Abstract
Objective
To determine if higher levels of partial pressure of arterial oxygen are associated with in-hospital mortality and poor neurologic status at hospital discharge in patients treated with mild therapeutic hypothermia after sudden cardiac arrest.
Design
Retrospective analysis of a prospective cohort study
Patients
A total of 170 consecutive patients treated with therapeutic hypothermia in the cardiovascular care unit of an academic tertiary care hospital.
Interventions
None.
Measurements and Main Results
Of 170 patients, 77 (45.2%) survived to hospital discharge. Survivors had a significantly lower maximum partial pressure of arterial oxygen(198 mmHg, IQR 152.5–282) measured in the first 24 hours following cardiac arrest compared to nonsurvivors (254 mmHg, IQR 172–363, p = .022). A multivariable analysis including age, time to return of spontaneous circulation, the presence of shock, bystander CPR, and initial rhythm revealed that higher levels of the partial pressure of arterial oxygen were significantly associated with increased in-hospital mortality (odds ratio 1.439, 95% confidence interval 1.028–2.015, p = 0.034) and poor neurologic status at hospital discharge (odds ratio 1.485, 95% confidence interval 1.032–2.136, p = 0.033).
Conclusions
Higher levels of the maximum measured partial pressure of arterial oxygen are associated with increased in-hospital mortality and poor neurologic status on hospital discharge in patients treated with mild therapeutic hypothermia after sudden cardiac arrest.
Keywords: therapeutic hypothermia, hyperoxia, sudden cardiac arrest, cerebral performance category, mortality, neurological outcomes
Introduction
Even in patients who achieve return of spontaneous circulation (ROSC), survival to hospital discharge after sudden cardiac arrest has been reported to range from 5% to 40% (1,2). Given such poor outcomes likely depend on the neurologic insult incurred during the arrest, recent focus has turned to potential interventions aimed at improving post-arrest neurologic function and, ultimately, mortality. Mild therapeutic hypothermia (TH) has been shown in a number of studies to improve neurologic outcomes, decrease mortality (3,4), and is now recommended for patients having suffered an out-of-hospital cardiac arrest from a ventricular arrhythmia (5,6). Because TH may modulate the systemic inflammatory response seen after cardiac arrest (7), its use in clinical practice has been extended to patients after cardiac arrest from non-ventricular arrhythmias, such as asystole and pulseless electrical activity (PEA).
Hyperoxia may play a role in the pathogenesis of the post-cardiac arrest syndrome (8) and is a potentially modifiable risk factor for poor neurologic outcomes and death in patients who achieve ROSC (9). The pathophysiologic mechanisms underlying these poor outcomes are unclear, but some evidence suggests increased formation of reactive oxygen species (ROS) resulting in lipid peroxidation of neuronal cells (10–12), impairment in cerebral oxidative energy metabolism (13), and cerebral and myocardial vasoconstriction in the setting of increased arterial oxygen tension (14, 15). TH is also thought to have protective effects against damaging ROS (16,17); however it is unknown if hyperoxia is associated with mortality or poor neurological outcomes in patients undergoing TH.
We conducted a retrospective analysis of a prospective cohort to test the hypothesis that increasing levels of the maximum measured partial pressure of arterial oxygen (PaO2) in the 24 hours after sudden cardiac arrest is associated with an increased risk of in-hospital mortality and poor neurologic outcome in patients treated with TH.
Materials and Methods
Study Design
The study population included 173 consecutive comatose patients who were treated with mild therapeutic hypothermia following sudden cardiac arrest at Vanderbilt University Medical Center between May 15, 2007 and January 2, 2012. Patients determined by their treating physician to be suitable for mild therapeutic hypothermia were externally cooled to maintain a target temperature of 32–34 degrees Celsius for 24 hours following ROSC, after which they were rewarmed passively at a rate of 0.25 degrees Celsius per hour. After approval from the institutional review board, data were collected prospectively on these patients, including initial rhythm, time to ROSC, receipt of bystander CPR, Cerebral Performance Category (CPC) score at hospital discharge, and hospital mortality. We returned to these patients’ records and retrospectively collected the highest measured PaO2 in the first 24 hours following cardiac arrest.
The primary analysis for this study was in-hospital mortality. The secondary analysis was poor neurologic outcome defined as a CPC score of 3 or greater. The Cerebral Performance Category score was developed as a measure of central nervous system function after cardiac arrest and has become the most commonly used outcome tool for this purpose (3, 4, 18). A CPC score between 3 and 5 has been recommended for use as defining a poor neurologic outcome in study patients and clinically represents a range of neurologic function from severe neurologic disability to death (18).
