering with recycling will lead to receptor accumulation at the plasma membrane. We performed experiments to block endocytosis with sucrose (450 mM) or methyl-β-cyclodextrin (MβCD) (5 mM), both of which inhibit clathrin-mediated endocytosis but do not affect intracellular receptor recycling or the steady-state distribution of clathrin (Subtil et al., 1999; Paing et al., 2002; Leterrier et al., 2004; Xia et al., 2004). After incubation with sucrose or MβCD for 2 h, the amount of Δ329–343-EGFP at the cell surface appeared to increase with a concomitant decrease in intracellular Δ329–343-EGFP (Fig. 6c). The same results were observed by ELISA (Fig. 6d), indicating that the formation of the intracellular receptor pool results from constitutive endocytosis.
The role of arrestin2/3 and clathrin in the regulation of constitutive endocytosis of the Δ329–343 mutant was investigated using specific siRNAs to knock down the expression of arrestin2/3 and clathrin in Δ329–343 stably expressing HEK-293 cells. The endogenous expression of arrestin2/3 or clathrin was effectively and specifically knocked down by the specific siRNA treatment but was unaffected in cells treated with nonspecific or control siRNA (Fig. 6, e and f). Silencing clathrin but not arrestin2/3 effectively inhibited ligand-independent endocytosis of Δ329–343 as analyzed by microscopy (Fig. 6g). Taken together, these data suggest that Δ329–343 undergoes constitutive endocytosis in a clathrin-dependent and arrestin2/3-independent pathway.
To further evaluate whether the Δ329–343 mutant is constitutively active in receptor signaling, we examined the ligand-independent levels of cAMP in cells expressing the mutant or the wild-type receptor. HEK-293 cells transiently cotransfected with the HCA2 wild-type or Δ329–343 mutant receptor and pCRE-Luc were grown to 90 to 95% confluence and stimulated with dimethyl sulfoxide alone or 10 μM forskolin in DMEM without fetal bovine serum. After incubation for 4 h at 37°C, luciferase activity was detected. We found that expression of the Δ329–343 mutant resulted in significantly lower basal and forskolin-stimulated cAMP levels (P < 0.01) than expression of the wild-type receptor (Fig. 6h). This result was confirmed using a nonradioactive competitive binding cAMP kit (Fig. 6i), indicating that the Δ329–343 mutant is constitutively active in Gi-coupled signaling. Our findings suggest that a sequence between residues 329 and 343 within the C-terminal tail of HCA2 probably plays a crucial role in keeping HCA2 in an inactive conformation.
Discussion
The rapid internalization or sequestration of receptors into intracellular membrane compartments after agonist activation is important for the regulation of GPCR signaling. Our previous results indicated the involvement of GRK2 and arrestin3 in the regulation of HCA2 receptor internalization. Intracellular GPCR domains, particularly the C terminus, are generally thought to regulate receptor desensitization, internalization, and intracellular trafficking by facilitating association of the receptor with various cellular proteins. To understand more about the structural requirements for the regulation of HCA2 internalization and desensitization, we first constructed four mutants, each with a deletion of 10 to 15 amino acids at the C-terminal tail, which were distinct from progressive truncated mutants. We used these mutants to successfully identify different domains responsible for receptor export, constitutive activity, desensitization, and internalization. A comprehensive series of mutants with alanine substitutions replacing conserved serine and threonine residues within the C-terminal tail was also generated to pinpoint the key residues essential for GRK2-mediated phosphorylation and arrestin3 association.
