Abstract
Proteoglycan-4 (Prg4) protects synovial joints from arthropathic changes by mechanisms that are incompletely understood. Parathyroid hormone (PTH), known for its anabolic actions in bone, increases Prg4 expression and has been reported to inhibit articular cartilage degeneration in arthropathic joints. To investigate the effect of Prg4 and PTH on articular cartilage, 16-week-old Prg4 mutant and wildtype mice were treated with intermittent PTH (1–34) or vehicle control daily for six weeks. Analyses included histology of the knee joint, micro-CT of the distal femur, and serum biochemical analysis of type II collagen fragments (CTX-II). Compared to wildtype littermates, Prg4 mutant mice had an acellular layer of material lining the surfaces of the articular cartilage and menisci, increased articular cartilage degradation, increased serum CTX-II concentrations, decreased articular chondrocyte apoptosis, increased synovium SDF-1 expression, and irregularly contoured subchondral bone. PTH-treated Prg4 mutant mice developed a secondary deposit overlaying the acellular layer of material lining the joint surfaces, but PTH-treatment did not alter signs of articular cartilage degeneration in Prg4 mutant mice. The increased joint SDF-1 levels and irregular subchondral bone found in Prg4 mutant mice introduce novel candidate mechanisms by which Prg4 protects articular cartilage.
Keywords: Proteoglycan-4, PTH, cartilage, degeneration, SDF-1
INTRODUCTION
The proteoglycan-4 (Prg4) protein products, lubricin and superficial zone protein, are secreted glycoproteins expressed in synovial joints by synoviocytes and superficial zone articular chondrocytes1. Prg4 has well known boundary lubricating properties in joints, and has actions inhibiting cell adhesion and suppressing synoviocyte proliferation1,2,3. Characterization of the joints in Prg4 mutant (Prg4−/−) mice demonstrated that Prg4 protects synovial joints from early arthropathic changes, which are non-inflammatory in nature1. Prg4−/− mice have hyperplasia of synovial intimal cells, disappearance of superficial zone chondrocytes, abnormal protein deposition on cartilage surfaces, articular cartilage degradation, and precocious joint failure1,3.
Studies demonstrating an increased coefficient of friction in loaded joints from Prg4−/− mice indicate that the boundary lubricating properties of Prg4 play an important role protecting cartilage from load induced wear4,5. In addition to validating the role of boundary lubrication, investigations of the Prg4−/− mice have introduced novel candidate mechanisms by which Prg4 protects articular cartilage. Coles et al. postulated that lubricin’s ability to form a surface covering maintains articular cartilage surface integrity by actions beyond boundary lubrication3. Based on findings that Prg4 restricts synovial intimal cell proliferation via an adhesion dependent mechanism, Rhee et al. speculated that Prg4 actions limiting synovial cell proliferation protect articular cartilage from physical invasion by hyperplastic synovium1.
PRG4 loss-of-function mutations in humans result in an autosomal recessive disorder, camptodactyly-arthropathy-coxa vara-pericarditis (CACP) syndrome, which is characterized by precocious joint failure recapitulated in the Prg4−/− mice6. In addition to CACP syndrome, reduced PRG4 levels have been associated with osteoarthritis (OA) and rheumatoid arthritis (RA), the two most common degenerative joint conditions7,8. These findings of decreased PRG4 levels in arthropathic joints, coupled with studies demonstrating that exogenous lubricin administration inhibits the onset and progression of OA in rats suggest that PRG4 plays a regulatory role in the pathogenesis of degenerative joint disease9,10.
Intermittent parathyroid hormone (PTH) (1–34) injection, currently the only FDA approved anabolic therapy for osteoporosis, has been reported to inhibit the progression of articular cartilage degeneration in rodent OA models11,12. Subcutaneous intermittent PTH injection has recently been shown to increase Prg4 and proteoglycan expression in articular cartilage of degenerating joints responsive to PTH treatment12. While it has been postulated that PTH protects articular cartilage from arthropathic changes by restricting chondrocyte maturation, PTH actions increasing Prg4 and proteoglycan expression in articular chondrocytes are strong candidate mechanisms mediating PTH chondroprotective properties11,12.
Prg4−/− mice were studied to further elucidate Prg4 functions protecting articular cartilage in vivo. In light of recent in vivo findings that Prg4 is a PTH-responsive gene in degenerating articular cartilage protected by intermittent PTH-treatment12, we investigated the impact of PTH on articular cartilage degeneration in the arthropathic joints of adult Prg4−/− mice.
