Abstract
Background
Age-related disparities in colon cancer treatment exist, with older patients less likely to receive recommended therapy. However, few studies have focused on receipt of surgery. The objective was to describe patterns of surgery in colon cancer patients ≥80 years and examine outcomes with and without colectomy.
Methods
Medicare beneficiaries ≥80 years with colon cancer diagnosed from 1992–2005 were identified from the Surveillance, Epidemiology and End Results- Medicare database. Multivariable logistic regression analysis was utilized to assess factors associated with non-operative management. Kaplan-Meier survival analysis determined one-year overall and colon cancer-specific survival.
Results
Of 31,574 patients, 80% underwent colectomy. 46% occurred during an urgent/emergent admission, with decreased 1-year overall survival (70% vs. 86% during an elective admission). Factors most predictive of non-operative management include older age, black race, more hospital admissions, use of home oxygen, use of a wheel chair, being frail and dementia. For both operative and non-operative patients, one-year overall survival was lower than colon cancer-specific survival (colectomy 78% vs. 89%; no colectomy 58% vs. 78%).
Conclusions
Most older colon cancer patients are receiving surgery, with improved outcomes compared to non-operative management. However, many patients not selected for surgery die of unrelated causes, reflecting good surgical selection. Patients undergoing surgery during an urgent/emergent admission have an increased short-term mortality. As earlier detection of colon cancer may increase the proportion of older patients undergoing elective surgery, these findings have policy implications for colon cancer screening and suggest that age should not be the only factor driving cancer screening recommendations.
Keywords: colon cancer, elderly, colectomy, octogenarian, nonagenarian, aged
INTRODUCTION
The United States population is aging1 and in 2010 included over 11 million individuals over the age of 80 years. The growing numbers of the “oldest-old” are at least partially a reflection of increasing life-expectancy, as the average life-expectancy for an 80-year-old alive today exceeds eight years.2 These changing demographics have a significant impact on health care as a whole and cancer care in particular. For cancers such as colon cancer, which are largely a disease of the elderly, the impact may be especially large.
Prior research has demonstrated that age-related disparities in the treatment of colon cancer exist, with older patients less likely to receive recommended therapy.3 Most studies examining age-related disparities have focused on adjuvant chemotherapy, with several studies demonstrating that older adults are less likely to be recommended or to receive adjuvant treatment.4–8 Only one study to date has focused on receipt of surgery.9 This study demonstrated that the majority of older colon cancer patients undergo cancer-directed surgery. However, this study excluded patients with regional disease or who were unstaged. Considering older patients are more likely to be unstaged,10 the conclusions from this study may not be reflective of surgical treatment for the majority of older Americans. Additionally, this study did not examine factors associated with selection for surgery in these older patients. We initiated the current research with the goal of examining current practices in the United States with regards to the surgical management of colon cancer in patients 80 years of age and older.
METHODS
This study was approved by the University of Wisconsin Institutional Review Board and given a waiver of consent.
Data Source
We examined data from the linked SEER-Medicare database for patients diagnosed with colon cancer between 1992 and 2005. The SEER cancer registries include information on patient demographics, tumor characteristics, first course of treatment, and survival for persons newly diagnosed with cancer. For individuals who are eligible for Medicare services, the SEER-Medicare database includes claims for covered health care services, including hospital, physician, outpatient, home health, and hospice bills. The SEER-Medicare dataset has successfully linked 93% of individuals over age 65 at diagnosis to their Medicare record.11,12 In 2000, SEER regions included approximately 26% of the US population.12
Patient Selection
All Medicare-enrolled patients aged 80 years and older diagnosed with primary colon adenocarcinoma within a SEER region during the years 1992 through 2005 were considered for study inclusion. Patients with colon cancer were identified by SEER anatomic site (18.0–18.9, 19.9) and histology (8140–8417, 8210–11, 8220–21, 8260–63, 8480–81, 8490) codes. Patients were excluded if they had distant metastases (American Joint Commission on Cancer [AJCC] Stage IV or SEER Summary Stage “distant”). Continuous enrollment in Medicare Part A and Part B was required for the three years preceding diagnosis through the three years following discharge, death, or December 31, 2005 (whichever came first) to allow ascertainment of comorbidities and survival. Patients were excluded if they were enrolled in a Health Maintenance Organization during the same time period. Patients were also excluded if they were diagnosed with another malignancy one year before or after the date of colon cancer diagnosis, or if their first diagnosis of colon cancer was made after death (i.e., on autopsy or death certificate). From an initial cohort of 42,873 colon cancer patients ≥80 years of age, the following patients were sequentially excluded: distant metastases (n=6755), discontinuous Medicare Part A and Part B coverage (n=1143), and another cancer within one year of colon cancer diagnosis (n=3401). The final sample size was 31,574 patients.
Outcome Variables
The primary outcome measure for this study was receipt of “curative surgery”. Surgery was considered to be of curative intent if it occurred within 90 days of the colon cancer diagnosis and included one of the following ICD-9 procedure codes: 45.7X and 45.8X. One-year overall and cancer-specific survival were examined based on dates of death recorded in the SEER Patient Entitlement and Diagnosis Summary File (PEDSF) according to Social Security administration data. Length of stay, 30-day readmission rates (readmission to any acute care hospital), and in-hospital complications were also examined. In-hospital complications included complications that resulted in reoperation or other procedural intervention.13
Patient-Related Variables
Basic patient-related variables included date of birth, gender, race/ethnicity, marital status, SEER registry region, and census tract median level of household income and median level of education (used as proxies for socioeconomic status). Geographic region was represented by SEER registry and rural/urban county of residence, based on 2003 Rural/Urban Continuum Codes identified from the PEDSF.