Inclusion/Exclusion Criteria
All patients treated with TH and who also had at least one PaO2 measured in the first 24 hours following cardiac arrest were included. This time period was chosen because it covers the time of active cooling in TH. Patients without a PaO2 measured in the first 24 hours following cardiac arrest were excluded.
Statistical Analysis
Univariable analyses were conducted using Wilcoxon rank sum test for continuous variables, Fisher’s exact test and chi square for a trend tests for categorical variables, and Kruskal-Wallis test for ordinal variables. Median values with interquartiles were used for continuous variables and frequencies for categorical variables. Multivariable logistic regression models were developed for the outcomes of in-hospital mortality and poor neurologic outcome; explanatory variables included known risk factors for poor outcomes in this patient population and those selected using data reduction methods. Pertinent variables with p-values ≤ 0.10 in univariable analyses were included in the final multivariable regression model. In addition, shock, as defined by the requirement of vasoactive medications to maintain a mean arterial blood pressure greater than or equal to 65 mmHg, was determined a priori to be an important potential confounder and included in the regression model. IBM® SPSS® Statistics (version 19.0) was used for statistical analysis; a two-sided significance level of 0.05 was used for statistical inference.
Results
Clinical Characteristics
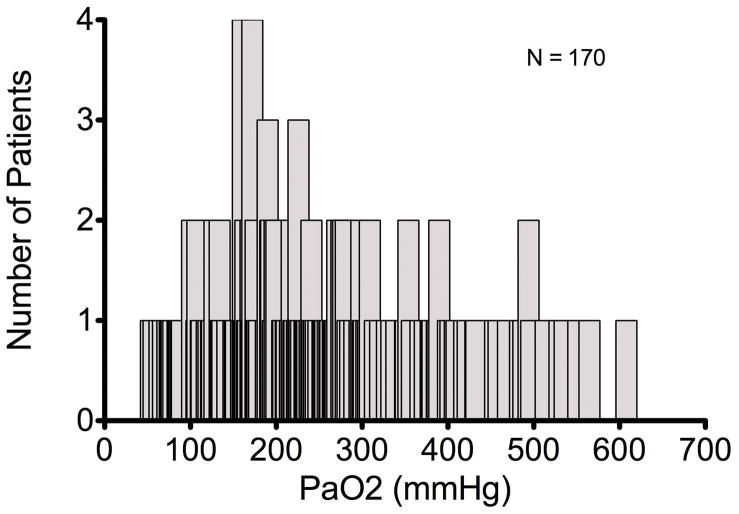
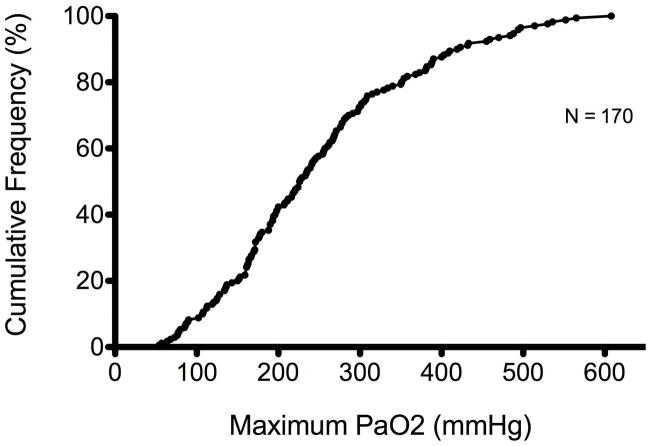
Of the 173 patients considered for inclusion, 170 had at least one PaO2 measured in the first 24 hours following cardiac arrest and, therefore, defined the study population. AllPaO2 measurements included in the analysis were obtained while patients were at the target temperature of 32–34 degrees Celsius. Table 1 displays a comparison of baseline characteristics. Overall, survivors were younger, were more likely to have had a ventricular arrhythmia as the cause of their arrest, had shorter ROSC time, and had a lower median PaO2. There was no significant difference in gender, presence of shock requiring vasoactive medications, number of patients who received bystander CPR, or the number of hours spent at the target TH temperature. The median highest PaO2 among all patients was 226.5 mmHg (IQR 162.7–309) with a range from 54 mmHg to 608 mmHg (Figure 1a and 1b).
Table 1.