METHODS
Breeding of Prg4 mutant mice, and administration of PTH
Prg4 mutant (Prg4−/−) mice, generated by homologous recombination in 129Sv/Ev-derived embryonic stem cells1, were backcrossed onto a C57BL6 genetic background. 16-week-old Prg4−/− and Prg4 wildtype (Prg4+/+) littermate mice were given daily subcutaneous injections of either recombinant human PTH (1–34) (50 μg/kg) (Bachem, Torrence, CA) or vehicle (0.9% NaCl) control for 6 weeks. The daily 50μg/kg PTH (1–34) dose, a moderate murine anabolic dose, was carried out for 6 weeks duration to thoroughly assess PTH actions on skeletal and joint remodeling. 24 hours following final injection, mice were sacrificed and tissues harvested.
Histology
Hindlimbs were fixed in 10% phosphate-buffered formalin (PBF) for 48 hours at 4°C, and then decalcified in 14% EDTA pH 7.2 for 14 days at room temperature. Knee joints were embedded in paraffin. 5 μm serial sagittal knee sections were cut, laterally to medially across the knee joint. H&E staining was performed to assess histopathology. Safranin O-fast green staining was carried out to evaluate proteoglycan content and articular cartilage degradation. The Osteoarthritis Research Society International (OARSI) osteoarthritis cartilage histopathology assessment system was adopted to score the severity and extent of articular cartilage degradation in the tibial plateau13,14. All histological scoring was performed in a blinded manner.
Immunohistochemistry (IHC)
IHC was carried out in sagittal knee sections to analyze the expression of type 1 collagen (col1), type 2 collagen (col2), and stromal cell-derived factor-1 (SDF-1). Staining was performed with antibodies to col1, col2, SDF-1, or an IgG matched isotype control (ABCAM, Cambridge, MA), in conjunction with a HRP Cell & Tissue Staining Kit (R&D Systems, Minneapolis, MN). Col1 and col2 labeling were evaluated to elucidate the cartilaginous make-up of the ectopic deposits lining the joint surfaces of Prg4−/− mice. SDF-1 labeling was assessed to characterize the localization and intensity of SDF-1 expression in synovial cells. Representative images (20X) of the synovium were acquired in the anterior aspect of the knee joint with Olympus FV-500 confocal microscope, and SDF-1 intensity was graded as (1) low, (2) moderate, (3) high.
Serum biochemical analyses
Via cardiac puncture at euthanasia, serum was isolated, and stored at −80°C. Serum Pre-Clinical CartiLaps ELISA (Immunodiagnostic Systems, Fountain Hills, AZ) was carried out in duplicate to assess degradation products of C-terminal telopeptides of type II collagen (CTX-II).
TUNEL staining
TUNEL staining was carried out in sagittal knee sections to assess chondrocyte apoptosis in articular cartilage. The region of interest was the full thickness cartilage of the tibial plateau, between the anterior and posterior menisci. Exposed ends of DNA fragments, induced by apoptotic signals, were bound by Klenow enzyme and subsequently detected following the protocol of the FragEL™ DNA Fragmentation Detection Kit (EMD Chemicals, Gibbstown, NJ).
Immunofluorescence (IF)
SDF-1 IF was carried out in sagittal knee sections to quantitatively assess SDF-1 expression in the synovium. SDF-1 IF was performed using Zenon Alexa Fluor 488 Rabbit Labeling Kit (Invitrogen, Carlsbad, CO) and SDF-1 alpha rabbit polyclonal AB (ABCAM). Sections were mounted with ProLong Gold antifade reagent with DAPI (Invitrogen). Representative images (20X) of the synovium were acquired in the anterior aspect of the knee joint with Olympus FV-500 confocal microscope, and analysis was performed using Image Pro Plus 5.1 Software.
Micro-computed tomography (micro-CT)
Femurs were fixed in 10% PBF for 48 hours at 4°C, stored in 70% EtOH, and scanned in H20 at an 18-μm isotropic voxel size via eXplore Locus SP (GE Healthcare Pre-Clinical Imaging, London, ON, Canada). Calibrated three-dimensional images were reconstructed, and distal femur subchondral bone was assessed via GE Medical Systems MicroView v2.2 software.
Statistical analyses
Unpaired t tests and the Mann-Whitney U test (semi-quantitative histological scoring) were performed using GraphPad Instat Software (GraphPad Inc., San Diego, CA). Significance was noted at p<0.05. Data are presented as mean ± standard error of the mean.