We assessed patients’ overall health by a number of mechanisms. The number of emergency room visits and hospital admissions in the year prior to diagnosis was examined to assess health care utilization. Specific comorbidities previously found to be associated with patient outcomes were identified.14 Given the importance of dementia in treatment-making for older adults, we assessed for dementia using a separate, validated algorithm based on the following ICD-9 codes: ICD-9 331.0–331.1, 331.11, 331.19, 331.7, 290.0, 290.10–290.13, 290.20–290.21, 290.3, 290.40–290.43, 294.0, 294.1, 294.10, 294.11, 294,8, and 797.15 Home oxygen use was recorded if patients had two codes related to home oxygen usage in the three years prior to diagnosis (DME A7017). Additionally, functional status was indirectly assessed by examining claims for mobility devices in the three years prior to diagnosis (assessment for device: 97755, 97542; cane: E0100, E0105; crutch: E0110-E0017; walker: E0130, E0135, E0140–E0141, E0143–E0144, E0147-E0149; wheelchair: K0001-K0007, E0983, E0984, E1210-E1213, E2368-E2370, K0010-K0012, K0014, K0800-K0899). Patient frailty (defined in this context as decreased physiologic reserve and resistance to stressors as a result of physiologic multi-system decline) was assessed using ICD-9 codes for 12 conditions as defined by the John Hopkins’ Adjusted Clinical Groups (ACG) case-mix system (e.g. difficulty walking, weight loss, frequent falls, malnutrition, impaired vision, decubitus ulcer, incontinence).16 Patients having billing codes for any of these conditions were considered to be frail. Of the 4233 meeting criteria for frailty, 75% met criteria with a single ICD-9 code. The most common single codes were difficulty walking (38%), weight loss (30%), frequent falls (16%), and decubitus ulcers (10%); the remaining codes occurred most frequently in combination.
Colon cancer stage was assessed using the SEER historic stage variable, which categorizes patients as local, regional, distant or unstaged; within SEER, staging is generated used a combination of the most precise clinical and pathological documentation of the extent of disease. Because our patient cohort included patients who did not undergo surgery, there was a significant proportion of unstaged patients. This proportion was smaller when the SEER historic stage variable was used rather than AJCC staging. All unstaged patients were kept in our cohort.
Treatment-Related Variables
In addition to receipt of “curative surgery”, we examined whether patients received a colectomy after the defined 90-day “curative window”. Receipt of a diverting stoma without accompanying colectomy (ICD-9 46.0, 46.01, 46.03, 46.1, 46.11, 46.13, 46.2, 46.20, 46.21, 46.23, CPT 44141, 44143, 44144, 44146, 44150, 44155, 44206, 44212, 44310, 44320, 44322) or a colonic stent (ICD-9 46.86, 46.87, 97.04, CPT 44397, 45327, 45387) any time after diagnosis was noted. We also examined whether patients had an intestinal obstruction (ICD-9 560.89 or 560.90) or an intestinal perforation (ICD-9 569.83) at the time of surgery, or were admitted under emergent or urgent conditions.
Whether patients received a surgical evaluation after diagnosis was examined by recording the date of service from a surgical specialist (defined as Centers for Medicare & Medicaid Services provider specialty codes 2 [general surgery], 28 [colorectal surgery], and 91 [surgical oncology]) with an appropriate Evaluation and Management codes (inpatient, 99025, 99218–99223, 99234–99236, 99251–99255, 99261–99263, 99281–99285, 99356, 99357; outpatient, 99058, 99201–99205, 99211–99215, 99241–99245, 99271–99275, 99354–99355, 99381–99387, 99391–99397, 99401–99404, 99411–99412, 99420, 99429, 99431–99432, G0101, G0245-G0250, G0344, G0375, G0376).
Analysis
Descriptive statistics of patient characteristics were generated. The frequency of all patient-related and treatment variables by receipt of surgery with curative intent were compared using Χ2 tests for categorical variables and two-way ANOVA tests for continuous variables. Multivariable logistic regression analysis was utilized to assess factors associated with not undergoing surgery. Additional surgical interventions (delayed colectomy, diverting stoma, or colon stent) for the group not undergoing curative surgery were examined. One-year overall and cancer-specific survival was assessed using Kaplan-Meier analysis. Cox regression analysis was used to identify factors associated with overall survival. Given the high percentage of unstaged patients in the “no surgery” group, sensitivity analyses were performed to evaluate the impact on cancer stage on survival. As the addition of cancer stage had minimal impact on the model, stage was not included in the final models.
RESULTS
Of the 31,574 patients ≥80 years of age diagnosed with colon cancer, 80% underwent colectomy within 90 days of diagnosis (Figure 1). Patient characteristics are described in Table 1. As would be expected, a number of differences between surgical and non-surgical patients existed, with patients treated non-operatively more likely to be older, male, non-white and unmarried. Additionally, non-operative patients were less “healthy”, with more hospital admissions in the prior year, more use of home oxygen, more claims for mobility assist devices, and greater comorbidities (Table 1). In adjusted analyses, factors most predictive of being managed non-operatively after a diagnosis of colon cancer include: older age, black race, more hospital admissions in the prior year, use of home oxygen, use of a wheel chair, being frail, and dementia (Table 2).