Baseline Characteristics
| Characteristic | Survivors (n = 77) | Nonsurvivors (n = 93) | p-value | Overall |
|---|---|---|---|---|
| Age (years) | 57 (49, 64) | 62 (54, 71) | 0.002 | 60.5 (51.7, 69) |
| Male (%) | 51 (66.2%) | 60 (64.5%) | 0.872 | 111 (65.3%) |
| Presence of Shock requiring vasopressors (%) | 29 (37.7%) | 46 (49.5%) | 0.162 | 75 (44.1%) |
| Initial Rhythm Vtach/Vfib (%) | 60 (77.9%) | 43 (46.2%) | <0.001 | 103 (60.6%) |
| Out-of-hospital Arrest (%) | 63 (81.8) | 72 (77.4%) | 0.569 | 135 (79.4%) |
| Received bystander CPR (%) | 45 (58.4%) | 42 (45.2%) | 0.092 | 87 (51.2%) |
| Time to ROSC (minutes) | 15 (9, 18) | 25 (15, 37.5) | <0.001 | 18 (12, 30) |
| Time spent at target temperature of 32–34 degrees Celcius (hours) | 17 (15, 22) | 20 (16, 23.5) | 0.071 | 18 (16, 23) |
| Highest PaO2 (mmHg) | 198 (152.5, 282) | 254 (172, 363) | 0.022 | 226.5 (162.7, 309) |
| CPC score at hospital discharge | 1 (1, 1.5) | 5 (5, 5) | <0.001 | 5 (1, 5) |
| Poor Neurologic Outcome (%) | 7 (9.1%) | 93 (100%) | <0.001 | 100 (58.8%) |
Data given as median (25th percentile, 75th percentile) or number (percentage) of patients.
Vtach = Ventricular Tachycardia; Vfib = Ventricular Fibrillation; CPR = Cardiopulmonary Resuscitation
Figure 1.
Figures 1a and 1b. Distribution and Cumulative Frequency Distribution Curves of Maximum PaO2 in the first 24 hours Following Sudden Cardiac Arrest.
Mortality
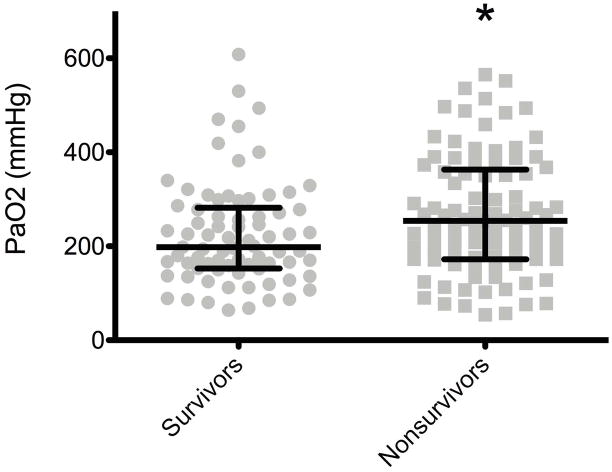
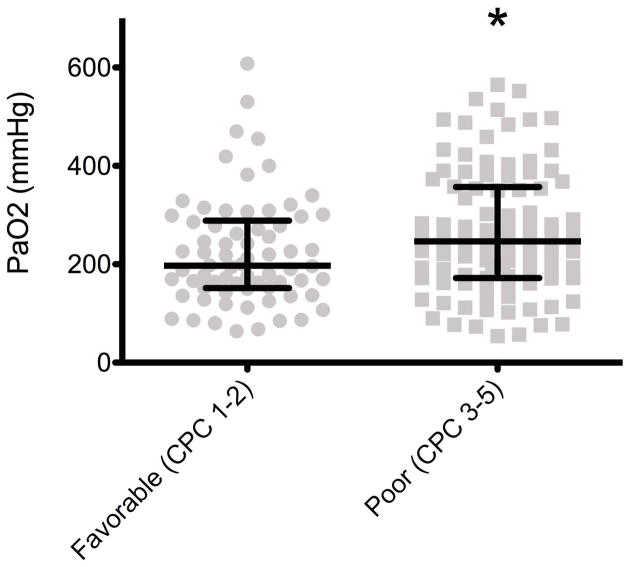
Of the 170 patients included in the final analysis, 93 (54.7%) died during hospitalization. Survivors had a significantly lower maximum PaO2 (198mmHg, IQR 152.5–282) measured in the 24 hours following cardiac arrest compared to nonsurvivors (254 mmHg, IQR 172–363, p = 0.022) (Figure 2).
Figure 2. Maximum PaO2 and In-Hospital Mortality.