A regulatory mechanism shared by most GPCRs involves GRK-mediated phosphorylation of the C terminus of the receptor followed by arrestin binding, which leads to receptor internalization (Lefkowitz and Shenoy, 2005). Prior studies have examined the role of the carboxyl-terminal tail and putative domains for GRK phosphorylation in the regulation of GPCR internalization. A previous study has demonstrated that a cluster of serine/threonine residues in the C-terminal tail is essential for receptor internalization and desensitization, serving as targets for agonist-induced phosphorylation and association of the arrestin adaptor proteins (Oakley et al., 2001). A bradykinin B2 receptor mutant with four alanine substitutions in a serine/threonine cluster in the C-terminal tail has also been shown to be deficient in arrestin binding and in receptor internalization (Zimmerman et al., 2011). Furthermore, substitution of serines 355, 356, and 364 of the β2-adrenergic receptor to alanines reduces GRK-mediated C-terminal phosphorylation by at least 90% and almost completely eliminates receptor internalization (Seibold et al., 2000). In the current study, using a series of mutants with C-terminal deletions of 15 to 20 amino acids out of a total of 69 residues, we showed that the sequence from Arg315 to Ser328 (Δ315–328) is required for agonist-mediated receptor phosphorylation and internalization. Further mutagenesis studies using alanine substitution defined the three residues (Ser326, Thr327, and Ser328) responsible for GRK-mediated phosphorylation and internalization. We previously showed that HCA2 internalization can be triggered by arrestin3, but not by arrestin2, using siRNA-mediated knockdown (Li et al., 2010). Our findings lead to the conclusion that, upon activation by niacin, GRK2 mediates the phosphorylation of Ser326, Thr327, and Ser328 residues in the carboxyl tail of HCA2. This then facilitates arrestin3 binding to the receptor, resulting in receptor internalization by targeting receptors to clathrin-coated pits. The recruitment of arrestins to phosphorylated receptors results in attenuation of the GPCR response by uncoupling receptors from heterotrimeric G proteins. This mechanism of GPCR desensitization is important in allowing cells to avoid agonist overstimulation. It is generally accepted that both the second messenger-dependent protein kinases, PKA and PKC, and GRKs are involved in the phosphorylation of serine and threonine residues within the intracellular loops and C-terminal tail domains of GPCRs (Ferguson, 2001). For GPCRs, phosphorylation of the PKA and PKC sites within the third intracellular loop and the carboxyl-terminal tail has been found to contribute to agonist-dependent desensitization (Yuan et al., 1994; Moffett et al., 1996; Liang et al., 1998; Tang et al., 1998), whereas mutation of all of the serine and threonine residues within the C-terminal tail of the β2-adrenergic receptor and the third intracellular loop of the m2 muscarinic acetylcholine receptor prevents the induction of GRK-mediated desensitization (Bouvier et al., 1988; Nakata et al., 1994). Results from our previous mutagenesis experiments demonstrated that a sequence of 14 amino acids, from residues 315 to 328 (Δ315–328) at the C-terminal end of the HCA2 receptor is responsible for GRK-mediated phosphorylation and arrestin recruitment and internalization (Li et al., 2011). In the present study, mutant receptors with C-terminal deletions between Arg315 to Ser328 (Δ315–328) or with alanine substitutions for the three Ser/Thr residues in this region (Ser326, Thr327, and Ser328) not only exhibited deficiencies in arrestin3 binding and receptor internalization and phosphorylation (Figs. 3, c and d, and 4, a and b) but also exhibited reduced agonist-induced desensitization during the inhibition of forskolin-induced cAMP accumulation (Fig. 4c). These results are consistent with our previous observation that the Δ315–328 mutant exhibited sustained activation of ERK1/2 from 10 to 30 min compared with wild-type HCA2 (Li et al., 2011). From our current data, we cannot exclude a potential role for PKA and PKC in regulating HCA2 desensitization. However, GRK-mediated phosphorylation of Ser326, Thr327, and Ser328 is also likely to contribute to the agonist-dependent desensitization of the HCA2 receptor.