Figure 1.
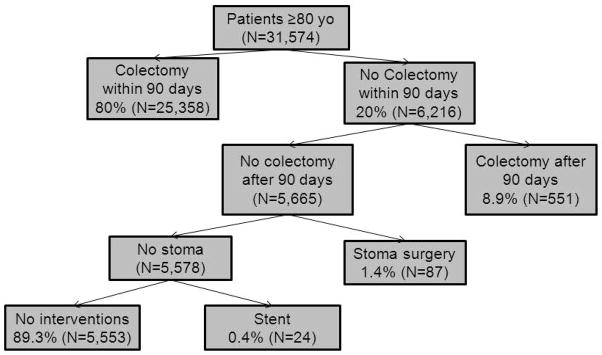
Surgical Treatment of Colon Cancer Patients ≥80 Years of Age.
Table 1.
Characteristics of Colon Cancer Patients Eighty Years of Age and Older With and Without Colectomy
| Colectomy N=25358 |
No Colectomy N=6216 |
P value | |
|---|---|---|---|
| Demographics | |||
| Age at diagnosis, mean (SD) | 85.0 (4.0) | 86.0 (4.6) | <0.005 |
| Male | 36.0% | 38.3% | 0.001 |
| Race | |||
| White | 88.7% | 83.8% | <0.005 |
| Black | 5.1% | 9.3% | |
| Other | 6.2% | 7.0% | |
| Marital Status | |||
| Married | 36.5% | 31.6% | <0.005 |
| Widowed | 50.4% | 51.2% | |
| Single, separated, divorced | 9.7% | 11.5% | |
| Cancer Stage | |||
| Localized | 46.4% | 46.7% | <0.005 |
| Regional | 52.3% | 23.2% | |
| Unstaged | 1.3% | 30.1% | |
| Health Factors | |||
| # of hospital admissions in prior year, mean (SD) | 1.49 (0.90) | 1.70 (1.2) | <0.0005 |
| # or ER visits in prior year, mean (SD) | 3.62 (4.1) | 3.53 (4.1) | 0.280 |
| Home Oxygen | 2.4% | 3.7% | <0.005 |
| Mobility Assist Device | |||
| No assessment | 91.7% | 87.2% | <0.005 |
| Crutch/cane | 4.6% | 6.1% | |
| Wheelchair | 3.7% | 6.8% | |
| Frail | 6.8% | 14.6% | <0.005 |
| Comorbidities | |||
| Dementia | 1.8% | 6.1% | <0.005 |
| Congestive heart failure | 38.6% | 42.8% | <0.005 |
| Valvular disease | 14.2% | 13.7% | <0.005 |
| Hypertension | 75.1% | 71.6% | <0.005 |
| Peripheral Vascular Disease | 31.0% | 33.3% | <0.005 |
| Paralysis | 3.7% | 5.1% | <0.005 |
| Other neurologic disorders | 14.2% | 16.8% | <0.005 |
| Chronic Pulmonary Disease | 25.6% | 26.9% | <0.005 |
| Diabetes (uncomplicated) | 15.1% | 15.4% | <0.005 |
| Diabetes (with complications) | 8.7% | 9.9% | <0.005 |
| Renal failure | 5.7% | 6.2% | <0.005 |
| Weight loss | 6.7% | 7.1% | <0.005 |
Table 2.
Multi-variable Analysis of Factors Associated with Not Undergoing Colectomy for Colon Cancer in Patients ≥ 80 Years of Age
| Adjusted Odds Ratio | 95% CI | P value | |
|---|---|---|---|
| Demographics | |||
| Age at diagnosis, mean (SD) | 1.04 | 1.03–1.05 | <0.005 |
| Male | 1.04 | 0.93–1.16 | 0.46 |
| Race | |||
| White | Reference | ||
| Black | 1.42 | 1.18–1.70 | <0.005 |
| Other | 1.07 | 0.86–1.34 | 0.52 |
| Marital Status | |||
| Married | Reference | ||
| Widowed | 1.07 | 0.95–1.21 | 0.23 |
| Single, separated, divorced | 1.19 | 1.0–1.41 | 0.05 |
| Health Factors | |||
| # of hospital admissions in prior year, mean (SD) | 1.10 | 1.05–1.14 | <0.005 |
| # or ER visits in prior year, mean (SD) | 0.99 | 0.98–1.01 | 0.37 |
| Home Oxygen | 1.44 | 1.15–1.78 | 0.001 |
| Mobility Assist Device | |||
| No assessment | Reference | ||
| Crutch/cane | 1.16 | 0.98–1.37 | 0.08 |
| Wheelchair | 1.24 | 1.05–1.48 | 0.01 |
| Frail | 1.79 | 1.56–2.05 | <0.005 |
| Comorbidities | |||
| Dementia | 2.22 | 1.80–2.73 | <0.005 |
| Congestive heart failure | 1.16 | 1.05–1.28 | 0.01 |
| Valvular disease | 1.07 | 0.94–1.21 | 0.32 |
| Hypertension | 0.91 | 0.81–1.03 | 0.13 |
| Peripheral Vascular Disease | 1.05 | 0.95–1.16 | 0.32 |
| Paralysis | 1.37 | 1.12–1.67 | 0.002 |
| Other neurologic disorders | 1.07 | 0.95–1.22 | 0.28 |
| Chronic Pulmonary Disease | 1.09 | 0.98–1.21 | 0.11 |
