In this issue of Circulation, Koval et al.1 show that two arrhythmogenic human cardiac Na+ channel (hNaV1.5) variants mimic the altered channel gating effects induced by Ca2+-calmodulin dependent protein kinase (CaMKII). They also show that phosphorylation of an adjacent CaMKII target site on NaV1.5 is enhanced in human heart failure (HF) samples and in the border zone of post-infarcted canine hearts.
The cardiac Na+ channel, NaV1.5, is responsible for inward Na+ current (INa) that drives the cardiac action potential (AP) upstroke and electrical impulse propagation.2 Genetic variants of the SCN5A gene encoding NaV1.5 are associated with long QT syndrome-3 (LQTs; gain of function), Brugada syndrome (BRs; loss of function), conduction system disease, SIDS, sick sinus syndrome, and dilated cardiomyopathy.3,4 These inherited channelopathies have been tremendously important to our understanding of normal NaV1.5 function and arrhythmia mechanisms. However, “acquired” forms of altered NaV1.5 function due to post-translational modification (e.g. phosphorylation or oxidation) may have pathophysiological consequences during ischemia/ reperfusion or HF, and thus reach a larger patient population. Indeed, half of all HF deaths are sudden and presumed to be due to lethal ventricular arrhythmias.5,6
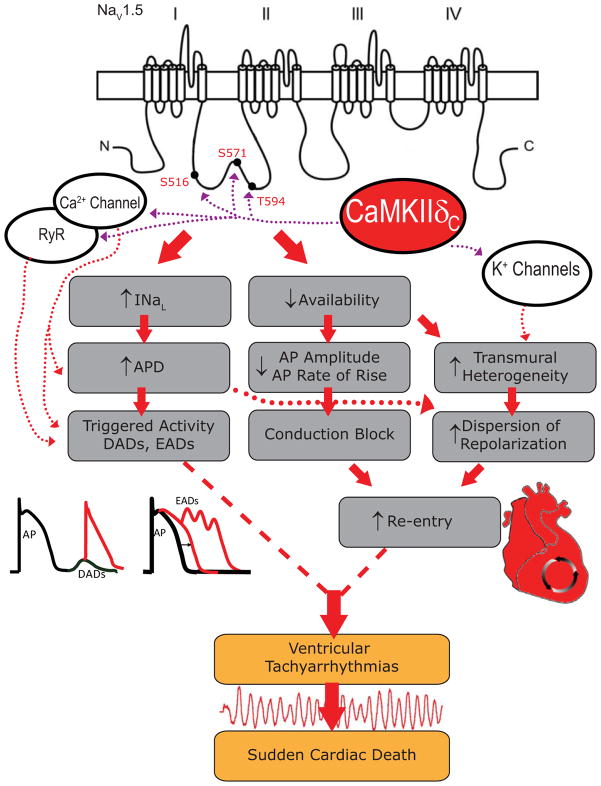
The pore forming α subunit (~220Kd predicted MW; NaV1.5) has four homologous domains (I–IV) with six transmembrane segments each (S1–S6; Figure 1), is glycosylated and has auxiliary regulatory β subunits (β1–β4, ~30–35Kd).7 The S5–S6 linker includes the P-loops or pore region, the four S4 segments serve as voltage sensors (involved in activation gating), while an IFM motif in the DIII–IV linker is important for fast inactivation gating. Importantly, NaV1.5 forms a macromolecular complex with interacting proteins that can regulate channel gating and localization, and mutations in many of these proteins can be pro-arrhythmic (reviewed in 3,7,8).
Figure 1.
CaMKII Phosphorylation of NaV1.5 is Pro-Arrhythmogenic. Arrhythmogenic mechanism of CaMKII based regulation of INa, showing different CaMKII based alterations in cardiac ion channel targets and contributions. The emphasis is on CaMKII sites on NaV1.5 at S571, S516, and T594 on the DI–II loop of NaV1.5. When loss- and gain-of-function effects combine with other CaMKII ion channel targets, this can further enhance ventricular arrhythmias and sudden cardiac death.