Another interesting observation of the current study was that mutant HCA2 receptors with a 15-amino acid C-terminal deletion from Val329 to Glu343 showed significant constitutive endocytosis (Fig. 6, a and b) compared with wild-type HCA2. Moreover, this ligand-independent internalization of the Δ329–343 mutant underwent a clathrin-dependent and arrestin2/3-independent pathway (Fig. 6g). It is now well established that GPCRs can be activated spontaneously or constitutively in the absence of an agonist. The first observation of constitutive GPCR activity was obtained for the δ-opioid receptor (Koski et al., 1982) and the β2-adrenoceptor (Cerione et al., 1984). During the past decades, more than 60 GPCRs have been shown to be constitutively active in both recombinant cells and native cells (Costa and Herz, 1989; Cotecchia et al., 1990; Smit et al., 2007). In addition, naturally occurring mutant GPCRs with constitutive activities greater than wild-type activity are responsible for a variety of human diseases (Seifert and Wenzel-Seifert, 2002). Considerable evidence has accumulated in recent years to suggest that specific sequences in the C-terminal regions of GPCRs play a role in mediation of constitutive receptor activity. Deletion of the last several residues of the thyrotropin-releasing hormone receptor and the prostaglandin E receptor results in constitutive receptor activity (Matus-Leibovitch et al., 1995; Hasegawa et al., 1996), and shorter C-terminal variants of 5-HT4 receptors have also been shown to have greater constitutive activity (Claeysen et al., 1999). In addition, deletion of the C-terminal regions of the β-adrenergic receptor (Parker and Ross, 1991) and the CB1 cannabinoid receptor (Nie and Lewis, 2001) promotes their constitutive activity. Mutation of the proximal palmitoylation site C404S of the 5-HT7(a) receptor leads to agonist-independent constitutive activity (Kvachnina et al., 2009). A sequence within the C-terminal tail, upstream of the Leu358 residue, that is rich in serine and threonine residues was also shown to be important for maintaining 5-HT4 receptors in an inactive conformation (Claeysen et al., 1999). However, it is quite likely that the sequence of 15 amino acids from Val329 to Glu343 of HCA2 plays a crucial role in constraining the HCA2 receptor in an inactive conformation in the absence of an agonist.
The export trafficking and precise localization of GPCRs on the cell surface play critical roles in the functional response of GPCRs to ligand binding, and extensive efforts have been made to elucidate the molecular mechanisms underlying the export trafficking of GPCRs. Studies have suggested that GPCRs are exported from the ER and that transport to the Golgi and the cell surface is tightly regulated at multiple steps (Dong et al., 2007). We have demonstrated that the mutant HCA2 receptor lacking the C-terminal region between Tyr295 and Gln314 fails to export from the ER to the cell surface. This finding is consistent with the previous observation that the membrane-proximal portion of the C terminus is required for ER export for a number of GPCRs, including the α2B-adrenergic, dopamine D1, and angiotensin II type 1 receptors (Gáborik et al., 1998; Bermak et al., 2001; Duvernay et al., 2004). Mutagenesis studies have also identified several motifs, such as E(X)3LL, F(X)3F(X)3F, and FN(X)2LL(X)3L, that are essential for GPCR export from the ER (Dong et al., 2007). With use of progressive truncation strategies and alanine-scanning mutagenesis, Phe436, Ile443, and Leu444 in the C terminus of the α2B-adrenergic receptor and Phe309, Leu316, and Leu317 in the C terminus of the angiotensin II type 1 receptor have been shown to be critical for receptor export from the ER (Duvernay et al., 2004). In the present study, we have shown that Phe311, Leu318, and Ile319 within the membrane-proximal C terminus of the human HCA2 receptor also seem likely to play a critical role in regulating receptor export from the ER to the cell surface.
We conclude that several distinct C-terminal domains within the C-terminal tail of the human HCA2 receptor directly link this receptor to interaction with the intracellular sorting machinery and to the phosphorylation, internalization, and desensitization pathways. Our findings provide a structural and mechanistic basis for better understanding the regulation of desensitization and internalization of GPCRs.
Supplementary Material
Acknowledgments
We thank Aiping Shao, Ming Ding, and Hanmin Chen for their technical assistance and equipment usage.
The online version of this article (available at http://molpharm.aspetjournals.org) contains supplemental material.
This work was supported by the Ministry of Science and Technology [Grants 2012CB910402, 2012AA020303-05]; National Natural Science Foundation of China [Grant 81173106]; Zhejiang Natural Science Foundation [Grant Z2080207]; and National Institutes of Health National Institute of General Medical Sciences [Grant GM44944].
Article, publication date, and citation information can be found at http://molpharm.aspetjournals.org.
