Abstract
Objective
Patients with schizophrenia speak with blunted vocal affect but little is known regarding the prosody of persons with schizotypal personality disorder (SPD). This work examined expressive prosody in SPD, its relationship to brain structure, and outlined a framework for measuring elements of prosody in clinical populations.
Methods
Twenty-eight antipsychotic-naïve SPD subjects were matched with 27 healthy comparison (HC) subjects. Subjects read aloud short sentences and responded to probes to record both predetermined and self-generated speech samples. Samples were analyzed acoustically (pause proportion, duration, attack, and pitch variability) and subjectively by raters (amount of pauses, degree of emotion portrayed, and how much they wanted to hear more from the subjects) on paragraph, sentence, word, word-fragment, and syllable levels. Alexithymia and ability to self-monitor behavior was compared between groups. The pars opercularis was manually traced on structural MRI data.
Results
SPD subjects' speech had significantly more pauses, was slower, had less pitch variability, and expressed less emotion than HC subjects. Pitch variability correlated with socio-economic status achievement. There was no difference between groups in left or right pars opercularis volumes. A statistically significant correlation suggested smaller left pars opercularis volumes in SPD subjects correlated with more pauses and less emotion. SPD subjects reported more alexithymia and difficulty self-monitoring their behavior compared with controls. In SPD subjects the high alexithymia correlated with raters not wanting to hear more from them and SPD subjects' inability to modulate their social behavior correlated with their having fewer friends. Thus, the SPD subjects exhibited insight.
Conclusions
SPD subjects displayed significant prosodic deficits that were measurable in speech samples as brief as a word-fragment. The determinants of these deficits are not known although may include a dysfunctional pars opercularis. These data add to the nascent literature describing social cognition deficits in SPD.
Keywords: Schizotypal personality disorder, schizophrenia, prosody, vocal affect, pars opercularis, inferior frontal lobe, alexithymia, social cognition
Introduction
Prosody is the vocal expression of one's internal emotional state or intent. Key components or elements include pitch, pitch variability, attack, pauses between sounds, and duration of the utterance (Leentjens et al. 1998) (Shaw et al. 1999) (Leitman et al. 2010) (Leitman et al. 2011) (Table 1, Elements). By varying these elements in speech, people transmit subtle distinctions of meaning. Pitch variability may be the most important aspect of prosody (Leitman et al. 2010) (Ross et al. 2001). For example, simply modulating pitch can change the expressed emotion from anger to sadness. Similar alterations in amount of pauses, duration of an utterance, or attack, can dramatically affect the sound and cadence of speech and, hence, the expression of emotion and meaning (Monnot et al. 2003). The ability to modulate one's voice slightly to reflect accurately one's intention is important for social communication.
Table 1.
Elements, Measurements, and Hypotheses. Variables were listed along with how they were measured, comments for clarification of the variable or the measurement, the type of analysis used (either acoustic or subjective), the level of the analysis (speech sample length), the rationale behind inclusion of the variable, and the specific a priori hypotheses associated with the variable. Note that some variables, such as attack, were added post-hoc, and were indicated as such. This table was included for clarification.
| Prosodic Element | Measurement | Comments | Type of Analysis | Level of Analysis and Sample Used | Rationale | Hypotheses |
|---|---|---|---|---|---|---|
| Acoustic Analysis | ||||||
| Pause Proportion | Percentage of time the sample contained a pitch above baseline from time of first utterance to final utterance. | This would include utterances such as “uh”, for example, as well as proper words. | Acoustic | Paragraph (Admire, Store) Sentence (Nutmeg) |
Self-generated sentences selected as the natural flow of speech was of interest. Nutmeg included in contrast as was neutral and pre-determined sentence. Analyses performed “blind” to content. |
Admire, Store SPD > HC Nutmeg SPD = HC |
| Duration | Time from beginning to end of a defined speech sample. Beginning and end were determined by detecting a change in pitch. | Analogous to how fast someone speaks a given word. | Acoustic | Word (“adorable”) Word-fragment (“dora”) |
Emotional word “adorable” selected as wanted to measure impact of emotion on speech speed. “Dora” also included as unknown how short an utterance would be necessary to see group differences. |
“adorable” “dora” No hypothesis |
| Attack | Rise in amplitude (loudness) over time for a given phoneme. Beginning defined as change in slope from baseline. Occasionally slope changed direction. To ensure reproducibility, end defined by maximum amplitude. |
Analogous to with how much “punch” or stress an utterance was said. Element added post-hoc as wished to explore as many variables as possible. |
Acoustic | Syllable (“Nut”, “do”) | “Nut” selected as beginning of neutral sentence. “do” was isolated from “adorable” as visual inspection of data blind to diagnostic group suggested most variation in frequency. |
“Nut” “do” No hypotheses |
| Pitch Variability | Standard deviation in Hertz of the pitch maximum and minimum across an utterance, same as the standard deviation of the fundamental frequency | Change in pitch Analogous to amount of variation in inflection |
Acoustic | Sentence (Nutmeg, Puppy) Word (“adorable”) Word-fragment (“dora”) |
Predetermined sentences used in order to control for content. “adorable” and “dora” were included as it was unknown how short an utterance could be and still demonstrate group differences. |
Nutmeg SPD = HC Puppy SPD < HC “adorable” “dora” No hypotheses |
| Subjective Analysis | ||||||
| Rating of Amount of Pauses | Mean amount of pauses along Likert scale perceived by raters. | Tapped into same aspect of speech as pause proportion above, but subjectively rated | Subjective | Paragraph (Admire, Store) | Self-generated paragraphs used in order to have naturalistic sample. Raters likely unable to “blind” themselves to content, that is, their assessments may be affected by content. Unknown the degree to which raters “tuned out” the “ums” and “ahs” that occur naturally in speech. |
Admire SPD > HC Store SPD > HC |
| Emotion Portrayed | Mean degree along Likert scale that the raters thought the subject spoke with emotion. | How emotional the subject sounded Admire minus Store added post-hoc |
Subjective | Paragraph (Admire, Store) Paragraph (Admire minus Store) |
Self-generated paragraphs used in order to have naturalistic sample. Admire minus Store was examined to see if subjects altered the emotional valence of their speech with the more evocative probe, Admire, than they did with the less evocative probe, Store. Raters likely unable to “blind” themselves to content, that is, their assessments may be affected by content. |
Admire SPD < HC Store SPD < HC Admire – Store SPD < HC |
| Hear More | Mean degree along Likert scale that the raters said that they wanted to hear more from the subject. | Subjective | Paragraph (Admire, Store) | Self-generated paragraphs used in order to have naturalistic sample. Raters likely unable to “blind” themselves to content, that is, their assessments may be affected by content. |
Admire SPD < HC Store SPD < HC |
|
Although the hallmark feature of abnormal speech in schizophrenia is in the domain of semantics clinically encapsulated by the term “formal thought disorder”, abnormal vocal affect has been demonstrated as well (Borod et al. 1989) (Ross et al. 2001) (Hoekert et al. 2007) (Murphy and Cutting 1990) (Leentjens et al. 1998). Prosodic deficits initially described by Beuler included abnormalities of duration, amplitude, and pitch(Bleuler 1911, 1950) (Baltaxe and Simmons 1995). Further work identified reduced speech intonation and impaired emotional mimicry(Alpert et al. 2000) (Mitchell and Crow 2005) (Leentjens et al. 1998) (Murphy and Cutting 1990, Ross et al. 2001) (Murphy and Cutting 1990). Indeed, a meta-analysis of five studies of expressive prosody in schizophrenia revealed a large effect size (Cohen's d = −1.11)(Hoekert et al. 2007). None of these studies, however, included fine-grained analyses of the various elements of prosody.