RESULTS
Histological abnormalities in the cartilage and meniscus of Prg4−/− mice
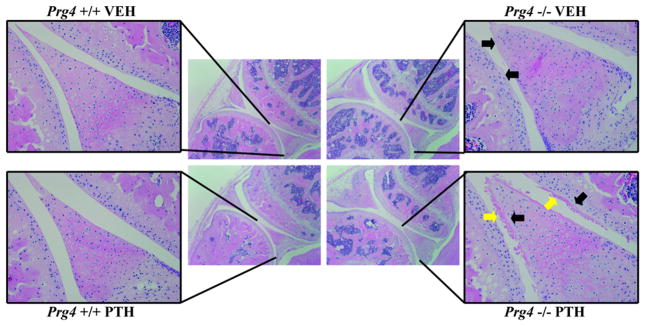
Vehicle- and PTH-treated Prg4−/− mice had an acellular layer of material lining the surfaces of the articular cartilage and menisci (Fig. 1). Rhee et al. speculated that the acellular layer of material lining the Prg4−/− joint surfaces is composed of adsorbed proteins from the synovial fluid1. Interestingly, the formation of a secondary deposit overlaying the acellular layer of material lining the surfaces of the articular cartilage and posterior meniscus was detected in 5 of 10 PTH-treated Prg4−/− mice, whereas 0 of 10 vehicle-treated Prg4−/− mice demonstrated these deposits. PTH-treatment had no observed histologic effects on the joints of Prg4+/+ mice (Fig. 1).
Figure 1.
Knee joint histopathology. 16-week-old Prg4 mutant (Prg4−/−) and wildtype (Prg4+/+) mice were administered intermittent PTH (1–34) (50μg/kg) or vehicle (VEH) (0.9% NaCl) control subcutaneous injection daily for 6 weeks. Knee joints were isolated from 22-week-old Prg4 mice for histological evaluation. Representative images of H&E stained sagittal knee sections (4X), with (inset) 20X image of the articular cartilage and posterior meniscus (black arrows indicate the acellular layer lining joint surfaces; yellow arrows indicate the secondary deposit associated with PTH-treatment).
Nature of the deposits overlying the joint surfaces of Prg4−/− mice
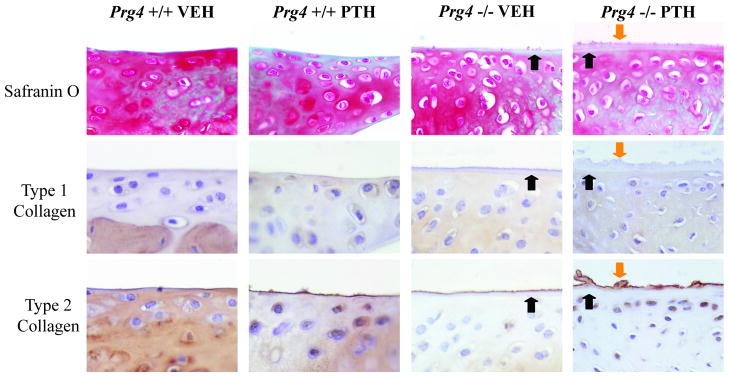
The acellular layer lining the surfaces of articular cartilage and menisci in Prg4−/− mice, and the overlaying secondary deposit noted in PTH-treated Prg4−/− mice were weakly labeled by Safranin O staining, suggesting the deposits were not primarily proteoglycan in nature (Fig. 2). Col2 labeled the most outward layer of the joint surfaces, but failed to stain underlying layers (Fig. 2). Similar to a narrow intense band of col2 lining physiologic joint surfaces in Prg4+/+ mice, a thin intense band of col2 labeled the periphery of the acellular layer lining the joint surfaces in vehicle-treated Prg4−/− mice. Col2 labeling of the secondary deposit in PTH-treated Prg4−/− mice was not restricted superficially to the periphery but instead permeated through the deposit. Col1 staining was not localized to the ectopic deposits in vehicle- or PTH-treated Prg4−/− mice, demonstrating that the deposits do not have a fibrocartilaginous composition (Fig. 2).
Figure 2.
Nature of the deposits overlaying the joint surfaces of Prg4 mutant mice. Representative images of meniscal surfaces (40X) in sagittal knee sections labeled with Safranin O-fast green stain, type 1 collagen IHC stain, type 2 collagen IHC stain (black arrows indicate labeling of the acellular layer lining joint surfaces; orange arrows indicate labeling of the secondary deposit associated with PTH-treatment).
Articular cartilage degradation in Prg4−/− mice
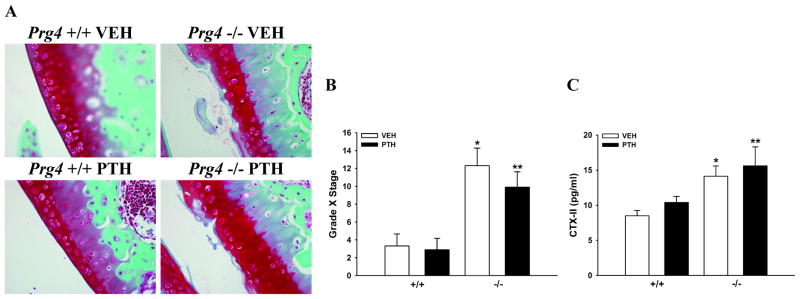
Prg4−/− mice had articular cartilage surfaces characterized by fibrillations, fissures, and mid-zone excavations (Fig. 3A). Histologic scoring indicated that the severity and extent of articular cartilage degradation was greater in Prg4−/− mice, and that degradation was not altered by PTH-treatment (Fig. 3B). Serum C-terminal telopeptides of type II collagen (CTX-II), were assayed as a systemic marker for articular cartilage degradation. Serum CTX-II levels were increased in Prg4−/− vs. Prg4+/+ mice, and were not affected by PTH-treatment (Fig. 3C).