| Diabetes (uncomplicated) | 1.07 | 0.94–1.22 | 0.27 |
| Diabetes (with complications) | 1.23 | 1.06–1.42 | 0.01 |
| Renal failure | 1.09 | 0.91–1.31 | 0.34 |
| Weight loss | 0.80 | 0.67–0.96 | 0.02 |
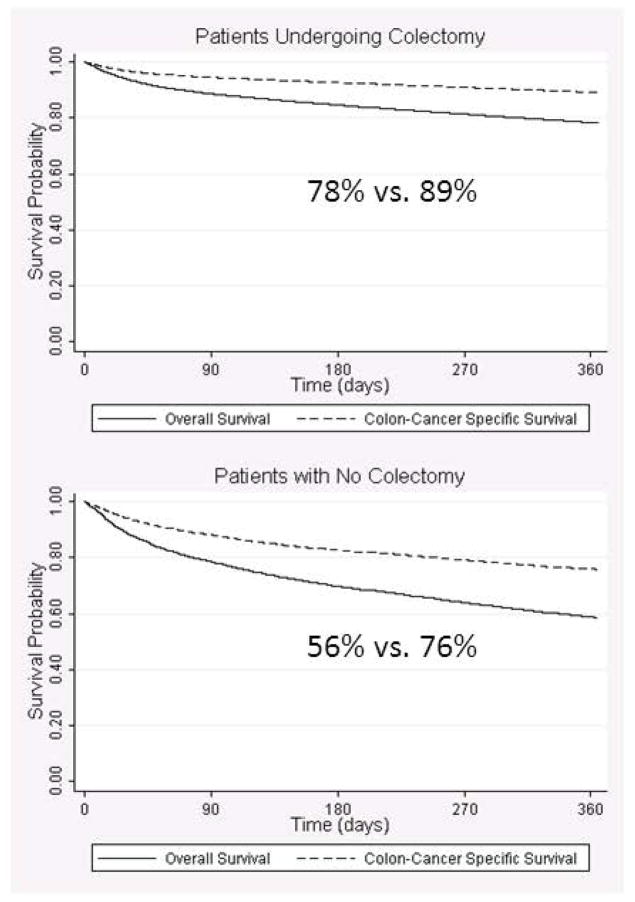
For patients undergoing curative colectomy, one-year overall and colon cancer-specific survival was 78% vs. 89% (Figure 2). Colon cancer-specific survival was similarly higher for non-surgical patients (58% vs. 76%). Factors associated with decreased overall and colon cancer-specific survival included older age, being widowed, having more hospitalizations or ER visits in the prior year, use of home oxygen, being frail, dementia, and not receiving elective surgery (Table 3). In addition, male gender, being single/separated/divorced, and use of a wheelchair were associated with decreased overall survival. Some specific comorbidities (such as hypertension, peripheral vascular disease, chronic pulmonary disease) were associated with improved overall and cancer-specific survival.
Figure 2.
Overall and Colon Cancer-Specific Survival for Patients ≥80 Years of Age, With and Without Curative Colectomy.
Table 3.
Predictors of Overall and Cancer-Specific Survival in Colon Cancer Patients ≥80 years of Age
| Overall Survival | Cancer-Specific Survival | |||||
|---|---|---|---|---|---|---|
| Adjusted Hazard Ratios | 95% CI | P value | Adjusted Hazard Ratios | 95% CI | P value | |
| Demographics | ||||||
| Age at diagnosis, mean (SD) | 1.04 | 1.03–1.05 | <0.005 | 1.04 | 1.03–1.05 | <0.005 |
| Male | 1.26 | 1.16–1.36 | <0.005 | 1.08 | 0.96–1.21 | 0.23 |
| Race | ||||||
| White | Reference | |||||
| Black | 1.13 | 0.99–1.29 | 0.07 | 1.12 | 0.93–1.37 | 0.23 |
| Other | 0.97 | 0.83–1.14 | 0.74 | 1.05 | 0.83–1.33 | 0.67 |
| Marital Status | ||||||
| Married | Reference | |||||
| Widowed | 1.21 | 1.11–1.32 | <0.005 | 1.20 | 1.05–1.36 | 0.01 |
| Single, separated, divorced | 1.17 | 1.03–1.32 | 0.01 | 1.09 | 0.91–1.31 | 0.37 |
| Health Factors | ||||||
| # of hospital admissions in prior year, mean (SD) | 1.13 | 1.10–1.16 | <0.005 | 1.08 | 1.03–1.12 | 0.001 |
| # or ER visits in prior year, mean (SD) | 1.02 | 1.01–1.02 | <0.005 | 1.02 | 1.01–1.03 | 0.001 |
| Home oxygen | 1.52 | 1.32–1.76 | <0.005 | 1.28 | 1.01–1.61 | 0.04 |
| Mobility Assist Device | ||||||
| No assessment | Reference | |||||
| Crutch/cane | 0.90 | 0.79–1.02 | 0.11 | 0.91 | 0.75–1.09 | 0.31 |
| Wheelchair | 1.18 | 1.03–1.33 | 0.01 | 1.10 | 0.91–1.33 | 0.34 |
| Frail | 4.74 | 4.33–5.18 | <0.005 | 5.24 | 4.6–5.97 | <0.005 |
| Comorbidities | ||||||
| Dementia | 1.90 | 1.64–2.19 | <0.005 | 2.05 | 1.70–2.48 | <0.005 |
| Congestive heart failure | 1.00 | 0.93–1.08 | 0.92 | 0.85 | 0.76–0.94 | 0.003 |
| Valvular disease | 0.89 | 0.81–0.97 | 0.01 | 0.81 | 0.70–0.94 | 0.01 |
| Hypertension | 0.66 | 0.60–0.71 | <0.005 | 0.60 | 0.53–0.67 | <0.005 |
| Peripheral Vascular Disease | 0.77 | 0.72–0.83 | <0.005 | 0.71 | 0.64–0.80 | <0.005 |
| Paralysis | 0.93 | 0.81–1.08 | 0.37 | 0.87 | 0.69–1.10 | 0.25 |
| Other neurologic disorders | 0.80 | 0.73–0.88 | <0.005 | 0.75 | 0.66–0.87 | <0.005 |
| Chronic Pulmonary Disease | 0.90 | 0.83–0.97 | 0.01 | 0.79 | 0.70–0.89 | <0.005 |