Interpretation of such studies is complicated by schizophrenic subjects' use of antipsychotic medications, as antipsychotics may affect prosody and brain morphology (Keshavan et al. 1998) (Kee et al. 1998). Schizotypal personality disorder (SPD) subjects may represent an ideal population for study of prosody given that SPD and schizophrenic subjects are epidemiologically related (Kendler et al. 1993), yet SPD subjects have not been prescribed antipsychotic medications. Moreover, both patients with schizophrenia and SPD subjects demonstrate social cognition deficits including impaired facial affect recognition and expression(Dickey et al. 2011); fail to achieve expected social roles (Dickey et al. 2005); and have odd speech and formal thought disorder (Dickey et al. 1999).
Although expressive prosodic speech has not been studied in SPD, auditory perception has been. SPD subjects have deficits in the processing of simple auditory percepts (tones) and higher-order phoneme matching (Dickey et al. 2010, Dickey et al. 2008). Although SPD subjects may interpret prosody correctly, in a recent fMRI study they were shown to have “inefficient” activation in the superior temporal gyrus (STG) while hearing sentences with happy, sad, sarcastic, or neutral intonation (Dickey et al. 2010). Specifically, for healthy comparison subjects, an increase in the extent of activation in the STG in either the left or right correlated with more prosodic sentences correctly identified. For SPD subjects, there was no such relationship between extent of activation and accuracy (Dickey et al. 2010). The STG, superior temporal sulcus, and other brain regions considered important for auditory and prosodic processing, thus may have morphological and functional abnormalities in SPD, particularly on the left(Downhill et al. 2001, Dickey et al. 2003, Dickey et al. 1999, Dickey et al. 2010).
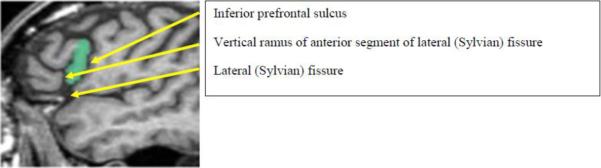
Abnormalities in the frontal lobe have also been found in SPD subjects, albeit less extensively than in schizophrenic patients (for review see Hazlett et al., 2012 (Hazlett, Goldstein and Kolaitis 2012), and Dickey et al, 2002 (Dickey, McCarley and Shenton 2002)). Specifically, SPD subjects have larger bilateral middle frontal gyri, smaller right straight gyri (Suzuki et al. 2005), and smaller left inferior frontal gyri compared with healthy control subjects(Kawasaki et al. 2004). The posterior part of the left inferior frontal gyrus, (Brodmann Area 44, Broca's area), the pars opercularis, is important for motoric speech generation (Foundas et al. 1998, Tomaiuolo et al. 1999), Figure 1).
Figure 1.
Left Pars OpercularisTracing. Boundaries were defined on lateral sagittal images as follows. The medial boundary was defined by the first appearance of insula white matter; posterior boundary by demarcating the inferior prefrontal sulcus; anterior boundary by the vertical ramus of the anterior segment of the lateral fissure (i.e.: Sylvian fissure); inferior boundary by the lateral fissure; and superior boundary by the superior extent of the pars opercularis, except in cases of contiguity with other prefrontal structures, in which case a line was drawn to separate it. In those instances in which the extent of pars opercularis was unclear, guidance came from outlining the vertical ramus and the pre-central sulcus on axial images with coronal images used for confirmation.
The pars opercularis has not been evaluated in SPD and deserves attention. It is thought to be critical for sequencing motor movements involved in phoneme production (Koechlin and Jubault 2006)), and for verbal fluency, the progression from one phoneme to the next (Foundas et al. 1998, Tomaiuolo et al. 1999). The requirement of appropriate sequencing and fluency in speech suggests that executive functioning has a major role in speech production (Burns and Fahy 2010). Note that verbal fluency and phoneme processing are impaired in SPD (Dickey et al. 2010) as well as in schizophrenia (Kugler and Caudrey 1983) (Johnson-Selfridge and Zalewski 2001). Most germane to the current report, however, is the pars opercularis involvement in prosody production (Sahin et al. 2009) (Aziz-Zadeh, Sheng and Gheytanchi 2010) and perception (Aziz-Zadeh et al. 2010, Wildgruber et al. 2005). Prosody perception also increases the functional coupling of the “emotional voice areas” of the STG with the inferior frontal gyrus, suggesting that the STG and the inferior frontal gyrus work in concert (Ethofer et al. 2012). Abnormal cortical gyrification of the pars opercularis (Wisco et al. 2007)and bilateral volume reduction of the inferior frontal gyrus (Suzuki et al. 2005) have suggested abnormal in utero development, and possibly, abnormal cortical connectivity with consequent language disturbances (Wisco et al. 2007) in schizophrenic patients.
One of the goals of the current report was to examine the pars opercularis. If the left pars opercularis is reduced in volume in SPD, then that might provide a clue regarding the pathogenesis of abnormal speech production in SPD.
Another objective was to investigate whether SPD subjects have deficits expressing prosody, and, if so, with which specific elements and in how short an utterance. To address this objective two types of speech samples were recorded: predetermined and self-generated. Predetermined sentences (one with neutral and one with emotional content) were read aloud to obtain a consistent sample across all subjects. Self-generated samples were responses to probes that gave rise to a longer and more naturalistic speech samples. Both types of samples were evaluated in two ways: acoustic analysis and subjective ratings (Table 1, Type of Analysis). Acoustic analyses were undertaken to examine the pitch variability, attack, pause proportion, and duration of speech, devoid of any influence from semantic content or gender effects. Subjective ratings by raters were included to examine the social effectiveness of language, including the degree of emotion, the amount of pauses, and extent that the raters wished to hear more from the speaker. The length of the utterance examined--word fragment, word, sentence, or paragraph--differed based on which element was being evaluated (Table 1, Rationale).
Subjects' self-assessments of alexithymia (Vorst and Bermond 2001) and ability to self-monitor their behavior were assessed (Snyder and Gangestad 1986, Penn et al. 1999), to determine whether SPD subjects, like persons with schizophrenia, recognized their difficulties in the realm of alexithymia (Kubota et al. 2011, Sasamoto et al. 2011). Alexithymia refers to difficulties in the realms of experiencing, identifying, and expressing emotions, particularly verbally (Swart, Kortekaas and Aleman 2009, Bermond, Vorst and Moormann 2006) (Mattila et al. 2009). Alexithymia may reflect right hemisphere dysfunction (Bermond et al. 2006) and can affect quality of life in healthy persons (Mattila et al. 2009). Alexithymia is present in a range of psychiatric disorders, including other personality disorders (Loas et al. 2012, Loas 2012, Domes et al. 2011).
The ability to recognize such deficits in oneself—insight--in schizophrenia can have profound yet variable effects on medication compliance, suicidal risk, and social functioning (Melle and Barrett 2012). Evidence of poor insight in SPD comes mainly from the obsessive compulsive disorder (OCD) literature. OCD subjects with co-morbid SPD, have been shown to have less insight and poorer outcome than those without co-morbid SPD (Catapano et al. 2010) (Poyurovsky et al. 2008). In the current study insight was indirectly assessed by correlating SPD subjects' assessment of their own alexithymia with rater's desire to hear more; and subjects' ability to monitor their social behavior with self-report of number of meaningful friendships or confidants.
It was predicted that SPD subjects compared with healthy control (HC) subjects would demonstrate abnormalities of prosodic elements acoustically and subjectively (Table 1, Hypotheses), and that raters would be less likely to want to hear more from SPD subjects than HC subjects. SPD subjects were predicted to have reduced volumes of the left pars opercularis based on findings in other language-related imaging studies (Dickey et al. 1999) (Dickey et al. 2010). Further prediction included SPD subjects, similar to schizophrenic subjects, would have preserved insight regarding their alexithymia and difficulty monitoring their behavior (Penn et al. 1999, Kubota et al. 2011).