CaMKII Regulation of Cardiac Na+ Channels
CaMKII was shown to associate with and phosphorylate NaV1.5, causing characteristic INa gating changes in mouse and rabbit ventricular myocytes.9 Specifically, CaMKII shifted INa availability to more negative potentials, enhanced entry into intermediate inactivation and slowed recovery from inactivation, all of which are loss-of function effects (analogous to BRs). CaMKII also increased late INa (INaL), an acquired LQTs gain-of-function effect. These potentially arrhythmogenic INa effects were acutely abolished by CaMKII inhibitors KN93 or AIP in rabbit myocytes. CaMKII expression and activity are both increased in HF.10,11 and CaMKIIδ overexpressing mice exhibit enhanced arrhythmogenesis.9 Notably, the full set of CaMKII- induced changes in INa gating almost exactly phenocopies a human point mutation (Ins1795D in the C-terminus) that is linked with combined LQTs and BRs in the same patients.12 In these contexts, the seminal Wagner et al.9 study fueled the search for critical CaMKII target sites on NaV1.5 that could explain these gating effects and identify potential therapeutic targets for arrhythmias in cardiac disease.
Based on the above, one might look for a CaMKII target site in the C-terminal tail (near residue 1795), but Aiba et al.13 provided evidence that the I–II loop might be a major CaMKII phosphorylation target. Utilizing a computer based scan for the traditional CaMKII consensus sequence, RXXS/T, Hund et al.14 identified S571 as a potential CaMKII target (Figure 1). They demonstrated that CaMKII phosphorylates S571 in vitro and that, in a heterologous cell system expressing NaV1.5, CaMKII shifts WT steady state inactivation to negative potentials. This effect on channel inactivation was abolished when S571 was mutated to a non-phosphorylatable alanine and mimicked when S571 was mutated to a phospho-mimetic glutamine reside. Our group15 found that only the I–II loop of hNaV1.5 was substantially phosphorylated by CaMKII (i.e. neither other loops nor N-or C-tail were targets), and systematic analysis of the entire I–II loop showed that S516 and T594 were the main in vitro CaMKII phosphorylation sites. In patch-clamp analysis, we found that alanine substitution of S516, S571 and T594 could all inhibit the CaMKII-dependent negative shift in INa availability and accumulation of intermediate inactivation observed in myocytes. However, only S516E and T594E phospho-mimetic mutants could recapitulate CaMKII effects on INa availability. Thus there may be three sites in this stretch of the I–II loop that participate in CaMKII-dependent regulation of cardiac INa gating (Figure 1).
There are two major points raised by Koval et al.1 in extending their work on the S571 site. First, they provide evidence that phosphorylation of S571 on NaV1.5 is increased in the canine post-infarct border zone, may be slightly increased in human HF and is increased in isolated mouse myocytes after acute stress (isoproterenol + phosphatase inhibition), but not in mice expressing CaMKII inhibitor AC3-I. These observations have potentially important implications for the genesis of acquired arrhythmias in cardiac disease. Second, they show that two rare, naturally occurring NaV1.5 point mutations (A572D and Q573E) might functionally mimic the negative charge induced by S571 phosphorylation, resulting in similar channel biophysics. While these experiments only indirectly suggest this mimicry, electrostatic potential maps are also consistent with this idea. These channels also evoked AP prolongation in cultured neonatal mouse ventricular myocytes. The authors thus posit that the region comprising residues 571–573 functions as a sort of negatively charged “hot spot”, with a negative charge at any of these three residues (due to phosphorylation or a naturally occurring mutation) conferring similar results on channel gating and function. The 571–573 hot spot referred to here could extend to encompass the other CaMKII modulation sites (S516 and T594), which may also have a local charge basis such that multiple local sites might contribute to altered INa gating. Importantly, this may provide a new therapeutic target downstream of CaMKII for heart failure and arrhythmias.16 Further studies will be required to clarify these molecular mechanisms.
CaMKII and Arrhythmias in HF
CaMKII transgenic mice exhibited tachyarrhythmias9 and CaMKII inhibition may be protective against electrical17 and structural18 remodeling in HF. Interestingly, heterozygous SCN5A+/− knock-out19 and Ins1795D knock-in20 mice also have conduction disturbances and increased propensity to arrhythmias. Since CaMKII can cause both LQTs-like INaL and loss of function BRs-like effects,3,4 it is worth considering how CaMKII effects contribute to arrhythmogenesis and increase the risk of SCD in HF. Channelopathies associated with NaV1.5 loss of function, such as BRs, and gain of function, LQT3, provide a nice framework for discussion.