Figure 3.
Articular cartilage degradation. A: Representative images of Safranin O-fast green stained articular cartilage (40X) in sagittal knee sections. B: Bar graph represents the OARSI score (Grade X Stage) for the articular cartilage in the tibial plateau (n=8–10/gp). *p<0.01 vs. +/+ VEH; **p<0.01 vs. +/+ PTH. C: Bar graph represents serum C-terminal telopeptides of type II collagen (CTX-II) levels (n=8–10/gp). *p<0.01 vs. +/+ VEH; **p=0.08 vs. +/+ PTH.
Decreased articular chondrocyte apoptosis in Prg4−/− mice
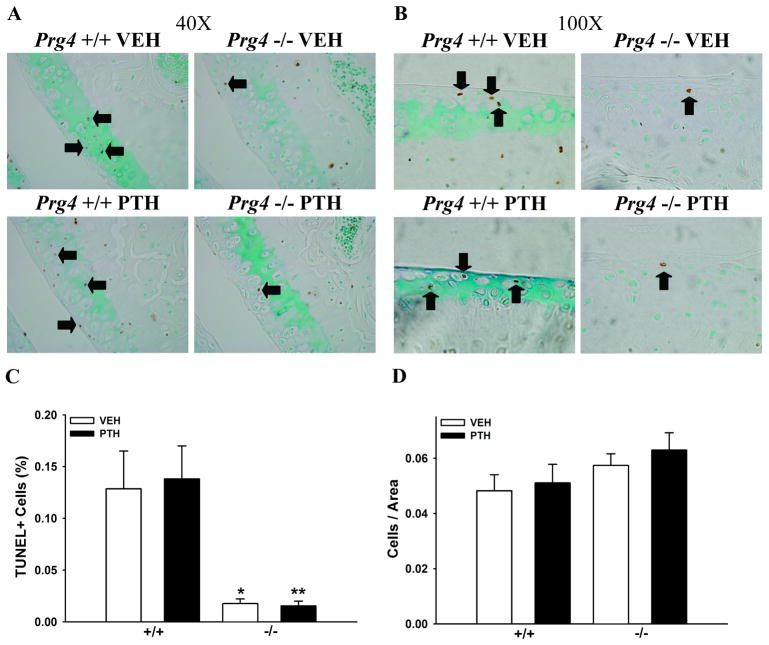
TUNEL staining of sagittal knee sections was performed to evaluate the effect of Prg4 and the impact of PTH-treatment on articular chondrocyte apoptosis. The frequency of TUNEL+ chondrocytes was significantly lower in Prg4−/− mice. There was no effect of PTH on the frequency of TUNEL+ chondrocytes in the articular cartilage of either Prg4−/− or Prg4+/+ mice (Figs. 4A–C). Total number of chondrocytes per cartilage area were similar in Prg4−/− vs. Prg4+/+ mice, and PTH did not alter total number of chondrocytes per cartilage area (Fig. 4D).
Figure 4.
Articular chondrocyte apoptosis. Representative images of TUNEL stained articular chondrocytes in sagittal knee sections: (A) 40X, (B) 100X (black arrows point to selected TUNEL+ chondrocytes). C: Bar graph represents the number of TUNEL+ chondrocytes per total chondrocytes (n≥5/gp). *p<0.01 vs. +/+ VEH; **p<0.001 vs. +/+ PTH. D: Bar graph represents the total number of chondrocytes per cartilage area (n≥5/gp).