Survivors had a significantly lower maximum PaO2 (198 mmHg) in the first 24 hours than non-survivors (254 mmHg)(p = 0·022)*. Values are medians (middle long horizontal line) and interquartile ranges (IQR: upper and lower caps).
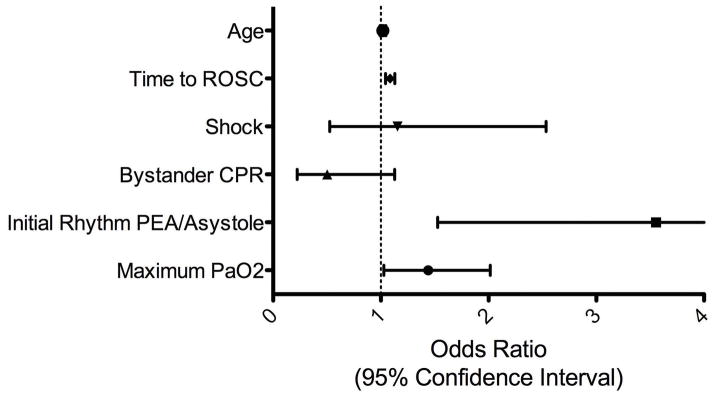
Using data reduction methods and accounting for known risk factors for in-hospital mortality, a multivariable logistic regression model was developed to examine the association between highest measured PaO2 and mortality. After controlling for age, time to ROSC, presence of shock, bystander CPR, and initial rhythm, higher levels of PaO2 were significantly associated with an increased risk of in-hospital mortality (OR 1.439, 95% CI 1.028–2.015, p = 0.034)(Table 2)(Figure 3).
Table 2.
Logistic Regression Model for In-Hospital Mortality
| Characteristic | Odds Ratio | 95% Confidence Interval | p-value |
|---|---|---|---|
| Age (years) | 1.019 | 0.991–1.047 | 0.182 |
| Time to ROSC (minutes) | 1.086 | 1.044–1.130 | <0.001 |
| Shock | 1.154 | 0.525–2.534 | 0.722 |
| Bystander CPR | 0.502 | 0.223–1.128 | 0.095 |
| Initial Rhythm PEA/Asystole | 3.554 | 1.527–8.275 | 0.003 |
| Maximum PaO2 (mmHg) | 1.439 | 1.028–2.015 | 0.034 |
The odds ratio for maximum PaO2 is for an increment of every 100mmHgabove 54 mmHg.
ROSC = Return of Spontaneous Circulation; CPR = Cardiopulmonary Resuscitation; PEA = Pulseless Electrical Activity; Shock = requirement of vasoactive medications to maintain a mean arterial blood pressure greater than or equal to 65 mmHg.
Figure 3. Risk of In-Hospital Mortality associated with Maximum PaO2.
Odds ratio adjusted for age, time to ROSC, shock, bystander CPR, and initial rhythm. The odds ratio for maximum PaO2 is for an increment of every 100mmHgabove 54 mmHg. Shock = requirement of vasoactive medications to maintain a mean arterial blood pressure greater than or equal to 65 mmHg.
Poor Neurologic Outcome
Of the 170 patients in the final analysis, 100 (58.8%) had a poor neurologic outcome on hospital discharge, defined as a CPC greater than or equal to 3. Patients with favorable neurologic outcomes (CPC score of 1 or 2) had a significantly lower maximum PaO2 (197 mmHg, IQR 151.5–288.7) measured in the 24 hours following cardiac arrest compared to patients with poor neurologic outcomes (246.5 mmHg, IQR 172–357, p = .026)(Figure 4). After controlling for the same variables used in the mortality analysis, there was a significant association between higher levels of PaO2 and poor neurologic outcome at hospital discharge (OR 1.485, 95% CI 1.032–2.136, p = .033)(Table 3).
Figure 4. Maximum PaO2 and Neurologic Status at Hospital Discharge.
Patients with favorable neurological outcomes had significantly lower maximum PaO2 (197 mmHg) in the first 24 hours than patients with poor neurological outcomes (246·5 mmHg)(p = 0·026)* at hospital discharge. Values are medians (middle long horizontal line) and interquartile ranges (IQR: upper and lower caps).
Table 3.