A final objective of this study was to provide a novel yet easily applicable method to measure prosody in clinical populations. In addition to schizophrenia, many disorders manifest signs of usual prosody and pragmatics including autism, William's syndrome, primary aprosodias, social phobia, and right-hemisphere stroke (Hubbard and Trauner 2007, Ross and Monnot 2008) (Ross et al. 2001, Heilman, Leon and Rosenbek 2004, Rosenbek et al. 2004, Mervis and John 2010, Laukka et al. 2011). The simple techniques employed here can be applied to measure specific prosodic deficits or to track improvement during clinical trials targeting social remediation.
Methods
Subject Recruitment
Subjects were recruited from the community (Dickey et al. 2005). Inclusion criteria were right-handed; age 18–55; no history of psychosis, ECT, loss of consciousness more than 5 minutes, neurologic disorder, treatment with antipsychotics, active psychotropic usage, substance dependence in the last five years or abuse in the last year as determined by self-report; English as the first-language; and IQ greater than 80. Additional criteria for HC subjects were no first degree relative with mental illness per subject report and no personal history of mental illness or personality disorder.
All subjects participated in SCID and SCID II interviews. Groups were one-to-one matched on age and group-matched on parental socio-economic status (PSES), IQ, and gender. Groups were matched on PSES not personal SES as SPD has been shown to hinder persons from achieving their expected social and occupational roles (Dickey et al. 2005), similar to what has been shown in schizophrenia. Eleven SPD and 12 HC subjects previously studied also participated in prior experiments of prosody perception (Dickey et al. 2010). After complete description of the study to the subjects, written informed consent was obtained. This work had approval from local Institutional Review Boards.
Voice Recordings
Two different types of speech samples were recorded: predetermined, with all subjects saying the same sentences; and self-generated, with all subjects responding to a probe using their own words and concepts. Predetermined speech samples were acquired by asking subjects to read aloud: “Nutmeg is a spice”, an emotionally neutral sentence (referred to as Nutmeg); and “The puppies are adorable”, a positively valenced sentence (Puppies). Note that the reading of Puppies was added after the study had already begun to include an emotional sentence resulting in a lower subject N. Both sentences were of equal word count (four words). These predetermined sentences allowed for the evaluation of prosody without the potential confound of variability of content across subjects as sentences were the same across subjects.
Self-generated speech samples were acquired by asking subjects to respond to the following probes: “Tell me about a recent trip to a store”, designed to generate neutral prosody (referred to as Store); and “Whom do you most admire and why?”, considered likely to generate emotion (Admire). Subjects had unlimited time to respond to these probes. This subject-generated speech, as opposed to the predetermined sentences, allowed for the recording of more naturalistic speech patterns.
Speech Sample Analyses
Two separate methods for analyzing the speech samples were performed. First, samples were analyzed acoustically using freely-available Praat software (Boersma and Weenink 2005) (www.ling.lu.se/persons/Sidney/frames.html). Variables examined included pause proportion, duration, attack and pitch variability (Table 1, Measurement).
Second, and more germane to social interactions, samples were rated subjectively by raters. Sentence order was counter-balanced across raters to minimize priming effects. The four raters were college-educated laboratory members blind to diagnostic group. Raters evaluated the samples for perceived amount of pauses, degree of emotion portrayed, and how much they wanted to hear more from a subject along a seven-point Likert scale (1= not at all, 4 = moderate, 7 = extreme) with ratings averaged across raters (Table 1). Interrater reliability was calculated using Intraclass correlation coefficient.
One goal of these experiments was to determine at what level of speech or in how short a sample of speech--paragraph, sentence, word, word-fragment, or phoneme level--could a deficit be demonstrated in SPD subjects (Table 1, Level of Analysis). Common sense guided the choice of level for each of the variables (Table 1, Rationale). For example, raters were not asked to judge the duration of a word-fragment as it would occur too quickly for reliable evaluation. However, raters were asked to evaluate the amount of pauses and degree of emotion in a subject's description of whom they admired.
Alexithymia
The Bermond-Vorst Alexithymia Questionnaire is a self-administered questionnaire to probe a subject's experience, fantasy, identification, consideration, and expression of their emotions (Vorst and Bermond 2001) (Bermond et al. 2006). It included statements such as, “I find it difficult to express my feelings verbally”, and “When I am upset, I know whether I am afraid or sad or angry”. This questionnaire was given to determine whether SPD subjects knew that they had difficulties vocalizing their inner experiences and emotions, in other words, whether the SPD subjects exhibited insight.
Self-Monitoring Scale
The Self-Monitoring Scale (SMS) (Snyder and Gangestad 1986) is a true/false self-administered questionnaire that inquired whether a subject believed that he/she can alter their behavior based on perceived expectations. Examples included: “I have trouble changing my behavior to suit different people and different situations”, and, “I may deceive people by being friendly when I really dislike them”. A high score means more effective paralinguistic social skills. This scale has been used reliably with subjects with schizophrenia, who have been shown to have low scores on this measure (Penn et al. 1999).
Volumetric Measures of Pars Opercularis
The pars opercularis was manually delineated bilaterally for 20 SPD and 19 HC subjects on previously-acquired high-resolution MRI images (1.5 Tesla, 124 coronal SPGR slices 1.5 mm thick, TR 35 ms, TE 5 ms, FOV 24 cm) using 3D slicer (www.slicer.org) (Figure 1). Volumes were corrected for brain size by regressing for intracranial content and using the saved residuals in subsequent analyses (Dickey et al. 2000).
Statistics and Interpretation
Groups were compared with ANOVA, two-tailed with alpha set at 0.05 for significance, on each of the prosodic elements (Table 1, Elements) as well as Alexithymia, Self-monitoring, and pars opercularis volumes. Effect sizes were measured by Cohen's d. “Expected SES” or the SES subjects were expected to obtain based on their parents' status (SES regressed for PSES), was computed(Dickey et al. 2005). Exploratory correlations were performed between Expected SES and pitch variation, the element possibly most critical to prosody (Leitman et al. 2010) (Ross et al. 2001). In order to discern whether SPD subjects had insight into their social communication problems, a second exploratory correlation was performed between scores on Alexithymia and raters wanting to hear more from subjects. A negative correlation (more alexithymia and raters less interested in hearing more) would suggest that the SPD subjects had insight. Insight was further explored by correlating scores on the Self-monitoring Scale and the DSM IV SPD criteria of “no close friends”. A positive correlation (poorer self-monitoring and fewer friends) would support the contention of persevered insight. Finally, exploratory correlations between pars opercularis volumes and each of the seven prosodic elements (Table 7) were performed. A Bonferroni correction of the alpha level for consideration of statistical significance was not applied. Fisher Z transformations were used to compare groups on their correlation coefficients.
Certain statistical tests were added post-hoc (Table 1, Comments). The purpose of these tests was to demonstrate measurement methods and to provide data for future hypothesis generation. This data set of SPD subjects' speech samples, to the best of the authors' knowledge, is unique, thus warranting thorough investigation.
Results
Pause Proportion and Rating of the Amount of Pauses
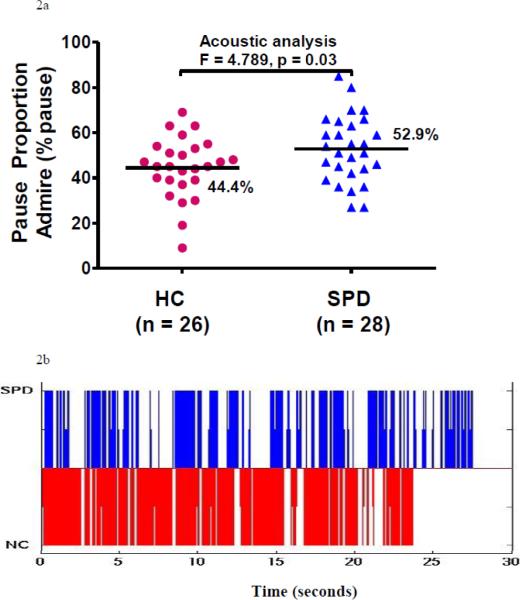
Compared with HC subjects, SPD subjects spoke with more pauses in their self-generated speech samples (Table 2, Admire, Figure 2a), best illustrated by spectrographs of individual subjects (Figure 2b). Responses to the Store probe did not show group differences. Subjective analysis by raters showed a statistically significant difference between groups for both probes with good interrater reliability (Table 2). Indeed, there was a medium effect size for raters' evaluation of Store. As expected, there was no difference between groups in pause proportion when subjects read the “Nutmeg” sentence (Table 2).