Increased SDF-1 expression in the synovium of Prg4−/− mice
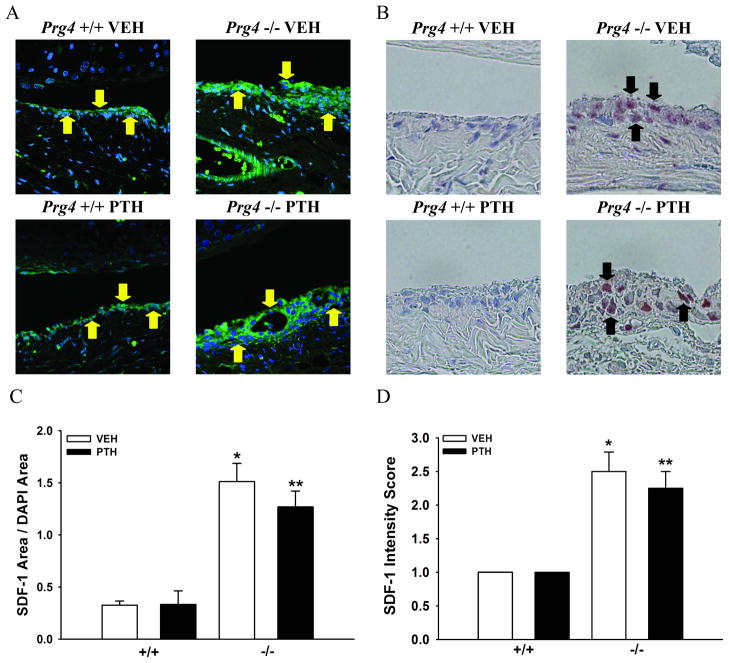
Expression of SDF-1, a chemokine best known for physiologic roles regulating the homing and mobilization of hematopoietic cells in the bone marrow15, was assessed in the knee joint since increased SDF-1 levels have been reported to play a multifaceted role in degenerative joint disease16,17,18. Additional rationale for evaluating SDF-1 expression in the joints of Prg4−/− mice was based on findings that Prg4−/− mice have altered SDF-1 expression in bone marrow19.
SDF-1 IF labeling of sagittal knee sections revealed increased SDF-1 in the synovium of Prg4−/− mice (Figs. 5A, C). While the Prg4+/+ synovium had low to moderate SDF-1 expression limited to the surface synovium approximating lining synovial intimal cells, the Prg4−/− synovium had intense SDF-1 expression throughout the hyperplastic tissue matrix. SDF-1 IHC labeling demonstrated the increased SDF-1 expression in the Prg4−/− synovium was localized to synoviocytes within the hyperplastic tissue matrix (Figs. 5B, D). There was no difference in SDF-1 expression in the synovium of vehicle- vs. PTH-treated mice (Figs. 5A–D).
Figure 5.
Synovium SDF-1 expression. A: Representative images of stromal cell-derived factor-1 (SDF-1) immunofluorescence labeled synovium (60X) (SDF-1/Green and DAPI/Blue) (yellow arrows indicate SDF-1+ synovium) in sagittal knee sections. B: Representative images of SDF-1 immunohistochemistry labeled synovium (60X) (SDF-1/Brown) (black arrows indicate SDF-1+ synovial cells) in sagittal knee sections. C: Bar graph represents SDF-1 area/DAPI area in the synovium (n≥4/gp). *p<0.001 vs. +/+ VEH; **p<0.01 vs. +/+ PTH. D: Bar graph represents synovial cell SDF-1 intensity score (n=4/gp). *p<0.05 vs. +/+ VEH; **p<0.05 vs. +/+ PTH.
Irregular subchondral bone in Prg4−/− mice
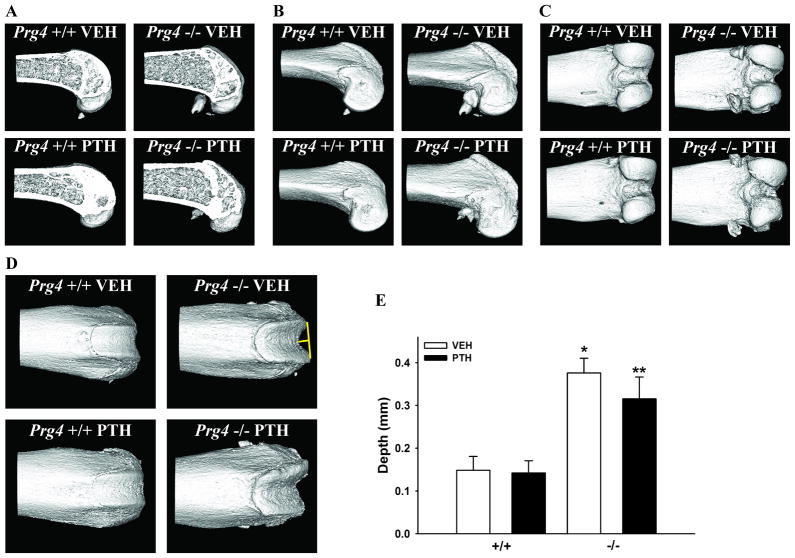
Based on reports that Prg4 is expressed in bone20 and radiographic findings that Prg4−/− mice and humans afflicted by CACP have periarticular osteopenia1,6, micro-CT analysis was employed to assess subchondral bone morphology in the distal femur of Prg4−/− mice (Fig. 6). Sagittal section images of the distal femur confirmed the expected bone forming anabolic effects of PTH in the metaphyseal trabecular and cortical bone of Prg4−/− and Prg4+/+ mice (Fig. 6A). While a side view (Fig. 6B) and posterior view (Fig. 6C) demonstrated no obvious morphological differences, the anterior view of the distal femur (Fig. 6D) revealed abnormalities in the surface contour of the subchondral bone in Prg4−/− mice. Specifically, the subchondral bone of the patellar groove had irregular contours characterized by a medial clefting with elevated lateral ridges (Fig. 6D). Perpendicular to a line drawn through the height of the lateral ridges, the depth of the patellar groove was greater in Prg4−/− vs. Prg4+/+ mice (Figs. 6D, E).