Koval et al.1 found that A572D and Q573E arrhythmia variants expressed in cultured neonatal mouse cardiomyocytes exhibited enhanced INaL and APD that was sensitive to TTX and the INaL specific inhibitor Ranolazine. Early afterdepolarizations were even seen in 2 of the 9 A572D neonatal mouse myocytes, and their computer simulations produced comparable results, and resemble earlier LQT3 models of INaL effects gain of function mutations in SCN5A.21 In our computational model of CaMKII effects on INa in rabbit,22 an LQT3-like phenotype was present at low heart rates (where APD is long) and thus integrated INaL is large. At higher heart rates (> 1Hz), the APD effect disappeared, and a BRs-like loss of INa availability and slower AP rate of rise was more apparent. Several studies have demonstrated increased INaL in HF 23,24 and some of this may be dependent on CaMKII activity.25 More mechanistic work will be needed to test which (if any) of the CaMKII phosphorylation sites is critical for enhanced INaL. Regardless, selective INaL inhibition may be an important therapeutic strategy to treat some LQT-like arrhythmias in HF.26
What about the INa loss of function effects caused by CaMKII? CaMKII and all of the identified CaMKII sites (S571, T594, and S516) seem to mediate loss of INa function effects (reduced INa availability, increased intermediate inactivation and slowed recovery from inactivation). These effects may also contribute to arrhythmogenesis analogous to BRs, but have attracted less attention than the INaL and LQT3-like effects. The loss of INa function induced by CaMKII is likely to be exacerbated at high heart rates22 and could slow conduction, create conduction block in vulnerable tissue, shorten APD and enhance the substrate for reentrant arrhythmias (Figure 1). In that sense, the CaMKII effects would match the Ins1795D and ΔK1500 human mutations, where patients exhibit LQT symptoms at low heart rate and Brugada-like symptoms at high heart rate.12,27
CaMKII effects on Other Channels Contribute to Arrhythmias
CaMKII also modulates ICa and Ito and IK1 properties16 (Figure 1). CaMKII mediates ICa facilitation, which enhances peak ICa and slows inactivation (gain-of-function), and tends to prolong APD (along with INaL), favoring EADs. CaMKII also enhances Ito recovery from inactivation, thereby hastening repolarization. When the CaMKII effects on ICa, Ito, and INa are combined, APD shortening is predicted in epicardial rabbit myocytes.22 However, in endocardial or HF myocytes, with reduced Ito expression and function, APD would be prolonged because of INa and ICa effects. This would enhance the normal transmural dispersion of repolarization, thereby enhancing the substrate for reentrant arrhythmias. Additionally, regional differences in CaMKII activity, such as around myocardial infarct zones, may translate into heterogeneity of phosphorylated channels. Re-entrant arrhythmias could thus ensue as in BRs. Clearly the combined effects of CaMKII in cardiac electrical remodeling are complex and further studies are needed.
CaMKII also phosphorylates phospholamban (PLN) to stimulate SR Ca-ATPase (enhancing SR Ca2+ loading) and the SR Ca2+ release channel (RyR2) enhancing arrhythmogenic spontaneous SR Ca2+ release.16 Moreover, when the putative RyR2 CaMKII phosphorylation is mimicked (S2814D) or prevented (S2818A) in knock-in mice, there is increased or decreased (respectively) DADs and triggered arrhythmias.28 Indeed, the combination of enhanced SR Ca2+ uptake and sensitized RyR2 may synergize in developing CaMKII-dependent DADs. In the setting of HF, where Na/Ca exchange is increased and IK1 is reduced (both of which increase DAD amplitude), there is an increased propensity for triggered arrhythmias.29
All of the above pathways could degenerate into lethal ventricular tachyarrhythmias (Figure 1). These mechanisms are certainly not mutually exclusive, and a mixture of several factors plays a role. Inherited mutations at Ins1795D and ΔK1500 in hNaV1.5 with combined LQTs and BRs and RyR2 sensitizing inherited mutations linked to catecholaminergic polymorphic ventricular tachycardia (CPVT), both of which phenocopy CaMKII effects, may identify these channel targets as especially important.
Future Studies
Koval et al.1 furthers our understanding of the role and importance of CaMKII based NaV1.5 phosphorylation at S571, but several key questions remain. What is the relative role of S571 and the other two CaMKII phosphorylation sites S516 and T594 (additive, synergistic, redundant, antagonistic)? Differences in the mode or location of CaMKII activation could differentially influence targets and their impact. For example, binding of CaMKII to β-spectrin may enhance site phosphorylation14 and activation state of CaMKII may alter its binding affinities for certain substrates.30 Further studies will are needed to evaluate the multiple phosphorylatable CaMKII sites on the Na+ channel.