Table 2.
Subject Demographics, Prosody Variables, Clinical Variables and Gray Matter Volumes. There was no difference between groups on gender (Chi-Square 1.817, df = 1, p <0.3). Means for SPD and HC given with standard deviations (s.d.). Numbers to right of variable name indicate number of SPD and HC subjects used in the analysis. For pars opercularis volumes absolute volumes are given in Table but statistics were calculated based on regressed volumes.
| SPD | s.d. | NC | s.d. | F | P | Effect size, d | |
|---|---|---|---|---|---|---|---|
| Demographics | |||||||
| Age (28/27) | 36.6 | 9.6 | 32.0 | 11.4 | 2.633 | 0.1 | 0.19 |
| SES (26/25) | 3.3 | 1.1 | 4.1 | .5 | 11.390 | 0.001 | 0.62 |
| PSES (27/25) | 3.9 | 1.1 | 3.9 | 1.1 | .024 | <0.9 | 0.0 |
| “Expected SES” (25/23) | −0.4 | 1.0 | 0.4 | 0.6 | 11.224 | 0.002 | 0.55 |
| Vocabulary (27/27) | 12.7 | 4.1 | 13.7 | 3.0 | 1.264 | 0.3 | 0.16 |
| Education (27/27) | 14.5 | 2.6 | 15.9 | 2.5 | 3.626 | 0.06 | 0.27 |
| Prosody Elements, Acoustic analysis | |||||||
| Pause proportion (Acoustic analysis) | |||||||
| Admire (28/26) | 52.9 | 14.8 | 44.4 | 13.4 | 4.789 | 0.03 | 0.3 |
| Store (27/27) | 53.1 | 15.0 | 48.7 | 10.2 | 1.590 | 0.2 | 0.2 |
| Nutmeg (27/26) | 39.5 | 14.0 | 40.6 | 12.2 | .086 | <0.8 | 0.04 |
| Duration (Acoustic analysis) | |||||||
| “adorable” (17/17) | 520.2 | 229.6 | 397.8 | 82.1 | 4.283 | <0.05 | 0.59 |
| “dora” (17/17) | 333.2 | 108.5 | 296.1 | 47.6 | 1.663 | 0.2 | 0.2 |
| “nut” (26/27) | 248.2 | 53.9 | 241.0 | 58.1 | .218 | 0.6 | .06 |
| Attack (ms) (Acoustic analysis) | |||||||
| “nut” (26/27) | 141.9 | 41.2 | 134.0 | 43.6 | .462 | 0.5 | .09 |
| “do” (17/17) | 49.6 | 22.2 | 41.5 | 13.0 | 1.683 | 0.2 | 0.3 |
| Pitch variability (Hz)(s.d.) (Acoustic analysis) | |||||||
| Puppy (19/19) | 51.4 | 38.7 | 84.6 | 47.0 | 5.633 | 0.02 | 0.33 |
| “adorable” (17/17) | 44.9 | 48.4 | 71.6 | 64.2 | 1.870 | <0.2 | 0.2 |
| “dora” (17/17) | 14.8 | 15.4 | 55.9 | 63.7 | 6.704 | 0.01 | 0.3 |
| Nutmeg (27/27) | 70.7 | 48.7 | 76.3 | 40.5 | .209 | <0.7 | 0.07 |
| Prosody Elements, Subjective analysis | |||||||
| Rating of Amount of Pauses (Subjective analysis) | |||||||
| Admire (28/26) Interrater reliability (kappa = .850) |
2.3 | 1.3 | 1.8 | .6 | 4.300 | 0.04 | 0.38 |
| Store (28/27) Interrater reliability (kappa = .846) |
2.5 | 1.2 | 1.8 | .6 | 7.034 | 0.01 | 0.5 |
| Degree of Emotion portrayed (Subjective analysis) | |||||||
| Admire (28/28) Interrater reliability, (kappa = 0.677) |
2.1 | .8 | 2.6 | .7 | 5.347 | <0.03 | 0.34 |
| Store (28/27) Interrater reliability, (kappa = 0.778) |
2.1 | .9 | 1.9 | .9 | .238 | 0.6 | 0.1 |
| Admire minus Store (28/27) | .06 | .8 | .6 | .9 | 5.832 | <0.02 | 0.29 |
| Clinical Variables | |||||||
| Raters wanting to hear more Admire (28/26) | 1.9 | .5 | 2.0 | .5 | 1.578 | 0.2 | 0.1 |
| Raters wanting to hear more Store (28/27) | 1.7 | .5 | 1.7 | .5 | .124 | 0.7 | 0.0 |
| Alexithymia (27/27) | 98.9 | 21.3 | 86.2 | 15.1 | 6.323 | <0.02 | 0.33 |
| Self-Monitoring Scale (27/26) | 6.4 | 4.0 | 10.0 | 2.4 | 15.390 | <0.0005 | 0.6 |
| Gray Matter Volumes | |||||||
| Left pars opercularis volume (mls) (17/19) Interrater reliability (10 cases, 4 raters, 0.992) |
2.2 | .7 | 2.4 | .7 | .991 | 0.3 | 0.1 |
| Right pars opercularis volume (mls) (17/19) Interrater reliability (10 cases, 4 raters, 0.921) |
2.1 | .6 | 2.4 | .8 | 2.470 | 0.1 | 0.18 |
Figure 2.
Prosody Analyses. 2a. Pause proportion (Admire). SPD subjects (blue triangles) spoke with a higher proportion of pauses in their speech compared with HC subjects (red circles). Indeed, for SPD subjects, over half of the recorded time was devoid of actual sound. Means indicated by bars with pause percentage given. 2b. Spectrograph of speech sound. Areas with color demonstrate time during which subjects spoke, blank areas represented silence. The individual SPD subject (blue) had many pauses in his speech as he spoke about how much he admired Abraham Lincoln and the difficult life Lincoln had. This was in contrast to the HC subject (red) as he spoke about how he admired his wife and her ability to raise their children. Figure 2c. Matlab (www.mathworks.com) created waveforms of pitch variability as two subjects read, “The puppies are adorable”, standardized for duration. The red line demonstrated pitch fluctuation across the utterance from a single HC subject. For the HC subject one could easily match the text, “The puppies are adorable”, with the waveform as the HC raised his pitch at the start of “puppies”, “a”, and “do”. There was not a natural matching of text with waveform derived from the single SPD subject (blue line). This illustrated differences in the pattern of pitch variability across these two subjects. Figure 2d. Matlab created waveforms of individual subjects' pitch variation as they read “adorable”, standardized for duration. Each horizontal pseudo-color waveform represented a single subject's data with yellow representing highest pitch, red medium pitch, and blue lowest. Among the HC subjects (on right) there was a relatively consistent pattern of rising and falling pitch which could be tracked to the word, “adorable”. Among the SPD subjects, there was less coherence with some subjects demonstrating higher pitch much later in time. Also, it appeared as though SPD subjects spent more time with medium pitch (red pseudo-color) compared with HC subjects who demonstrate little red and more pronounced yellow. Figure 2e. Pitch Variability for Puppy. Figure 2f. “Dora”. For Figures 2e and 2f, SPD subjects (blue triangles) spoke with less pitch variability compared with HC subjects (red circles) at the sentence (Puppy) and word-fragment (“dora”) levels. Means given and indicated by bars. Figure 2g. Correlation between “Expected SES and Pitch Variability for Puppy. Upper graph is SPD subjects' data, lower graph is HC data.