Figure 6.
Subchondral bone morphology. Representative micro-CT images of the distal femur subchondral bone: (A) sagittal section view, (B) side view, (C) posterior view, (D) anterior view (yellow line indicates depth of patellar groove). E: Bar graph represents patellar groove depth (n=7–8/gp). *p<0.001 vs. +/+ VEH; **p<0.01 vs. +/+ PTH.
DISCUSSION
The present study investigated Prg4 actions regulating articular cartilage protection, and tested whether intermittent PTH administration could inhibit articular cartilage degeneration in Prg4 mutant mice. Treatment began at 16 weeks, an age at which Prg4 mutant mice have an intermediate degree of joint failure, as indicated by a significant loss of cartilage structure, stiffness, and frictional properties1,3. The intermediate stage was evaluated since this is the period when the majority of arthropathic patients become symptomatic and present for clinical care. While intra-articular injection delivers a controlled local dose to a targeted joint, PTH was administered via subcutaneous injection to avoid the risk of iatrogenic trauma to the joints.
In bone PTH exerts its anabolic actions by signaling at stromal/osteoblastic cells, the predominant PTH/PTH-related protein (PTHrP) receptor (PPR) expressing cells21. During skeletal development the PPR is expressed in growth plate prehypertrophic zone chondrocytes, contributing to a signaling feedback loop involving indian hedgehog which regulates endochondral bone formation22. Basal PTHrP appears to regulate articular chondrocyte maintenance23 and PPR expression is upregulated in articular chondrocytes within OA joints12, yet the role of PPR signaling in articular cartilage is largely unknown. In vitro studies demonstrating that PTH administration inhibits terminal differentiation of articular chondrocytes, and in vivo OA studies reporting that PTH treatment significantly suppresses chondrocyte maturation and apoptosis imply that PTH protects articular cartilage from arthropathic changes by restricting chondrocyte maturation11,12. The recent report that PTH upregulates Prg4 and proteoglycan expression in articular cartilage within degenerating joints responsive to PTH, highlights Prg4 as a strong candidate regulator of PTH actions inhibiting articular cartilage degeneration in arthropathic joints12.
Surprisingly, the only observed PTH effect in the joints of Prg4 mutant mice was that PTH induced the formation of a secondary deposit overlaying the acellular layer of material lining the surfaces of the articular cartilage and menisci in half of the PTH-treated mutant animals. The col2 IHC labeling through the full thickness of the secondary deposit in PTH-treated Prg4 mutant mice vs. the restricted col2 labeling at the outward periphery of the acellular layer lining the joint surfaces in vehicle-treated Prg4 mutant mice substantiates that the secondary deposit was due to PTH-treatment. Taking into consideration that subcutaneous PTH administration increased Prg4 mRNA and proteoglycan expression in the articular cartilage of degenerating OA joints within wildtype mice12, the finding that the secondary deposit lining the joint surfaces of PTH-treated Prg4 mutant mice was weakly proteoglycan in nature suggests that Prg4 supports PTH actions increasing proteoglycan expression in arthropathic joints. Although the composition of this secondary deposit is unclear at present, its formation indicates that PTH signaling was functional within the joints of the Prg4 mutant mice. Similar to reports that intermittent PTH did not affect articular chondrocytes in the knee joints of healthy rats or sham operated mice11,12, intermittent PTH had no impact on the joints of Prg4 wildtype mice.
While the minimal impact of PTH in the arthropathic joints of Prg4 mutant mice implies that Prg4 is required for PTH to exert chondroprotective effects, it is unclear whether Prg4 supports PTH actions inhibiting articular cartilage degeneration. Keeping in mind the lack of Prg4 throughout development in Prg4 mutant mice is different than acutely reduced Prg4 levels in degenerating joints, it is possible that the damage caused by the complete absence of Prg4 cannot be modified by the actions of PTH.
The unexpected finding that TUNNEL staining was significantly decreased in articular chondrocytes in Prg4 mutant mice suggests that Prg4 mutant articular chondrocytes may have cell autonomous alterations protecting against apoptosis. Taking into consideration a proposed hypothesis that PTH protects articular cartilage from arthropathic changes by restricting chondrocyte maturation11,12, PTH may not have altered articular cartilage degeneration in Prg4 mutant mice due to the anti-apoptotic nature of the Prg4 mutant chondrocytes.