| Diabetes (uncomplicated) | 0.99 | 0.90–1.09 | 0.88 | 0.95 | 0.82–1.10 | 0.48 |
| Diabetes (with complications) | 0.99 | 0.88–1.12 | 0.93 | 0.82 | 0.67–0.99 | 0.04 |
| Renal failure | 1.00 | 0.88–1.15 | 0.92 | 0.92 | 0.74–1.15 | 0.49 |
| Weight loss | 0.88 | 0.78–1.00 | 0.05 | 0.85 | 0.70–1.02 | 0.09 |
| Receipt of surgery | ||||||
| Elective | Reference | |||||
| Urgent/Emergent | 1.79 | 1.65–1.95 | <0.005 | 2.09 | 1.83–2.38 | <0.005 |
| No surgery | 2.15 | 1.95–2.37 | <0.005 | 2.76 | 2.38–3.21 | <0.005 |
Of the 6,216 patients who did not undergo colectomy within 90 days, 8.9% required a delayed colectomy (Figure 1). An additional 87 (1.4%) required a diverting stoma and 24 (0.4%) received a colonic stent. Overall, 5,554 (89.3%) of the patients not selected for immediate colectomy did not require any procedural intervention for their colon cancer. Only 31.1% of non-operative patients had an evaluation and management code for a surgeon visit recorded. Outcomes between non-operative patients who did and did not see a surgeon were equivalent (data not shown).
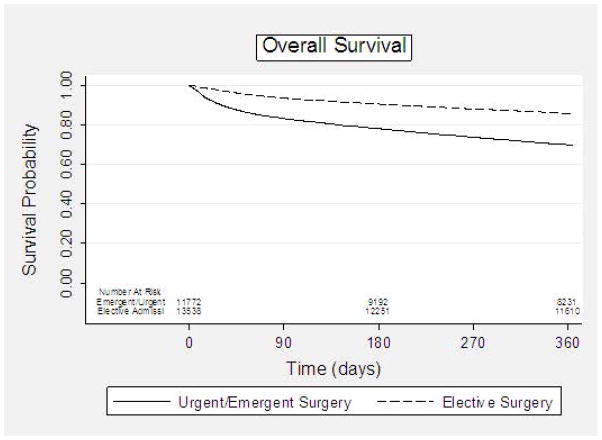
The majority of patients undergoing surgery did so during an elective admission (53.6%). Characteristics of patients undergoing elective versus urgent/emergent surgery differed significantly with patients undergoing urgent/emergent surgery more likely to be older (p<0.001), non-white (p<0.001), and married (<0.001). Additionally urgent/emergent operative patients had more hospitalizations in the prior year (p<0.001), higher cancer stages (p<0.001), more claims for mobility assist devices (<0.001), more use of home oxygen (p<0.001), and, in general, greater comorbidities (p<0.001). Although the overall rate of post-operative complications for the overall cohort was relatively low at 4.3%, the complication rate in patients undergoing surgery during an urgent/emergent admission was higher (5.4% versus 3.4%, p<0.0001). Similarly, length of stay (median 13 vs. 9 days, p<0.0001) and readmission rates (14.3% vs. 10.9%, p<0.0001) were increased. Overall one-year survival for patients undergoing elective surgery was 86% compared to 70% after an urgent or emergent admission (Figure 3). Of the 1,851 patients who died within 30 days of surgery, the majority of these deaths (70%) occurred after an urgent/emergent admission.
Figure 3.
Overall Survival of Colon Cancer Patients ≥80 Years of Age by Urgency of Surgery
DISCUSSION
We demonstrated that the majority of the “oldest-old” colon cancer patients in the United States are undergoing surgical resection. For both surgical and non-surgical patients, colon cancer-specific survival was higher than overall survival,17,18 demonstrating that many older patients diagnosed with colon cancer ultimately die from unrelated causes. Most patients selected for surgery do well, despite their advanced age and numerous comorbidities. This is especially true for older patients who undergo surgery under elective circumstances, with a 30-day mortality rate of only 3%. This suggests that for these “oldest-old” patients felt to be good candidates for elective surgical intervention, surgery should be considered as a standard of care.