Duration
SPD subjects, compared with HC, took longer to say the word “adorable” as detected in the acoustic analysis (Table 2, medium effect size), but not “nut” nor “dora”.
Pitch Variability and Emotion Portrayed
At the sentence level SPD subjects had less pitch variation reading the emotional sentence (Puppy), best illustrated by visually comparing the spectograms (Table 2, Figure 2c, 2d, 2e). There was no difference between groups on the neutral sentence, Nutmeg, nor on the word “adorable” (Table 2). SPD subjects had less pitch variability for “dora” compared with HC subjects (Table 2, Figure 2e).
Subjectively, raters found HC subjects expressed more emotion compared with SPD subjects as they described whom they most admired (Table 2). The two probes, Admire and Store, were designed such that Admire would elicit more emotion that would Store. Indeed, in a one-sample paired t test, HC subjects did portray more emotion on Admire than Store (t = 3.577, df = 25, p = 0.001). In contrast, in a one-sample paired t test, SPD subjects did not modulate their emotion between Admire and Store (t = .419, df = 27, p < 0.7).When comparing groups post-hoc on Admire minus Store, HC subjects modulated their voices more than did SPD subjects (Table 2).
“Expected SES” was used in a correlation analysis with the pitch variability from Puppy to examine the potential relationship between prosody and “expected SES”. One hypothesis was that if subjects spoke with less pitch variation, they may have less success in their communication and, thus, in their occupational advancement. SPD subjects showed a positive correlation (rho= .535, N = 16, p = 0.03, note that a negative value for “expected SES” means a lower SES) (Figure 2g, upper graph), in contrast to HC who showed a negative correlation (rho= −.523, N = 16, p < 0.04) (Figure 2g, lower graph). There was a statistically significant difference in correlation coefficients (Fisher Z transformation, p < 0.003).
Hear more
Contrary to predictions, raters were not more interested in hearing from HC than from SPD subjects (Table 2).
Alexithymia
SPD subjects had higher Alexithymia scores than the HC subjects (Table 2, Figure 3a). To gauge SPD subjects' insight into their relative abilities to verbally communicate effectively, self-assessed Alexithymia was correlated with whether raters wanted to hear more. The correlation was negative (Admire, rho = −.516, N = 27, p = 0.006); suggesting that SPD subjects had insight: the more trouble they had communicating, the less others wanted to hear more from them (Figure 3b). There was no such correlation for HC subjects (Admire, rho = .037, N = 26, p <0.9) (Figure 3b).
Figure 3.

Alexithymia. Figure 3a (Top graph) Alexithymia. SPD subjects (blue triangles) described more Alexithymia compared with HC subjects (red circles). Means given and indicated by bars. Figure 3b (Lower graphs) Alexithymia correlation with raters wanting to hear more. SPD subjects (lower right graph) had insight in that they knew that they had difficulty expressing their emotions verbally compared with HC subjects (lower left graph). The more alexithymic the SPD subject, the less raters wanted to hear more.
Self-Monitoring Scale (SMS)
SPD subjects scored lower on this measure compared with HC subjects, suggesting awareness of their inflexibility in response to their social environment (Table 2). SPD subjects who scored lower on this scale also had fewer friends (negative correlation between SMS and DSM IV criteria of “no close friends”) (rho = −414, N = 26, p < 0.04) (Figure 4). The correlation for HC subjects could not be performed as their scores on the SPD criteria of “no close friends” was a constant and at floor.
Figure 4.
Self-Monitoring Scale Correlation with DSM-IV Symptom of No Close Friends. SPD subjects only.
Pars opercularis volumes & correlation with clinical variables
There was no difference in right or left pars opercularis gray matter volumes between groups (Table 2). Interrater reliability for the tracings was high (Table 2).
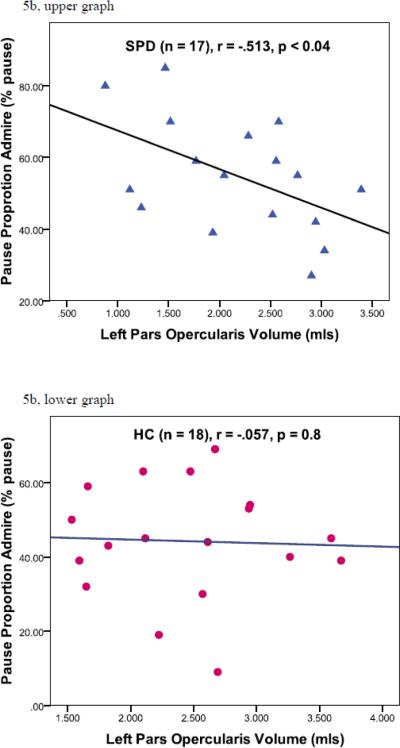
For the SPD subjects, the larger the left pars opercularis gray matter volume, the more raters thought the subject spoke with emotion (Admire, rho = .530, N = 17, p < 0.03) (Figure 5a, upper graph). For HC subjects, this relationship was not significant (Admire, rho = .126, N = 18, p = 0.6) (Figure 5a, lower graph) (Fisher Z transformation = 0.2). Similarly, for the SPD subjects, the larger the left pars opercularis gray matter volume, the fewer pauses (Admire, pause proportion: rho = −.513, N = 17, p < 0.04) (Figure 5b, upper graph). For HC subjects, this relationship was not significant (rho = −.057, N = 18, p = 0.8) (Figure 5b, lower graph). (Fisher Z transformation = −1.37, p < 0.2).
Figure 5.
Correlations with Pars Opercularis Volumes. Upper graphs depict SPD subject data (blue triangles) and lower graphs depict HC subject data (red circles). Figure 5a. Correlation between left pars opercularis volume and degree of emotion portrayed (Admire). Figure 5b. Correlation between left pars opercularis volumes and pause proportion (Admire, acoustic analysis). Figure 5c. Correlation between right pars opercularis volumes and Alexithymia.
For the HC subjects the smaller the right pars opercularis gray matter volume the more they had trouble describing their feelings (higher Alexithymia scores; rho = −.467, N = 19, p = 0.04) (Figure 5c, lower graph). For SPD subjects there was no such relationship: the ability to describe one's feelings was unrelated to right pars volumes (rho = .189, N = 16, p < 0.5) (Figure 5c, upper graph). There was a statistically significant different correlation coefficient (Fisher Z = −.1.97, p < 0.05) for Alexithymia and right pars volumes between HC and SPD subjects.
Discussion
The main findings of this report were that SPD subjects, compared with healthy controls, had statistically significant more periods devoid of speech, spoke more slowly, had less fluctuation in pitch, and expressed less emotion. Abnormalities were demonstrable in samples as long as a paragraph and as short as a word-fragment. SPD subjects had insight into their difficulties: on self-reflection they appreciated that they had difficulties communicating their feelings and modulating their expressions based on social context. These deficits were not due to volumetric abnormalities of the pars opercularis. Correlation analyses, however, raised the possibility of functional abnormalities in this region.
These group differences were measureable objectively using acoustic analyses (pause proportion, duration, and pitch variability). This is important as it suggested that the deficits in vocal communication for SPD subjects were beyond formal thought disorder (Dickey et al. 1999). Moreover, through the use of acoustic analyses, more fine-grained examination of the speech sample could be performed, for example, the measurement of the duration of a word-fragment. The demonstration of statistically significant abnormalities in duration or pitch variability in as short a sample of speech as a word-fragment reinforces the profundity of the deficit. Indeed, the reduced pitch variation in Puppy—just four short words—correlated with SPD subjects' inability to reach the socio-economic status of their parents (“expected SES”).