SDF-1 is expressed and secreted by synoviocytes, and the SDF-1 receptor, CXCR4, is expressed by articular chondrocytes24. While there is no known physiologic role for SDF-1/CXCR4 paracrine signaling in synovial joints, increased SDF-1 levels in the joints of OA and RA patients has been implicated in the degeneration of articular cartilage16,17,18. In light of in vitro articular chondrocyte studies demonstrating that supra-physiologic SDF-1 levels stimulate the secretion of matrix metalloproteinase-3 (MMP-3), MMP-9, and MMP-1316,17, we speculate that increased SDF-1 levels in the joints of Prg4 mutant mice stimulate articular chondrocytes to release MMPs which mediate the accelerated degradation of articular cartilage. Since MMP mediated degradation of articular cartilage is required for the release of type 2 collagen fragments into the circulation, the increased serum CTX-II levels observed in this study provides evidence that MMP activity is upregulated in the joints of Prg4 mutant mice.
The irregularly contoured subchondral bone surface of the patellar groove in Prg4 mutant mice illustrates that Prg4 loss-of-function mutation-induced changes in the joints are not limited to the synovium and cartilage. Of interest, prior work has suggested that changes in the shape and contour of the subchondral bone adversely impact the mechanical loading properties of the adjacent articular cartilage, which has been speculated to induce pathologic changes in cartilage structure and integrity25. Regardless of whether the irregularly contoured subchondral bone in Prg4 mutant mice is due to Prg4 actions supporting skeletogenesis or protecting articular cartilage, the altered subchondral bone morphology likely contributes to the degradation of articular cartilage in Prg4 mutant mice.
This investigation, which did not demonstrate a protective effect of PTH in the arthropathic joints of mature Prg4 mutant mice, uncovered novel candidate mechanisms by which Prg4 protects articular cartilage. Increased SDF-1 levels in the joints of Prg4 mutant mice suggest that Prg4 functions as an as an upstream regulator of catabolic cytokine expression in degenerating joints. Although it is unknown whether changes in subchondral bone precede or are secondary to alterations in articular cartilage, the irregularly contoured subchondral bone likely contributes to the disruption of the articular cartilage in Prg4 mutant mice. In summary, Prg4 is a dynamic factor that protects articular cartilage by diverse mechanisms.
Acknowledgments
This work was supported by the National Institutes of Health: DE019395, DK53904, DE007057, DE021298. The authors thank Dr. Matthew Warman for providing the Prg4 mutant mice and scientific discussions, and thank Chris Strayhorn for assistance with histological procedures.
Footnotes
The authors had no competing interests.
References
- 1.Rhee DK, Marcelino J, Baker M, et al. The secreted glycoprotein lubricin protects cartilage surfaces and inhibits synovial cell overgrowth. J Clin Invest. 2005;115:622–631. doi: 10.1172/JCI200522263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schmidt TA, Gastelum NS, Nguyen QT, et al. Boundary lubrication of articular cartilage: role of synovial fluid constituents. Arthritis Rheum. 2007;56:882–891. doi: 10.1002/art.22446. [DOI] [PubMed] [Google Scholar]
- 3.Coles JM, Zhang L, Blum JJ, et al. Loss of cartilage structure, stiffness, and frictional properties in mice lacking PRG4. Arthritis Rheum. 2010;62:1666–1674. doi: 10.1002/art.27436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jay GD, Torres JR, Rhee DK, et al. Association between friction and wear in diarthrodial joints lacking lubricin. Arthritis Rheum. 2007;56:3662–3669. doi: 10.1002/art.22974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Drewniak EL, Jay GD, Fleming BC, et al. Cyclic loading increases friction and changes cartilage surface integrity in lubricin mutant mouse knees. Arthritis Rheum. 2012;64:465–473. doi: 10.1002/art.33337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marcelino J, Carpten JD, Suwairi WM, et al. CACP, encoding a secreted proteoglycan, is mutated in camptodactyly-arthropathy-coxa vara-pericarditis syndrome. Nat Genet. 1999;23:319–322. doi: 10.1038/15496. [DOI] [PubMed] [Google Scholar]