- GRK
- G protein-coupled receptor kinase
- ERK1/2
- extracellular signal-regulated kinase1/2
- siRNA
- small interfering RNA
- GPCR
- G protein-coupled receptor
- DiI
- 1,1′-Dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate
- FITC
- fluorescein isothiocyanate
- HRP
- horseradish peroxidase
- HEK
- human embryonic kidney
- EGFP
- enhanced green fluorescent protein
- ELISA
- enzyme-linked immunosorbent assay
- WT
- wild-type
- TBS
- Tris-buffered saline
- BSA
- bovine serum albumin
- DMEM
- Dulbecco's modified Eagle's medium
- PBS
- phosphate-buffered saline
- CRE
- cAMP response element
- ER
- endoplasmic reticulum
- MβCD
- methyl-β-cyclodextrin
- PKA
- protein kinase A
- PKC
- protein kinase C
- 5-HT
- 5-hydroxytryptamine.
Authorship Contributions
Participated in research design: Li, Zhou, J.Lu, and Benovic.
Conducted experiments: Li, Zhou, Yu, Shi, and J.Luo.
Contributed new reagents or analytic tools: Li, Zhou, and J.Luo.
Performed data analysis: Li, Zhou, Yu, Chen, and Shi.
Wrote or contributed to the writing of the manuscript: Zhou, J.Lu, Benovic, and Li.
References
- Benyó Z, Gille A, Kero J, Csiky M, Suchánková MC, Nüsing RM, Moers A, Pfeffer K, Offermanns S. (2005) GPR109A (PUMA-G/HM74A) mediates nicotinic acid-induced flushing. J Clin Invest 115:3634–3640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bermak JC, Li M, Bullock C, Zhou QY. (2001) Regulation of transport of the dopamine D1 receptor by a new membrane-associated ER protein. Nat Cell Biol 3:492–498 [DOI] [PubMed] [Google Scholar]
- Bouvier M, Hausdorff WP, De Blasi A, O'Dowd BF, Kobilka BK, Caron MG, Lefkowitz RJ. (1988) Removal of phosphorylation sites from the β2-adrenergic receptor delays onset of agonist-promoted desensitization. Nature 333:370–373 [DOI] [PubMed] [Google Scholar]
- Cerione RA, Codina J, Benovic JL, Lefkowitz RJ, Birnbaumer L, Caron MG. (1984) The mammalian β2-adrenergic receptor: reconstitution of functional interactions between pure receptor and pure stimulatory nucleotide binding protein of the adenylate cyclase system. Biochemistry 23:4519–4525 [DOI] [PubMed] [Google Scholar]
- Claeysen S, Sebben M, Becamel C, Bockaert J, Dumuis A. (1999) Novel brain-specific 5-HT4 receptor splice variants show marked constitutive activity: role of the C-terminal intracellular domain. Mol Pharmacol 55:910–920 [PubMed] [Google Scholar]
- Costa T, Herz A. (1989) Antagonists with negative intrinsic activity at delta opioid receptors coupled to GTP-binding proteins. Proc Natl Acad Sci USA 86:7321–7325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotecchia S, Exum S, Caron MG, Lefkowitz RJ. (1990) Regions of theα1-adrenergic receptor involved in coupling to phosphatidylinositol hydrolysis and enhanced sensitivity of biological function. Proc Natl Acad Sci USA 87:2896–2900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delhaye M, Gravot A, Ayinde D, Niedergang F, Alizon M, Brelot A. (2007) Identification of a postendocytic sorting sequence in CCR5. Mol Pharmacol 72:1497–1507 [DOI] [PubMed] [Google Scholar]
- Dong C, Filipeanu CM, Duvernay MT, Wu G. (2007) Regulation of G protein-coupled receptor export trafficking. Biochim Biophys Acta 1768:853–870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duvernay MT, Dong C, Zhang X, Zhou F, Nichols CD, Wu G. (2009) Anterograde trafficking of G protein-coupled receptors: function of the C-terminal F(X)6LL motif in export from the endoplasmic reticulum. Mol Pharmacol 75:751–761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duvernay MT, Zhou F, Wu G. (2004) A conserved motif for the transport of G protein-coupled receptors from the endoplasmic reticulum to the cell surface. J Biol Chem 279:30741–30750 [DOI] [PubMed] [Google Scholar]