SPD subjects' prosodic abnormalities were similarly perceivable by raters. Both the subjective raters (Admire and Store) and the acoustic analyses (Admire) showed SPD subjects to have more pauses and less emotion (Admire) or pitch variability (Puppy, “dora”) in their speech compared to healthy controls. Raters' ability to assess these abnormalities in the laboratory may suggest that persons in the real-world may be similarly likely to notice the SPD subjects' relatively slow and flat speech.
How these findings may affect SPD subjects' functioning in the real-world is salient. Contrary to the initial prediction and fortunately for the SPD subjects, there was no difference between groups in raters wanting to hear more from them. Unfortunately, however, what was confirmed was that raters did not want to hear more from SPD subjects who described themselves as more alexithymic. Those SPD subjects who acknowledged difficulties verbally conveying their inner lives and emotions were less likely to have raters want to hear more from them. This accurate self-perception, or insight, on the part of the SPD subjects, might have been potentially clinically useful if they could use or apply this self-perception to monitor and modulate their social behavior accordingly. SPD subjects had trouble modulating their responses to others in social situations (lower scores on the SMS). SPD subjects were aware that they had deficits both in conveying their emotions verbally and modulating their behavior in social situations. Furthermore, SPD subjects' difficulty in modulating their social behavior correlated with their inability to forge meaningful friendships and have confidants. Successful social interaction depends on situation-specific modulation of behavior. For patients with schizophrenia, poor social interaction affects their sense of well-being (Perlick et al. 1992).
The final consideration of this study was whether the measures explored had neuroanatomic correlates. Current neurocognitive theory posits a right hemisphere specialization for prosody in HC(Ross and Monnot 2008) (Ross et al. 2001, Heilman et al. 2004) (Schlaug et al. 2010) (Ozdemir, Norton and Schlaug 2006). Although there was no difference between groups in pars opercularis gray matter volumes, correlations with prosody measures reinforced the concept of pars involvement with prosody production. For HC subjects, the greater their alexithymia, the smaller their right pars opercularis volumes. This suggested that, in HC subjects, larger right pars opercularis volumes may have facilitated the motor production of prosodic speech. This finding was interesting in light of a similar correlation for the SPD subjects, albeit not on the right, but on the left. The larger or more normal the SPD subjects' left pars opercularis volumes, the more the raters thought that they spoke with emotion and had fewer pauses. Why the correlation between gray matter volumes and prosody was on the left for SPD subjects was unclear, but is consistent with hemisphere lateralization functional abnormalities in schizophrenia spectrum subjects (Crow 2000).
One limitation of the current study was the relatively small subject number thus restricting generalizability and precluding a gender by diagnosis analysis. Recruiting for SPD subjects directly from the community is challenging given their inherent suspiciousness, social anxiety and the lack of an evidence-based treatment option. Another disadvantage was the scant schizophrenia literature evaluating prosody production, thus limiting the discussion of how the current findings in SPD compare with schizophrenia. Providing this easy-to-use method of measuring prosody may aid in advancing the field of social cognition remediation for a variety of clinical disorders. Future work exploring the relationship between prosody perception and identification (Dickey et al. 2010) with vocal affect production may elucidate the underlying mechanisms of these SPD subjects' social deficits.
Persons suffering from schizophrenia and SPD seek social connections and friendships. Current pharmacologic treatments do not address schizophrenia spectrum patients' lack of relationships and diminished prosody, yet prosody is important for the active engagement with another person and for achieving social and economic goals. Future social remediation programs designed for persons with schizophrenia spectrum disorders may benefit from a focus on prosodic speech production.
Acknowledgements
The authors would like to thank Paula Pelavin, Rebecca King, Daniel McCaffrey, and Talis Swisher for their participation as raters, and Ashley Henry for her administrative support.
Funding The project described was supported by the VA Boston Healthcare System and Award Number R21MH077979 (CCD) and VA Schizophrenia Center Award and Award Number RO1 MH52807 (RWM) from the National Institute of Mental Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Mental Health, the National Institutes of Health, or the VA.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributors: Dr. Dickey conceptualized the project, designed the tasks, analyzed the data, prepared the manuscript.
Ms. Vu prepared the graphs, helped prepare the data for analysis, and reviewed the manuscript.
Dr. Voglmaier reviewed the manuscript.
Dr. Niznikiewicz reviewed the manuscript.
Dr. McCarley reviewed the manuscript.
Dr. Panych prepared the data for acoustic analysis, ran the acoustic analyses, created the Matlab images, and reviewed the manuscript.
Disclosures: Dr. Dickey has no competing interests.
Ms. Vu has no competing interests.
Dr. Voglmaier has no competing interests.
Dr. Niznikiewicz has no competing interests.
Dr. McCarley has no competing interests.
Dr. Panych has no competing interests.
References
- Alpert M, Rosenberg SD, Pouget ER, Shaw RJ. Prosody and lexical accuracy in flat affect schizophrenia. Psych Res. 2000;97:107–118. doi: 10.1016/s0165-1781(00)00231-6. [DOI] [PubMed] [Google Scholar]
- Aziz-Zadeh L, Sheng T, Gheytanchi A. Common premotor regions for the perception and production of prosody and correlations with empathy and prosodic ability. PLoS ONE. 2010;5:e8759. doi: 10.1371/journal.pone.0008759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baltaxe CA, Simmons JQ., 3rd Speech and language disorders in children and adolescents with schizophrenia. Schizophr Bull. 1995;21:677–92. doi: 10.1093/schbul/21.4.677. [DOI] [PubMed] [Google Scholar]
- Bermond B, Vorst HC, Moormann PP. Cognitive neuropsychology of alexithymia: implications for personality typology. Cogn Neuropsychiatry. 2006;11:332–60. doi: 10.1080/13546800500368607. [DOI] [PubMed] [Google Scholar]
- Bleuler E. Dementia praecox of the group of schizophenias. International Universities Press; New York: 1911, 1950. [Google Scholar]
- Pratt: doing phonetics by computer. version 4.3.14.