- 7.Elsaid KA, Fleming BC, Oksendahl HL, et al. Decreased lubricin concentrations and markers of joint inflammation in the synovial fluid of patients with anterior cruciate ligament injury. Arthritis Rheum. 2008;58:1707–1715. doi: 10.1002/art.23495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ungethuem U, Haeupl T, Witt H, et al. Molecular signatures and new candidates to target the pathogenesis of rheumatoid arthritis. Physiol Genomics. 2010;42A:267–282. doi: 10.1152/physiolgenomics.00004.2010. [DOI] [PubMed] [Google Scholar]
- 9.Flannery CR, Zoller R, Corcoran C, et al. Prevention of cartilage degeneration in a rat model of osteoarthritis by intraarticular treatment with recombinant lubricin. Arthritis Rheum. 2009;60:840–847. doi: 10.1002/art.24304. [DOI] [PubMed] [Google Scholar]
- 10.Jay GD, Fleming BC, Watkins BA, et al. Prevention of cartilage degeneration and restoration of chondroprotection by lubricin tribosupplementation in the rat following anterior cruciate ligament transection. Arthritis Rheum. 2010;62:2382–2391. doi: 10.1002/art.27550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chang JK, Chang LH, Hung SH, et al. Parathyroid hormone 1–34 inhibits terminal differentiation of human articular chondrocytes and osteoarthritis progression in rats. Arthritis Rheum. 2009;60:3049–3060. doi: 10.1002/art.24843. [DOI] [PubMed] [Google Scholar]
- 12.Sampson ER, Hilton MJ, Tian Y, et al. Teriparatide as a chondroregenerative therapy for injury-induced osteoarthritis. Sci Transl Med. 2011;3:101ra93. doi: 10.1126/scitranslmed.3002214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pritzker KP, Gay S, Jimenez SA, et al. Osteoarthritis cartilage histopathology: grading and staging. Osteoarthritis Cartilage. 2006;14:13–29. doi: 10.1016/j.joca.2005.07.014. [DOI] [PubMed] [Google Scholar]
- 14.Welch ID, Cowan MF, Beier F, et al. The retinoic acid binding protein CRABP2 is increased in murine models of degenerative joint disease. Arthritis Res Ther. 2009;11:R14. doi: 10.1186/ar2604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peled A, Petit I, Kollet O, et al. Dependence of human stem cell engraftment and repopulation of NOD/SCID mice on CXCR4. Science. 1999;283:845–848. doi: 10.1126/science.283.5403.845. [DOI] [PubMed] [Google Scholar]
- 16.Kanbe K, Takagishi K, Chen Q. Stimulation of matrix metalloprotease 3 release from human chondrocytes by the interaction of stromal cell-derived factor 1 and CXC chemokine receptor 4. Arthritis Rheum. 2002;46:130–137. doi: 10.1002/1529-0131(200201)46:1<130::aid-art10020>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 17.Kanbe K, Takemura T, Takeuchi K, et al. Synovectomy reduces stromal-cell-derived factor-1 (SDF-1) which is involved in the destruction of cartilage in osteoarthritis and rheumatoid arthritis. J Bone Joint Surg Br. 2004;86:296–300. doi: 10.1302/0301-620x.86b2.14474. [DOI] [PubMed] [Google Scholar]
- 18.Wei L, Sun X, Kanbe K, et al. Chondrocyte death induced by pathological concentration of chemokine stromal cell-derived factor-1. J Rheumatol. 2006;33:1818–1826. [PubMed] [Google Scholar]
- 19.Novince CM, Koh AJ, Michalski MN, et al. Proteoglycan-4, a novel immunomodulatory factor, regulates parathyroid hormone actions on hematopoietic cells. Am J Pathol. 2011;179:2431–2442. doi: 10.1016/j.ajpath.2011.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Novince CM, Michalski MN, Koh AJ, et al. Proteoglycan-4: a dynamic regulator of skeletogenesis and PTH skeletal anabolism. J Bone Miner Res. 2011;27:11–25. doi: 10.1002/jbmr.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kousteni S, Bilezikian JP. Cellular actions of parathyroid hormone. 2008;3:639–656. [Google Scholar]
- 22.Kronenberg HM. PTHrP and Skeletal Development. Ann N Y Acad Sci. 2006;1068:1–13. doi: 10.1196/annals.1346.002. [DOI] [PubMed] [Google Scholar]
- 23.Macica C, Liang G, Nasiri A, et al. Genetic evidence of the regulatory role of parathyroid hormone-related protein in articular chondrocyte maintenance in an experimental mouse model. Arthritis Rheum. 2011;63:3333–3343. doi: 10.1002/art.30515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pablos JL, Santiago B, Galindo M, et al. Synoviocyte-derived CXCL12 is displayed on endothelium and induces angiogenesis in rheumatoid arthritis. J Immnunol. 2003;170:2147–2152. doi: 10.4049/jimmunol.170.4.2147. [DOI] [PubMed] [Google Scholar]
- 25.Radin EL, Rose RM. Role of subchondral bone in the initiation and progression of cartilage damage. Clin Orthop Relat Res. 1986;213:34–40. [PubMed] [Google Scholar]