Conversely, surgical outcomes during an urgent/emergent admission were significantly poorer, with 10% 30-day mortality. These outcomes mirror the findings of prior retrospective studies.19–21 A number of factors may be contributing to the poorer outcomes observed. In our cohort, patients who underwent urgent/emergent surgery were overall “less healthy” compared to those patients selected for elective surgery. The poorer outcomes observed, therefore, may occur as a result of delays in intervention when these higher-risk older adults present with an urgent or emergent condition. Alternatively, a conscious decision may have been made to avoid elective procedures in these patients. If this included cessation of screening colonoscopy, delays in diagnosis may have occurred, necessitating urgent/emergent surgery due to serious complications such as bowel perforation or obstruction. Finally, a decision may have been made by the patient and their care provider to avoid an elective colectomy itself due to the individuals’ health status. It may be these higher risk patients who avoided an elective colectomy at the time of diagnosis but then presented emergently who are contributing to the poorer outcomes observed. The limitations of the SEER-Medicare dataset do not allow us to further explore these potential factors driving the outcomes after urgent/emergent surgery, but highlight the importance of clinical judgment in the management of older colon cancer patients.
It is important to note that few patients not selected for initial surgical resection required a delayed surgical intervention for symptom management. This is likely a result of the high mortality rate in this patient population and reflects the good judgment of clinicians in selecting patients for surgical resection. Patients selected for non-operative management were overall “less healthy” than were surgical patients, as reflected by more frequent hospital admissions, higher rates of home oxygen and wheelchair utilization, and increased frailty and dementia. Although only 31% of non-operative patients saw a surgeon for a pre-operative consultation, outcomes for these patients were equivalent to those who did not have a surgical evaluation. This suggests that primary care providers and oncologists are effectively choosing patients for a surgical referral. Overall, this observation highlights the fact that for select older adults felt to be poor operative candidates by their health care providers, risk of death from a competing cause may be higher than the risk of developing colon cancer-related symptoms. In these highly selected patients, a selective, symptom-directed approach to management of their colon cancer may be reasonable.
There are some limitations to this study. First, our use of Medicare claims data limits our assessment of patients’ overall health to those factors that are associated with a billing claim, such as the presence of specific comorbidities, claims for mobility assist devices such as walkers or wheelchairs, and use of home oxygen. However, our claims data cannot provide insight into the chronicity or severity of these factors. This is especially relevant when assessing comorbidities in patients ≥80 years of age. Although the past medical history for many patients in this age group will include a significant list of comorbidities, there is a selection bias associated with living to be an octo- or nonagenarian that suggests that the severity of an individual’s comorbidities may be relatively low. This selection bias may explain our observation that several specific comorbidities were associated with improved survival. Additionally, clinical factors not captured by billing claims will not be included in our analysis. Factors such as independent living22 and frailty22–25 have been shown to be significantly associated with outcomes in the older adult population. Although we were able to assess for frailty, patients identified as “frail” through billing codes likely represent only the most severely impaired, and we are likely underestimating the degree of frailty in this “oldest-old” patient cohort. Next, a significant proportion of our patients who were not selected to undergo surgery were unstaged (30% vs. 1.3% in the operative cohort); the remaining patients had clinical or radiologic staging alone. Because of this, we were unable to fully assess the relationship between cancer stage and survival. Finally, we were restricted to using survival as our primary outcome. Older adults may place relatively greater importance on other outcomes, such as quality of life, maintaining an ability to live independently, or surviving to a particular milestone, such as a birth or a wedding.26 Our claims based dataset cannot provide insight into these values-dependent outcomes.
However, this study does have a number of strengths. By using the SEER-Medicare database, we were able to examine population-level outcomes for all patients over 80 years of age diagnosed with colon cancer. Severity of illness was examined using a number of different surrogates, including hospital admission, emergency room visits, and use of home oxygen. We were able to indirectly assess functional status through billing claims for mobility assist devices. Finally, by using the SEER data, we were able to examine the cause of death and provide insight into competing mortalities in this population.
CONCLUSIONS
Our study represents the first population-based study to comprehensively examine outcomes for the “oldest-old” colon cancer patients. We demonstrated that most colon cancer patients over the age of 80 are undergoing surgical resection, with good short-term outcomes. Outcomes for the 20% of patients not selected for surgical intervention are markedly poorer; however, as many of the deaths are unrelated to the colon cancer diagnosis, these findings likely reflect good clinical judgment in selecting appropriate patients for surgical treatment. For the patients who did undergo surgical resection, almost 50% of operations occurred during an emergent or urgent admission, with an observed increase in short-term mortality.
Our findings lead to several future research directions. First, it is apparent that patients who undergo surgery under emergent or urgent conditions have poorer outcomes. Many different factors may be driving this observation. Some may represent an appropriate reflection of patients’ preferences and values, such as a decision reached by a patient and their care provider to avoid elective surgery given poor overall health status. Others, such as delay in diagnosis due to cessation of screening colonoscopies may be less appropriate. Earlier diagnosis of colon cancer in older adults represents a potentially modifiable means of decreasing high-risk emergent surgery by conversion to an elective procedure. This observation has important policy implications for colon cancer screening. Based on the currently available data, the United States Preventive Task Force recommends against routine colon cancer screening after the age of 75 except in patients at increased risk of colon cancer.27 However, the “oldest-old” adults have largely been excluded from clinical trials and limited data is available to guide screening recommendations for this age group. Additionally, the United States aging population is diverse and a patient’s age may not accurately reflect their overall health status; therefore, chronologic age should not be the only factor driving cancer screening recommendations. Future research should focus on how to account for increasing life-expectancy in the United States population in colon cancer screening recommendations.