- Borod JC, Alpert M, Brozgold A, Martin C, Welkowitz J, Diller L, Peselow E, Angrist B, Lieberman A. A preliminary comparison of flat affect schizophrenics and brain-damaged patients on measures of affective processing. J Commun Disord. 1989;22:93–104. doi: 10.1016/0021-9924(89)90026-9. [DOI] [PubMed] [Google Scholar]
- Burns MS, Fahy J. Broca's area: rethinking classical concepts from a neuroscience perspective. Top Stroke Rehabil. 2010;17:401–10. doi: 10.1310/tsr1706-401. [DOI] [PubMed] [Google Scholar]
- Catapano F, Perris F, Fabrazzo M, Cioffi V, Giacco D, De Santis V, Maj M. Obsessive-compulsive disorder with poor insight: a three-year prospective study. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34:323–30. doi: 10.1016/j.pnpbp.2009.12.007. [DOI] [PubMed] [Google Scholar]
- Crow TJ. Schizophrenia as the price that homo sapiens pays for language: a resolution of the central paradox in the origin of the species. Brain Res Brain Res Rev. 2000;31:118–29. doi: 10.1016/s0165-0173(99)00029-6. [DOI] [PubMed] [Google Scholar]
- Dickey C, McCarley R, Shenton M. The brain in schizotypal personality disorder: A review of the structural MRI and CT findings. Harvard Rev Psychiatry. 2002;10:1–15. doi: 10.1080/10673220216201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickey C, Shenton M, Fraone S, Niznikiewicz M, Voglmaier M, Seidman L, Hirayasu Y, Kwon J, Fischer I, Anderson J, Frumin M, McCarley R. Reduced left Heschl's gyrus volume in schizotypal personality disorder. Biological Psychiatry. 2000;47:13S. [Google Scholar]
- Dickey CC, McCarley RW, Niznikiewicz MA, Voglmaier MM, Seidman LJ, Kim S, Shenton ME. Clinical, cognitive, and social characteristics in a sample of neuroleptic-naive persons with schizotypal personality disorder. Schiz Res. 2005;78:297–308. doi: 10.1016/j.schres.2005.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickey CC, McCarley RW, Voglmaier MM, Niznikiewicz MA, Seidman LJ, Demeo S, Frumin M, Shenton ME. An MRI study of superior temporal gyrus volume in women with schizotypal personality disorder. Am J Psychiatry. 2003;160:2198–201. doi: 10.1176/appi.ajp.160.12.2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickey CC, McCarley RW, Voglmaier MM, Niznikiewicz MA, Seidman LJ, Hirayasu Y, Fischer I, Teh EK, Van Rhoads R, Jakab M, Kikinis R, Jolesz FA, Shenton ME. Schizotypal personality disorder and MRI abnormalities of temporal lobe gray matter. Biol Psychiatry. 1999;45:1393–402. doi: 10.1016/s0006-3223(99)00030-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickey CC, Morocz IA, Minney D, Niznikiewicz MA, Voglmaier MM, Panych LP, Khan U, Zacks R, Terry DP, Shenton ME, McCarley RW. Factors in sensory processing of prosody in schizotypal personality disorder: An fMRI experiment. Schizophr Res. 2010;121:75–89. doi: 10.1016/j.schres.2010.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickey CC, Morocz IA, Niznikiewicz MA, Voglmaier M, Toner S, Khan U, Dreusicke M, Yoo SS, Shenton ME, McCarley RW. Auditory processing abnormalities in schizotypal personality disorder: an fMRI experiment using tones of deviant pitch and duration. Schizophr Res. 2008;103:26–39. doi: 10.1016/j.schres.2008.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickey CC, Panych LP, Voglmaier MM, Niznikiewicz MA, Terry DP, Murphy C, Zacks R, Shenton ME, McCarley RW. Facial emotion recognition and facial affect display in schizotypal personality disorder. Schizophr Res. 2011 doi: 10.1016/j.schres.2011.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domes G, Grabe HJ, Czieschnek D, Heinrichs M, Herpertz SC. Alexithymic traits and facial emotion recognition in borderline personality disorder. Psychother Psychosom. 2011;80:383–5. doi: 10.1159/000325828. [DOI] [PubMed] [Google Scholar]
- Downhill J, Buchsbaum M, Hazlett E, Barth S, Roitman S, Nunn M, Lekarev O, Wei T, Shihabuddin L, Mitropoulou V, Silverman J, Siever L. Temporal lobe volume determined by magnetic resonance imaging in schizotypal personality disorder and schizophrenia. Schiz Res. 2001;48:187–199. doi: 10.1016/s0920-9964(00)00131-6. [DOI] [PubMed] [Google Scholar]
- Ethofer T, Bretscher J, Gschwind M, Kreifelts B, Wildgruber D, Vuilleumier P. Emotional voice areas: anatomic location, functional properties, and structural connections revealed by combined fMRI/DTI. Cereb Cortex. 2012;22:191–200. doi: 10.1093/cercor/bhr113. [DOI] [PubMed] [Google Scholar]
- Foundas AL, Eure KF, Luevano LF, Weinberger DR. MRI asymmetries of Broca's area: the pars triangularis and pars opercularis. Brain Lang. 1998;64:282–96. doi: 10.1006/brln.1998.1974. [DOI] [PubMed] [Google Scholar]
- Hazlett EA, Goldstein KE, Kolaitis JC. A review of structural MRI and diffusion tensor imaging in schizotypal personality disorder. Curr Psychiatry Rep. 2012;14:70–8. doi: 10.1007/s11920-011-0241-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilman KM, Leon SA, Rosenbek JC. Affective aprosodia from a medial frontal stroke. Brain Lang. 2004;89:411–6. doi: 10.1016/j.bandl.2004.01.006. [DOI] [PubMed] [Google Scholar]
- Hoekert M, Kahn RS, Pijnenborg M, Aleman A. Impaired recognition and expression of emotional prosody in schizophrenia: review and meta-analysis. Schizophr Res. 2007;96:135–45. doi: 10.1016/j.schres.2007.07.023. [DOI] [PubMed] [Google Scholar]
- Hubbard K, Trauner DA. Intonation and emotion in autistic spectrum disorders. J Psycholinguist Res. 2007;36:159–73. doi: 10.1007/s10936-006-9037-4. [DOI] [PubMed] [Google Scholar]
- Johnson-Selfridge M, Zalewski C. Moderator variables of executive functioning in schizophrenia: meta-analytic findings. Schizophr Bull. 2001;27:305–16. doi: 10.1093/oxfordjournals.schbul.a006876. [DOI] [PubMed] [Google Scholar]
- Kawasaki Y, Suzuki M, Nohara S, Hagino H, Takahashi T, Matsui M, Yamashita I, Chitnis XA, McGuire PK, Seto H, Kurachi M. Structural brain differences in patients with schizophrenia and schizotypal disorder demonstrated by voxel-based morphometry. Eur Arch Psychiatry Clin Neurosci. 2004;254:406–14. doi: 10.1007/s00406-004-0522-1. [DOI] [PubMed] [Google Scholar]
- Kee KS, Kern RS, Marshall BD, Jr., Green MF. Risperidone versus haloperidol for perception of emotion in treatment-resistant schizophrenia: preliminary findings. Schizophr Res. 1998;31:159–65. doi: 10.1016/s0920-9964(98)00026-7. [DOI] [PubMed] [Google Scholar]
- Kendler KS, McGuire M, Gruenberg AM, O'Hare A, Spellman M, Walsh D. The Roscommon family study I. methods, diagnosis of probands, and risk of schizophrenia in relatives. Archives of General Psychiatry. 1993;50:527–540. doi: 10.1001/archpsyc.1993.01820190029004. [DOI] [PubMed] [Google Scholar]
- Keshavan MS, Haas GL, Kahn CE, Aguilar E, Dick EL, Schooler NR, Sweeney JA, Pettegrew JW. Superior temporal gyrus and the course of early schizophrenia: progressive, static, or reversible? J Psychiatr Res. 1998;32:161–7. doi: 10.1016/s0022-3956(97)00038-1. [DOI] [PubMed] [Google Scholar]
- Koechlin E, Jubault T. Broca's area and the hierarchical organization of human behavior. Neuron. 2006;50:963–74. doi: 10.1016/j.neuron.2006.05.017. [DOI] [PubMed] [Google Scholar]
- Kubota M, Miyata J, Hirao K, Fujiwara H, Kawada R, Fujimoto S, Tanaka Y, Sasamoto A, Sawamoto N, Fukuyama H, Takahashi H, Murai T. Alexithymia and regional gray matter alterations in schizophrenia. Neurosci Res. 2011;70:206–13. doi: 10.1016/j.neures.2011.01.019. [DOI] [PubMed] [Google Scholar]