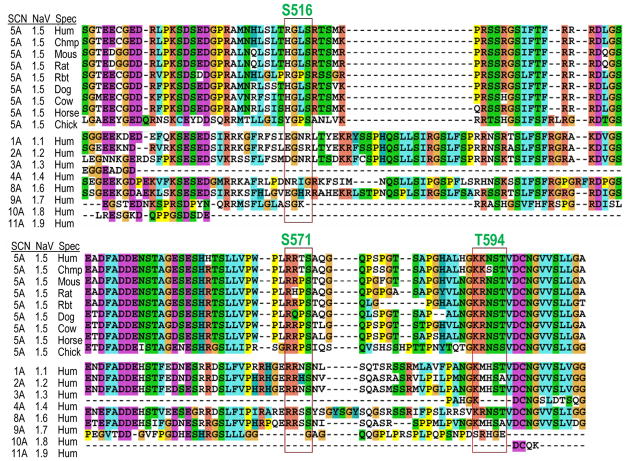
Going forward, it will be valuable to understand CaMKII effects on other Na+ channel isoforms (Figure 2), some of which may be expressed in the heart (NaV1.1, 1.3 and 1.6).8 The S571 and T594 CaMKII phospho-sites are well conserved among mammalian NaV1.5 (and in chicken). The S516 phospho-site, in contrast, appears to be exclusive to human NaV1.5 (in others there is no basic residue at the important P-3 position, except in rabbit). Skeletal muscle NaV1.4 (SCN4A) has none of these phosphorylation sites. Some neuronal Na+ channels (SCN1A, 2A, 8A and 9A or NaV1.1, 1.2, 1.6 and 1.7) contain the S571 site, but the T594 site is only the same in SCN8A (NaV1.6). This opens up new avenues of study about potential roles of CaMKII-dependent regulation of Na+ channels.
Figure 2.
Differential Conservation of CaMKII Phosphorylation Sites Across Species and Isoform. ClustalX2 protein sequence alignments of human NaV1.5 across species and NaV isoform. The I–II loop is shown with CaMKII consensus sequence (red bounding box) and Ser or Thr phospho-site highlighted. Species and isoform gene names as indicated.
It will also be important to understand which CaMKII channel targets are the most important arrhythmogenic influences (among Na+, K+ and Ca2+ channels or RyR2). Furthermore, studies are needed to determine if different populations of e.g. Na+ channels are present even within the same myocyte that differ in their signaling interactions and regulation by CaMKII. It may also be important to test whether these sites are targeted by other kinases relevant to cardiac pathophysiology, such as PKA and PKC. While much work remains to fully understand the complexities of phospho-regulation of voltage gated Na+ channels, the work from Koval et al.1 is an important step forward in our understanding of this process and its relevance to cardiac disease.
Acknowledgments
Sources of Funding: This study has been funded by NIH P01-HL080101, R37-HL030077 and training grant T32-GM099608.
Footnotes
Conflict of Interest Disclosures: None.
References
- 1.Koval OM, Snyder JS, Wolf RM, Pavlovicz RE, Cardona N, Glynn P, Leymaster ND, Dun W, Wright PJ, Qian L, Mitchell CC, Boyden PA, Binkley PF, Li C, Anderson ME, Mohler PJ, Hund TJ. CaMKII-based regulation of voltage-gated Na+ channel in cardiac disease. Circulation. 2012;126:XX–XXX. doi: 10.1161/CIRCULATIONAHA.112.105320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grant AO. Cardiac ion channels. Circ Arrhythm Electrophysiol. 2009;2:185–194. doi: 10.1161/CIRCEP.108.789081. [DOI] [PubMed] [Google Scholar]
- 3.Wilde AA, Brugada R. Phenotypical manifestations of mutations in the genes encoding subunits of the cardiac sodium channel. Circ Res. 2011;108:884–897. doi: 10.1161/CIRCRESAHA.110.238469. [DOI] [PubMed] [Google Scholar]
- 4.Zimmer T, Surber R. Scn5a channelopathies -an update on mutations and mechanisms. Prog Biophys Mol Biol. 2008;98:120–136. doi: 10.1016/j.pbiomolbio.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 5.Tomaselli GF, Zipes DP. What causes sudden death in heart failure? Circ Res. 2004;95:754–763. doi: 10.1161/01.RES.0000145047.14691.db. [DOI] [PubMed] [Google Scholar]
- 6.Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Makuc DM, Marcus GM, Marelli A, Matchar DB, Moy CS, Mozaffarian D, Mussolino ME, Nichol G, Paynter NP, Soliman EZ, Sorlie PD, Sotoodehnia N, Turan TN, Virani SS, Wong ND, Woo D, Turner MB American Heart Association Statistics C Stroke Statistics S. Heart disease and stroke statistics--2012 update: A report from the american heart association. Circulation. 2012;125:e2–e220. doi: 10.1161/CIR.0b013e31823ac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abriel H. Cardiac sodium channel Nav1.5 and interacting proteins: Physiology and pathophysiology. J Mol Cell Cardiol. 2010;48:2–11. doi: 10.1016/j.yjmcc.2009.08.025. [DOI] [PubMed] [Google Scholar]