- Ferguson SS. (2001) Evolving concepts in G protein-coupled receptor endocytosis: the role in receptor desensitization and signaling. Pharmacol Rev 53:1–24 [PubMed] [Google Scholar]
- Gáborik Z, Mihalik B, Jayadev S, Jagadeesh G, Catt KJ, Hunyady L. (1998) Requirement of membrane-proximal amino acids in the carboxyl-terminal tail for expression of the rat AT1a angiotensin receptor. FEBS Lett 428:147–151 [DOI] [PubMed] [Google Scholar]
- Hasegawa H, Negishi M, Ichikawa A. (1996) Two isoforms of the prostaglandin E receptor EP3 subtype different in agonist-independent constitutive activity. J Biol Chem 271:1857–1860 [DOI] [PubMed] [Google Scholar]
- Kamanna VS, Kashyap ML. (2007) Nicotinic acid (niacin) receptor agonists: will they be useful therapeutic agents? Am J Cardiol 100 (11A): S53–S61 [DOI] [PubMed] [Google Scholar]
- Koski G, Streaty RA, Klee WA. (1982) Modulation of sodium-sensitive GTPase by partial opiate agonists. An explanation for the dual requirement for Na+ and GTP in inhibitory regulation of adenylate cyclase. J Biol Chem 257:14035–14040 [PubMed] [Google Scholar]
- Kvachnina E, Dumuis A, Wlodarczyk J, Renner U, Cochet M, Richter DW, Ponimaskin E. (2009) Constitutive Gs-mediated, but not G12-mediated, activity of the 5-hydroxytryptamine 5-HT7(a) receptor is modulated by the palmitoylation of its C-terminal domain. Biochim Biophys Acta 1793:1646–1655 [DOI] [PubMed] [Google Scholar]
- Labasque M, Reiter E, Becamel C, Bockaert J, Marin P. (2008) Physical interaction of calmodulin with the 5-hydroxytryptamine2C receptor C-terminus is essential for G protein-independent, arrestin-dependent receptor signaling. Mol Biol Cell 19:4640–4650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefkowitz RJ, Shenoy SK. (2005) Transduction of receptor signals by β-arrestins. Science 308:512–517 [DOI] [PubMed] [Google Scholar]
- Leterrier C, Bonnard D, Carrel D, Rossier J, Lenkei Z. (2004) Constitutive endocytic cycle of the CB1 cannabinoid receptor. J Biol Chem 279:36013–36021 [DOI] [PubMed] [Google Scholar]
- Levoye A, Dam J, Ayoub MA, Guillaume JL, Couturier C, Delagrange P, Jockers R. (2006) The orphan GPR50 receptor specifically inhibits MT1 melatonin receptor function through heterodimerization. EMBO J 25:3012–3023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G, Deng X, Wu C, Zhou Q, Chen L, Shi Y, Huang H, Zhou N. (2011) Distinct kinetic and spatial patterns of protein kinase C (PKC)- and epidermal growth factor receptor (EGFR)-dependent activation of extracellular signal-regulated kinases 1 and 2 by human nicotinic acid receptor GPR109A. J Biol Chem 286:31199–31212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G, Shi Y, Huang H, Zhang Y, Wu K, Luo J, Sun Y, Lu J, Benovic JL, Zhou N. (2010) Internalization of the human nicotinic acid receptor GPR109A is regulated by Gi, GRK2, and arrestin3. J Biol Chem 285:22605–22618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang M, Eason MG, Jewell-Motz EA, Williams MA, Theiss CT, Dorn GW, 2nd, Liggett SB. (1998) Phosphorylation and functional desensitization of the α2A-adrenergic receptor by protein kinase C. Mol Pharmacol 54:44–49 [DOI] [PubMed] [Google Scholar]