Finally, our results demonstrate the importance of patient selection. Although significant peri-operative risk is associated with the “oldest-old” patients presenting with urgent/emergent problems, these patients often represent less of a clinical decision-making dilemma to surgeons than do patients who are evaluated in the clinic for an elective surgical intervention. This is especially true when outcomes other than survival (such as quality of life and independent living) are considered. Future research should focus on patients being considered for elective surgery to better identify those patients at increased risk of poor short-term outcomes who may be better managed with a non-operative approach.
Acknowledgments
This study used the linked SEER-Medicare database. The interpretation and reporting of these data are the sole responsibility of the authors. The authors acknowledge the efforts of the Applied Research Program, NCI; the Office of Research, Development and Information, CMS; Information Management Services (IMS), Inc.; and the Surveillance, Epidemiology, and End Results (SEER) Program tumor registries in the creation of the SEER-Medicare database. The collection of the California cancer incidence data used in this study was supported by the California Department of Public Health as part of the statewide cancer reporting program mandated by California Health and Safety Code Section 103885; the National Cancer Institute’s Surveillance, Epidemiology and End Results Program under contract N01-PC-35136 awarded to the Northern California Cancer Center, contract N01-PC-35139 awarded to the University of Southern California, and contract N02-PC-15105 awarded to the Public Health Institute; and the Centers for Disease Control and Prevention’s National Program of Cancer Registries, under agreement #U55/CCR921930-02 awarded to the Public Health Institute. The ideas and opinions expressed herein are those of the author(s) and endorsement by the State of California, Department of Public Health the National Cancer Institute, and the Centers for Disease Control and Prevention or their Contractors and Subcontractors is not intended nor should be inferred. The authors acknowledge the efforts of the Applied Research Program, NCI; the Office of Research, Development and Information, CMS; Information Management Services (IMS), Inc.; and the Surveillance, Epidemiology, and End Results (SEER) Program tumor registries in the creation of the SEER-Medicare database.
Footnotes
Financial Disclosure: Support for this project was provided by the Health Innovation Program and the Community-Academic Partnerships core of the University of Wisconsin Institute for Clinical and Translational Research (UW ICTR), grant 1UL1RR025011 from the Clinical and Translational Science Award program of the National Center for Research Resources, National Institutes of Health and by the University of Wisconsin Carbone Cancer Center, grant P30CA014520-34 from the National Cancer Institute, National Institutes of Health. Additional funding for this project was provided by the UW School of Medicine and Public Health from The Wisconsin Partnership Program.
References
- 1.Werner CA. The Older Population: 2010. Bureau USC. Census Briefs. 2010 http://www.census.gov/prod/cen2010/briefs/c2010br-09.pdf2010.
- 2.Kochanek KD, Xu J, Murphy SL, Minino AM, Kung HC. National Vital Statistics Reports. Services. USDoHaH. Deaths: Preliminary Data for 2009. 592009 [PubMed] [Google Scholar]
- 3.Townsley C, Pond GR, Peloza B, et al. Analysis of treatment practices for elderly cancer patients in Ontario, Canada. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2005 Jun 1;23(16):3802–3810. doi: 10.1200/JCO.2005.06.742. [DOI] [PubMed] [Google Scholar]
- 4.Krzyzanowska MK, Regan MM, Powell M, Earle CC, Weeks JC. Impact of patient age and comorbidity on surgeon versus oncologist preferences for adjuvant chemotherapy for stage III colon cancer. Journal of the American College of Surgeons. 2009 Feb;208(2):202–209. doi: 10.1016/j.jamcollsurg.2008.10.016. [DOI] [PubMed] [Google Scholar]
- 5.Winget M, Hossain S, Yasui Y, Scarfe A. Characteristics of patients with stage III colon adenocarcinoma who fail to receive guideline-recommended treatment. Cancer. 2010 Oct 15;116(20):4849–4856. doi: 10.1002/cncr.25250. [DOI] [PubMed] [Google Scholar]
- 6.Kahn KL, Adams JL, Weeks JC, et al. Adjuvant chemotherapy use and adverse events among older patients with stage III colon cancer. JAMA: the journal of the American Medical Association. 2010 Mar 17;303(11):1037–1045. doi: 10.1001/jama.2010.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sundararajan V, Hershman D, Grann VR, Jacobson JS, Neugut AI. Variations in the use of chemotherapy for elderly patients with advanced ovarian cancer: a population-based study. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2002 Jan 1;20(1):173–178. doi: 10.1200/JCO.2002.20.1.173. [DOI] [PubMed] [Google Scholar]