- 8.Meadows LS, Isom LL. Sodium channels as macromolecular complexes: Implications for inherited arrhythmia syndromes. Cardiovasc Res. 2005;67:448–458. doi: 10.1016/j.cardiores.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 9.Wagner S, Dybkova N, Rasenack EC, Jacobshagen C, Fabritz L, Kirchhof P, Maier SK, Zhang T, Hasenfuss G, Brown JH, Bers DM, Maier LS. Ca2+/calmodulin-dependent protein kinase II regulates cardiac Na+ channels. Journal Clin invest. 2006;116:3127–3138. doi: 10.1172/JCI26620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hoch B, Meyer R, Hetzer R, Krause EG, Karczewski P. Identification and expression of delta-isoforms of the multifunctional Ca2+/calmodulin-dependent protein kinase in failing and nonfailing human myocardium. Circ Res. 1999;84:713–721. doi: 10.1161/01.res.84.6.713. [DOI] [PubMed] [Google Scholar]
- 11.Ai X, Curran JW, Shannon TR, Bers DM, Pogwizd SM. Ca2+/calmodulin-dependent protein kinase modulates cardiac ryanodine receptor phosphorylation and sarcoplasmic reticulum Ca2+ leak in heart failure. Circ Res. 2005;97:1314–1322. doi: 10.1161/01.RES.0000194329.41863.89. [DOI] [PubMed] [Google Scholar]
- 12.Veldkamp MW, Viswanathan PC, Bezzina C, Baartscheer A, Wilde AA, Balser JR. Two distinct congenital arrhythmias evoked by a multidysfunctional Na+ channel. Circ Res. 2000;86:E91–97. doi: 10.1161/01.res.86.9.e91. [DOI] [PubMed] [Google Scholar]
- 13.Aiba T, Hesketh GG, Liu T, Carlisle R, Villa-Abrille MC, O’Rourke B, Akar FG, Tomaselli GF. Na+ channel regulation by Ca2+/calmodulin and Ca2+/calmodulin-dependent protein kinase II in guinea-pig ventricular myocytes. Cardiovasc Res. 2010;85:454–463. doi: 10.1093/cvr/cvp324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hund TJ, Koval OM, Li J, Wright PJ, Qian L, Snyder JS, Gudmundsson H, Kline CF, Davidson NP, Cardona N, Rasband MN, Anderson ME, Mohler PJ. A beta(iv)-spectrin/ CaMKII signaling complex is essential for membrane excitability in mice. J Clin Invest. 2010;120:3508–3519. doi: 10.1172/JCI43621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ashpole NM, Herren AW, Ginsburg KS, Brogan JD, Johnson DE, Cummins TR, Bers DM, Hudmon A. Ca2+/calmodulin-dependent protein kinase II (CaMKII) regulates cardiac sodium channel NaV1.5 gating by multiple phosphorylation sites. J Biol Chem. 2012;287:19856–19869. doi: 10.1074/jbc.M111.322537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Anderson ME, Brown JH, Bers DM. CaMKII in myocardial hypertrophy and heart failure. J Mol Cell Cardiol. 2011;51:468–473. doi: 10.1016/j.yjmcc.2011.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tsuji Y, Hojo M, Voigt N, El-Armouche A, Inden Y, Murohara T, Dobrev D, Nattel S, Kodama I, Kamiya K. Ca2+-related signaling and protein phosphorylation abnormalities play central roles in a new experimental model of electrical storm. Circulation. 2011;123:2192–2203. doi: 10.1161/CIRCULATIONAHA.110.016683. [DOI] [PubMed] [Google Scholar]
- 18.Zhang R, Khoo MS, Wu Y, Yang Y, Grueter CE, Ni G, Price EE, Jr, Thiel W, Guatimosim S, Song LS, Madu EC, Shah AN, Vishnivetskaya TA, Atkinson JB, Gurevich VV, Salama G, Lederer WJ, Colbran RJ, Anderson ME. Calmodulin kinase II inhibition protects against structural heart disease. Nature Med. 2005;11:409–417. doi: 10.1038/nm1215. [DOI] [PubMed] [Google Scholar]