- Mack WJ, Selzer RH, Hodis HN, Erickson JK, Liu CR, Liu CH, Crawford DW, Blankenhorn DH. (1993) One-year reduction and longitudinal analysis of carotid intima-media thickness associated with colestipol/niacin therapy. Stroke 24:1779–1783 [DOI] [PubMed] [Google Scholar]
- Matus-Leibovitch N, Nussenzveig DR, Gershengorn MC, Oron Y. (1995) Truncation of the thyrotropin-releasing hormone receptor carboxyl tail causes constitutive activity and leads to impaired responsiveness in Xenopus oocytes and AtT20 cells. J Biol Chem 270:1041–1047 [DOI] [PubMed] [Google Scholar]
- McCulloch CV, Morrow V, Milasta S, Comerford I, Milligan G, Graham GJ, Isaacs NW, Nibbs RJ. (2008) Multiple roles for the C-terminal tail of the chemokine scavenger D6. J Biol Chem 283:7972–7982 [DOI] [PubMed] [Google Scholar]
- Meyers CD, Liu P, Kamanna VS, Kashyap ML. (2007) Nicotinic acid induces secretion of prostaglandin D2 in human macrophages: an in vitro model of the niacin flush. Atherosclerosis 192:253–258 [DOI] [PubMed] [Google Scholar]
- Moffett S, Adam L, Bonin H, Loisel TP, Bouvier M, Mouillac B. (1996) Palmitoylated cysteine 341 modulates phosphorylation of the β2-adrenergic receptor by the cAMP-dependent protein kinase. J Biol Chem 271:21490–21497 [DOI] [PubMed] [Google Scholar]
- Moore CA, Milano SK, Benovic JL. (2007) Regulation of receptor trafficking by GRKs and arrestins. Annu Rev Physiol 69:451–482 [DOI] [PubMed] [Google Scholar]
- Nakata H, Kameyama K, Haga K, Haga T. (1994) Location of agonist-dependent-phosphorylation sites in the third intracellular loop of muscarinic acetylcholine receptors (m2 subtype). Eur J Biochem 220:29–36 [DOI] [PubMed] [Google Scholar]
- Nie J, Lewis DL. (2001) Structural domains of the CB1 cannabinoid receptor that contribute to constitutive activity and G-protein sequestration. J Neurosci 21:8758–8764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakley RH, Laporte SA, Holt JA, Barak LS, Caron MG. (2001) Molecular determinants underlying the formation of stable intracellular G protein-coupled receptor-β-arrestin complexes after receptor endocytosis. J Biol Chem 276:19452–19460 [DOI] [PubMed] [Google Scholar]
- Paing MM, Stutts AB, Kohout TA, Lefkowitz RJ, Trejo J. (2002) beta -Arrestins regulate protease-activated receptor-1 desensitization but not internalization or down-regulation. J Biol Chem 277:1292–1300 [DOI] [PubMed] [Google Scholar]
- Pankevych H, Korkhov V, Freissmuth M, Nanoff C. (2003) Truncation of the A1 adenosine receptor reveals distinct roles of the membrane-proximal carboxyl terminus in receptor folding and G protein coupling. J Biol Chem 278:30283–30293 [DOI] [PubMed] [Google Scholar]
- Parker EM, Ross EM. (1991) Truncation of the extended carboxyl-terminal domain increases the expression and regulatory activity of the avian β-adrenergic receptor. J Biol Chem 266:9987–9996 [PubMed] [Google Scholar]
- Richman JG, Kanemitsu-Parks M, Gaidarov I, Cameron JS, Griffin P, Zheng H, Guerra NC, Cham L, Maciejewski-Lenoir D, Behan DP, et al. (2007) Nicotinic acid receptor agonists differentially activate downstream effectors. J Biol Chem 282:18028–18036 [DOI] [PubMed] [Google Scholar]
- Seibold A, Williams B, Huang ZF, Friedman J, Moore RH, Knoll BJ, Clark RB. (2000) Localization of the sites mediating desensitization of the β2-adrenergic receptor by the GRK pathway. Mol Pharmacol 58:1162–1173 [DOI] [PubMed] [Google Scholar]
- Seifert R, Wenzel-Seifert K. (2002) Constitutive activity of G-protein-coupled receptors: cause of disease and common property of wild-type receptors. Naunyn Schmiedebergs Arch Pharmacol 366:381–416 [DOI] [PubMed] [Google Scholar]