- 8.Schrag D, Cramer LD, Bach PB, Begg CB. Age and adjuvant chemotherapy use after surgery for stage III colon cancer. Journal of the National Cancer Institute. 2001 Jun 6;93(11):850–857. doi: 10.1093/jnci/93.11.850. [DOI] [PubMed] [Google Scholar]
- 9.O’Connell JB, Maggard MA, Ko CY. Cancer-directed surgery for localized disease: decreased use in the elderly. Annals of surgical oncology. 2004 Nov;11(11):962–969. doi: 10.1245/ASO.2004.03.052. [DOI] [PubMed] [Google Scholar]
- 10.de Rijke JM, Schouten LJ, Schouten HC, Jager JJ, Koppejan AG, van den Brandt PA. Age-specific differences in the diagnostics and treatment of cancer patients aged 50 years and older in the province of Limburg, The Netherlands. Annals of oncology: official journal of the European Society for Medical Oncology/ESMO. 1996 Sep;7(7):677–685. doi: 10.1093/oxfordjournals.annonc.a010716. [DOI] [PubMed] [Google Scholar]
- 11.Potosky AL, Riley GF, Lubitz JD, Mentnech RM, Kessler LG. Potential for cancer related health services research using a linked Medicare-tumor registry database. Med Care. 1993 Aug;31(8):732–748. [PubMed] [Google Scholar]
- 12.Warren JL, Klabunde CN, Schrag D, Bach PB, Riley GF. Overview of the SEER-Medicare data: Content, research applications, and generalizability to the United States elderly population. Med Care. 2002 Aug;40(8 Suppl):IV-3–18. doi: 10.1097/01.MLR.0000020942.47004.03. [DOI] [PubMed] [Google Scholar]
- 13.Morris AM, Baldwin LM, Matthews B, et al. Reoperation as a quality indicator in colorectal surgery: a population-based analysis. Annals of surgery. 2007 Jan;245(1):73–79. doi: 10.1097/01.sla.0000231797.37743.9f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998 Jan;36(1):8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 15.Taylor DH, Jr, Ostbye T, Langa KM, Weir D, Plassman BL. The accuracy of Medicare claims as an epidemiological tool: the case of dementia revisited. J Alzheimers Dis. 2009;17(4):807–815. doi: 10.3233/JAD-2009-1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lieberman R, Abrams C, Weiner JP. Development and Evaluaton of the Johns Hopkins University Risk Adjustment Models for Medicare+Choice Plan Payment. Centers for Medicare and Medicaid Services. U.S. Department of Health and Human Services; 2003. [Google Scholar]
- 17.Surgery for colorectal cancer in elderly patients: a systematic review. Colorectal Cancer Collaborative Group. Lancet. 2000 Sep 16;356(9234):968–974. [PubMed] [Google Scholar]
- 18.Devon KM, Vergara-Fernandez O, Victor JC, McLeod RS. Colorectal cancer surgery in elderly patients: presentation, treatment, and outcomes. Diseases of the colon and rectum. 2009 Jul;52(7):1272–1277. doi: 10.1007/DCR.0b013e3181a74d2e. [DOI] [PubMed] [Google Scholar]
- 19.Heriot AG, Tekkis PP, Smith JJ, et al. Prediction of postoperative mortality in elderly patients with colorectal cancer. Diseases of the colon and rectum. 2006 Jun;49(6):816–824. doi: 10.1007/s10350-006-0523-4. [DOI] [PubMed] [Google Scholar]
- 20.Kunitake H, Zingmond DS, Ryoo J, Ko CY. Caring for octogenarian and nonagenarian patients with colorectal cancer: what should our standards and expectations be? Diseases of the colon and rectum. 2010 May;53(5):735–743. doi: 10.1007/DCR.0b013e3181cdd658. [DOI] [PubMed] [Google Scholar]
- 21.Violi V, Pietra N, Grattarola M, et al. Curative surgery for colorectal cancer: long-term results and life expectancy in the elderly. Diseases of the colon and rectum. 1998 Mar;41(3):291–298. doi: 10.1007/BF02237482. [DOI] [PubMed] [Google Scholar]
- 22.Robinson TN, Eiseman B, Wallace JI, et al. Redefining geriatric preoperative assessment using frailty, disability and co-morbidity. Annals of surgery. 2009 Sep;250(3):449–455. doi: 10.1097/SLA.0b013e3181b45598. [DOI] [PubMed] [Google Scholar]
- 23.Makary MA, Segev DL, Pronovost PJ, et al. Frailty as a predictor of surgical outcomes in older patients. Journal of the American College of Surgeons. 2010 Jun;210(6):901–908. doi: 10.1016/j.jamcollsurg.2010.01.028. [DOI] [PubMed] [Google Scholar]
- 24.Saxton A, Velanovich V. Preoperative frailty and quality of life as predictors of postoperative complications. Annals of surgery. 2011 Jun;253(6):1223–1229. doi: 10.1097/SLA.0b013e318214bce7. [DOI] [PubMed] [Google Scholar]
- 25.Chikwe J, Adams DH. Frailty: the missing element in predicting operative mortality. Seminars in thoracic and cardiovascular surgery. 2010 Summer;22(2):109–110. doi: 10.1053/j.semtcvs.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 26.Reuben DB. Medical care for the final years of life: “When you’re 83, it’s not going to be 20 years”. JAMA: the journal of the American Medical Association. 2009 Dec 23;302(24):2686–2694. doi: 10.1001/jama.2009.1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Calonge N, Petitti DB, DeWitt TG, et al. Screening for Colorectal Cancer: US Preventive Services Task Force Recommendation Statement. Ann Intern Med. 2008 Nov 4;149(9):627. doi: 10.7326/0003-4819-149-9-200811040-00243. [DOI] [PubMed] [Google Scholar]