- 19.Papadatos GA, Wallerstein PM, Head CE, Ratcliff R, Brady PA, Benndorf K, Saumarez RC, Trezise AE, Huang CL, Vandenberg JI, Colledge WH, Grace AA. Slowed conduction and ventricular tachycardia after targeted disruption of the cardiac sodium channel gene SCN5A. Proc Natl Acad Sci U S A. 2002;99:6210–6215. doi: 10.1073/pnas.082121299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Remme CA, Verkerk AO, Nuyens D, van Ginneken AC, van Brunschot S, Belterman CN, Wilders R, van Roon MA, Tan HL, Wilde AA, Carmeliet P, de Bakker JM, Veldkamp MW, Bezzina CR. Overlap syndrome of cardiac sodium channel disease in mice carrying the equivalent mutation of human SCN5A-1795insd. Circulation. 2006;114:2584–2594. doi: 10.1161/CIRCULATIONAHA.106.653949. [DOI] [PubMed] [Google Scholar]
- 21.Clancy CE, Tateyama M, Kass RS. Insights into the molecular mechanisms of bradycardia-triggered arrhythmias in long QT-3 syndrome. J Clin Invest. 2002;110:1251–1262. doi: 10.1172/JCI15928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grandi E, Puglisi JL, Wagner S, Maier LS, Severi S, Bers DM. Simulation of Ca-calmodulin-dependent protein kinase II on rabbit ventricular myocyte ion currents and action potentials. Biophys J. 2007;93:3835–3847. doi: 10.1529/biophysj.107.114868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maltsev VA, Reznikov V, Undrovinas NA, Sabbah HN, Undrovinas A. Modulation of late sodium current by Ca2+, calmodulin, and CaMKII in normal and failing dog cardiomyocytes: Similarities and differences. Am J Physiol Heart Circ Physiol. 2008;294:H1597–1608. doi: 10.1152/ajpheart.00484.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Valdivia CR, Chu WW, Pu J, Foell JD, Haworth RA, Wolff MR, Kamp TJ, Makielski JC. Increased late sodium current in myocytes from a canine heart failure model and from failing human heart. J Mol Cell Cardiol. 2005;38:475–483. doi: 10.1016/j.yjmcc.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 25.Wagner S, Ruff HM, Weber SL, Bellmann S, Sowa T, Schulte T, Anderson ME, Grandi E, Bers DM, Backs J, Belardinelli L, Maier LS. Reactive oxygen species-activated ca/calmodulin kinase iidelta is required for late INa augmentation leading to cellular Na and Ca overload. Circ Res. 2011;108:555–565. doi: 10.1161/CIRCRESAHA.110.221911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Belardinelli L, Shryock JC, Fraser H. Inhibition of the late sodium current as a potential cardioprotective principle: Effects of the late sodium current inhibitor ranolazine. Heart. 2006;92(Suppl 4):iv6–iv14. doi: 10.1136/hrt.2005.078790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grant AO, Carboni MP, Neplioueva V, Starmer CF, Memmi M, Napolitano C, Priori S. Long QT syndrome, brugada syndrome, and conduction system disease are linked to a single sodium channel mutation. J Clin Invest. 2002;110:1201–1209. doi: 10.1172/JCI15570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van Oort RJ, McCauley MD, Dixit SS, Pereira L, Yang Y, Respress JL, Wang Q, De Almeida AC, Skapura DG, Anderson ME, Bers DM, Wehrens XH. Ryanodine receptor phosphorylation by calcium/calmodulin-dependent protein kinase II promotes life-threatening ventricular arrhythmias in mice with heart failure. Circulation. 2010;122:2669–2679. doi: 10.1161/CIRCULATIONAHA.110.982298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pogwizd SM, Bers DM. Cellular basis of triggered arrhythmias in heart failure. Trends Cardiovasc Med. 2004;14:61–66. doi: 10.1016/j.tcm.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 30.Robison AJ, Bartlett RK, Bass MA, Colbran RJ. Differential modulation of ca2+/calmodulin-dependent protein kinase ii activity by regulated interactions with n-methyl-d-aspartate receptor nr2b subunits and alpha-actinin. J Biol Chem. 2005;280:39316–39323. doi: 10.1074/jbc.M508189200. [DOI] [PubMed] [Google Scholar]