- Kugler BT, Caudrey DJ. Phoneme discrimination in schizophrenia. Br J Psychiatry. 1983;142:53–9. doi: 10.1192/bjp.142.1.53. [DOI] [PubMed] [Google Scholar]
- Laukka P, Ahs F, Furmark T, Fredrikson M. Neurofunctional correlates of expressed vocal affect in social phobia. Cogn Affect Behav Neurosci. 2011;11:413–25. doi: 10.3758/s13415-011-0032-3. [DOI] [PubMed] [Google Scholar]
- Leentjens AF, Wielaert SM, van Harskamp F, Wilmink FW. Disturbances of affective prosody in patients with schizophrenia; a cross sectional study. J Neurol Neurosurg Psychiatry. 1998;64:375–8. doi: 10.1136/jnnp.64.3.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitman DI, Laukka P, Juslin PN, Saccente E, Butler P, Javitt DC. Getting the cue: sensory contributions to auditory emotion recognition impairments in schizophrenia. Schizophr Bull. 2010;36:545–56. doi: 10.1093/schbul/sbn115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitman DI, Wolf DH, Laukka P, Ragland JD, Valdez JN, Turetsky BI, Gur RE, Gur RC. Not Pitch Perfect: Sensory Contributions to Affective Communication Impairment in Schizophrenia. Biol Psychiatry. 2011 doi: 10.1016/j.biopsych.2011.05.032. [DOI] [PubMed] [Google Scholar]
- Loas G. Alexithymia and dependent personality disorder. Psychiatry Res. 2012;196:325–6. doi: 10.1016/j.psychres.2011.12.030. author reply 327–8. [DOI] [PubMed] [Google Scholar]
- Loas G, Speranza M, Pham-Scottez A, Perez-Diaz F, Corcos M. Alexithymia in adolescents with borderline personality disorder. J Psychosom Res. 2012;72:147–52. doi: 10.1016/j.jpsychores.2011.11.006. [DOI] [PubMed] [Google Scholar]
- Mattila AK, Saarni SI, Salminen JK, Huhtala H, Sintonen H, Joukamaa M. Alexithymia and health-related quality of life in a general population. Psychosomatics. 2009;50:59–68. doi: 10.1176/appi.psy.50.1.59. [DOI] [PubMed] [Google Scholar]
- Melle I, Barrett EA. Insight and suicidal behavior in first-episode schizophrenia. Expert Rev Neurother. 2012;12:353–9. doi: 10.1586/ern.11.191. [DOI] [PubMed] [Google Scholar]
- Mervis CB, John AE. Cognitive and behavioral characteristics of children with Williams syndrome: implications for intervention approaches. Am J Med Genet C Semin Med Genet. 2010;154C:229–48. doi: 10.1002/ajmg.c.30263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell RL, Crow TJ. Right hemisphere language functions and schizophrenia: the forgotten hemisphere? Brain. 2005;128:963–78. doi: 10.1093/brain/awh466. [DOI] [PubMed] [Google Scholar]
- Monnot M, Orbelo D, Riccardo L, Sikka S, Rossa E. Acoustic analyses support subjective judgments of vocal emotion. Ann N Y Acad Sci. 2003;1000:288–92. doi: 10.1196/annals.1280.027. [DOI] [PubMed] [Google Scholar]
- Murphy D, Cutting J. Prosodic comprehension and expression in schizophrenia. J Neurol Neurosurg Psychiatry. 1990;53:727–30. doi: 10.1136/jnnp.53.9.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozdemir E, Norton A, Schlaug G. Shared and distinct neural correlates of singing and speaking. Neuroimage. 2006;33:628–35. doi: 10.1016/j.neuroimage.2006.07.013. [DOI] [PubMed] [Google Scholar]
- Penn DL, Corrigan PW, Martin J, Ihnen G, Racenstein JM, Nelson D, Cassisi J, Hope DA. Social cognition and social skills in schizophrenia: the role of self-monitoring. J Nerv Ment Dis. 1999;187:188–90. doi: 10.1097/00005053-199903000-00009. [DOI] [PubMed] [Google Scholar]
- Perlick D, Stastny P, Mattis S, Teresi J. Contribution of family, cognitive and clinical dimensions to long-term outcome in schizophrenia. Schizophr Res. 1992;6:257–65. doi: 10.1016/0920-9964(92)90009-t. [DOI] [PubMed] [Google Scholar]
- Poyurovsky M, Faragian S, Pashinian A, Heidrach L, Fuchs C, Weizman R, Koran L. Clinical characteristics of schizotypal-related obsessive-compulsive disorder. Psychiatry Res. 2008;159:254–8. doi: 10.1016/j.psychres.2007.02.019. [DOI] [PubMed] [Google Scholar]
- Rosenbek JC, Crucian GP, Leon SA, Hieber B, Rodriguez AD, Holiway B, Ketterson TU, Ciampitti M, Heilman K, Gonzalez-Rothi L. Novel treatments for expressive aprosodia: a phase I investigation of cognitive linguistic and imitative interventions. J Int Neuropsychol Soc. 2004;10:786–93. doi: 10.1017/S135561770410502X. [DOI] [PubMed] [Google Scholar]
- Ross ED, Monnot M. Neurology of affective prosody and its functional-anatomic organization in right hemisphere. Brain Lang. 2008;104:51–74. doi: 10.1016/j.bandl.2007.04.007. [DOI] [PubMed] [Google Scholar]
- Ross ED, Orbelo DM, Cartwright J, Hansel S, Burgard M, Testa JA, Buck R. Affective-prosodic deficits in schizophrenia: comparison to patients with brain damage and relation to schizophrenic symptoms [corrected] J Neurol Neurosurg Psychiatry. 2001;70:597–604. doi: 10.1136/jnnp.70.5.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahin NT, Pinker S, Cash SS, Schomer D, Halgren E. Sequential processing of lexical, grammatical, and phonological information within Broca's area. Science. 2009;326:445–9. doi: 10.1126/science.1174481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasamoto A, Miyata J, Hirao K, Fujiwara H, Kawada R, Fujimoto S, Tanaka Y, Kubota M, Sawamoto N, Fukuyama H, Takahashi H, Murai T. Social impairment in schizophrenia revealed by Autism-Spectrum Quotient correlated with gray matter reduction. Soc Neurosci. 2011;6:548–58. doi: 10.1080/17470919.2011.575693. [DOI] [PubMed] [Google Scholar]
- Schlaug G, Norton A, Marchina S, Zipse L, Wan CY. From singing to speaking: facilitating recovery from nonfluent aphasia. Future Neurol. 2010;5:657–665. doi: 10.2217/fnl.10.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw RJ, Dong M, Lim KO, Faustman WO, Pouget ER, Alpert M. The relationship between affect expression and affect recognition in schizophrenia. Schizophr Res. 1999;37:245–50. doi: 10.1016/s0920-9964(98)00172-8. [DOI] [PubMed] [Google Scholar]
- Snyder M, Gangestad S. On the nature of self-monitoring: matters of assessment, matters of validity. J Pers Soc Psychol. 1986;51:125–39. doi: 10.1037/0022-3514.51.1.125. [DOI] [PubMed] [Google Scholar]
- Suzuki M, Zhou SY, Takahashi T, Hagino H, Kawasaki Y, Niu L, Matsui M, Seto H, Kurachi M. Differential contributions of prefrontal and temporolimbic pathology to mechanisms of psychosis. Brain. 2005;128:2109–22. doi: 10.1093/brain/awh554. [DOI] [PubMed] [Google Scholar]
- Swart M, Kortekaas R, Aleman A. Dealing with feelings: characterization of trait alexithymia on emotion regulation strategies and cognitive-emotional processing. PLoS ONE. 2009;4:e5751. doi: 10.1371/journal.pone.0005751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomaiuolo F, MacDonald JD, Caramanos Z, Posner G, Chiavaras M, Evans AC, Petrides M. Morphology, morphometry and probability mapping of the pars opercularis of the inferior frontal gyrus: an in vivo MRI analysis. Eur J Neurosci. 1999;11:3033–46. doi: 10.1046/j.1460-9568.1999.00718.x. [DOI] [PubMed] [Google Scholar]
- Vorst HCM, Bermond B. Validity and reliability of the Bermond-Vorst Alexithymia Questionnaire. Personality Indiv Diff. 2001;30:413–434. [Google Scholar]
- Wildgruber D, Riecker A, Hertrich I, Erb M, Grodd W, Ethofer T, Ackerman H. Identification of emotional intonation evaluated by fMRI. NeuroImage. 2005;24:1233–1241. doi: 10.1016/j.neuroimage.2004.10.034. [DOI] [PubMed] [Google Scholar]
- Wisco JJ, Kuperberg G, Manoach D, Quinn BT, Busa E, Fischl B, Heckers S, Sorensen AG. Abnormal cortical folding patterns within Broca's area in schizophrenia: Evidence from structural MRI. Schizophr Res. 2007 doi: 10.1016/j.schres.2007.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]