- Smit MJ, Vischer HF, Bakker RA, Jongejan A, Timmerman H, Pardo L, Leurs R. (2007) Pharmacogenomic and structural analysis of constitutive G protein-coupled receptor activity. Annu Rev Pharmacol Toxicol 47:53–87 [DOI] [PubMed] [Google Scholar]
- Soga T, Kamohara M, Takasaki J, Matsumoto S, Saito T, Ohishi T, Hiyama H, Matsuo A, Matsushime H, Furuichi K. (2003) Molecular identification of nicotinic acid receptor. Biochem Biophys Res Commun 303:364–369 [DOI] [PubMed] [Google Scholar]
- Subtil A, Gaidarov I, Kobylarz K, Lampson MA, Keen JH, McGraw TE. (1999) Acute cholesterol depletion inhibits clathrin-coated pit budding. Proc Natl Acad Sci USA 96:6775–6780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taggart AK, Kero J, Gan X, Cai TQ, Cheng K, Ippolito M, Ren N, Kaplan R, Wu K, Wu TJ, et al. (2005) d-β-Hydroxybutyrate inhibits adipocyte lipolysis via the nicotinic acid receptor PUMA-G. J Biol Chem 280:26649–26652 [DOI] [PubMed] [Google Scholar]
- Tang H, Guo DF, Porter JP, Wanaka Y, Inagami T. (1998) Role of cytoplasmic tail of the type 1A angiotensin II receptor in agonist- and phorbol ester-induced desensitization. Circ Res 82:523–531 [DOI] [PubMed] [Google Scholar]
- Taylor AJ, Sullenberger LE, Lee HJ, Lee JK, Grace KA. (2004) Arterial Biology for the Investigation of the Treatment Effects of Reducing Cholesterol (ARBITER) 2: a double-blind, placebo-controlled study of extended-release niacin on atherosclerosis progression in secondary prevention patients treated with statins. Circulation 110:3512–3517 [DOI] [PubMed] [Google Scholar]
- Tunaru S, Kero J, Schaub A, Wufka C, Blaukat A, Pfeffer K, Offermanns S. (2003) PUMA-G and HM74 are receptors for nicotinic acid and mediate its anti-lipolytic effect. Nat Med 9:352–355 [DOI] [PubMed] [Google Scholar]
- Tunaru S, Lättig J, Kero J, Krause G, Offermanns S. (2005) Characterization of determinants of ligand binding to the nicotinic acid receptor GPR109A (HM74A/PUMA-G). Mol Pharmacol 68:1271–1280 [DOI] [PubMed] [Google Scholar]
- Wise A, Foord SM, Fraser NJ, Barnes AA, Elshourbagy N, Eilert M, Ignar DM, Murdock PR, Steplewski K, Green A, et al. (2003) Molecular identification of high and low affinity receptors for nicotinic acid. J Biol Chem 278:9869–9874 [DOI] [PubMed] [Google Scholar]
- Xia S, Kjaer S, Zheng K, Hu PS, Bai L, Jia JY, Rigler R, Pramanik A, Xu T, Hökfelt T, et al. (2004) Visualization of a functionally enhanced GFP-tagged galanin R2 receptor in PC12 cells: constitutive and ligand-induced internalization. Proc Natl Acad Sci USA 101:15207–15212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan N, Friedman J, Whaley BS, Clark RB. (1994) cAMP-dependent protein kinase and protein kinase C consensus site mutations of the β-adrenergic receptor. Effect on desensitization and stimulation of adenylylcyclase. J Biol Chem 269:23032–23038 [PubMed] [Google Scholar]
- Zhou Q, Li G, Deng XY, He XB, Chen LJ, Wu C, Shi Y, Wu KP, Mei LJ, Lu JX, et al. (2012) Activated human hydroxy-carboxylic acid receptor-3 signals to MAP kinase cascades via the PLC-dependent PKC and MMP-mediated EGFR pathways. Br J Pharmacol 166:1756–1773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman B, Simaan M, Akoume MY, Houri N, Chevallier S, Séguéla P, Laporte SA. (2011) Role of βarrestins in bradykinin B2 receptor-mediated signalling. Cell Signal 23:648–659 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.