Logistic Regression Model for Poor Neurological Status at Hospital Discharge
| Characteristic | Odds Ratio | 95% Confidence Interval | p-value |
|---|---|---|---|
| Age (years) | 1.02 | 0.991–1.049 | 0.185 |
| Time to ROSC (minutes) | 1.121 | 1.065–1.181 | <0.001 |
| Shock | 1.24 | 0.539–2.854 | 0.612 |
| Bystander CPR | 0.509 | 0.215–1.203 | 0.124 |
| Initial Rhythm PEA/Asystole | 4.975 | 1.953–12.673 | 0.001 |
| Maximum PaO2 (mmHg) | 1.485 | 1.032–2.136 | 0.033 |
The odds ratio for maximum PaO2 is for an increment of every 100 mmHg above 54 mmHg. Poor neurological status is defined as CPC ≥ 3. ROSC = Return of Spontaneous Circulation; CPR = Cardiopulmonary Resuscitation; PEA = Pulseless Electrical Activity; Shock = requirement of vasoactive medications to maintain a mean arterial blood pressure greater than or equal to 65 mmHg.
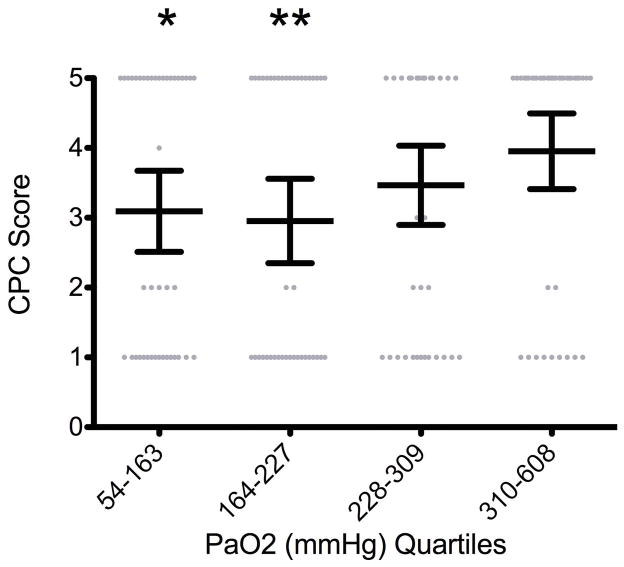
In an effort to determine if there is a threshold PaO2 level at which the CPC score significantly worsens, maximum PaO2 levels in the first 24 hours after cardiac arrest were divided into quartiles and CPC scores in each quartile were compared. Overall, there was no statistically significant association between increasing PaO2 and a change in CPC score (p = 0.08); however there were significant differences in CPC score between the first PaO2 quartile (54–163 mmHg) and second PaO2 quartile (164–227 mmHg) compared to the fourth quartile (310–608 mmHg)(p = .038 and .018, respectively)(Figure 5).
Figure 5. CPC Score at Hospital Discharge by PaO2 Quartiles.
There was no statistically significant association between increasing PaO2 and a change in CPC score (p = 0.08); however quartiles 1 and 2 had significantly better CPC scores at discharge than quartile 4 (p = 0·038* and 0·018**, respectively). Values are means (middle long horizontal line) and standard deviations (SD: upper and lower caps).
Discussion
In this retrospective analysis of a prospective cohort, there was a significant association between the maximum PaO2 measured, in-hospital mortality, and poor neurologic status on hospital discharge in victims of sudden cardiac arrest undergoing therapeutic hypothermia. These findings are similar to associations found in previous studies of patients with out-of-hospital cardiac arrest (9, 19), chronic obstructive pulmonary disease (20, 21), and acute myocardial infarction (22).
It is unclear what specific effects of hyperoxia result in poor neurologic outcomes and mortality. One proposed mechanism is hyperoxia-induced increase in the generation of reactive oxygen species (ROS) and their subsequent peroxidation of neuronal lipid membranes (11–12). To our knowledge, this study is the first to examine the effects of increasing PaO2 levels in patients undergoing TH and demonstrates an association between hyperoxia and worse outcomes in this population (19). Our study is unable to answer whether TH may still provide some protection against the oxidative effects of hyperoxia or if there is a threshold PaO2 above which exposure is more deleterious. In addition, we chose to account for the maximum PaO2 measured in the first 24 hours after cardiac arrest rather than using the first PaO2 measured as hyperoxia at any time point during TH should, theoretically, be harmful to patients and deleterious to the benefit of 24 hours of TH. This study is also unique in that we chose to analyze PaO2 as a continuous variable rather than dividing groups into hyperoxia and normoxia, adding strength to our statistical findings and biologic plausibility to our hypothesis. Hyperoxia has been shown to cause vasoconstriction (14, 15), potentially severe enough to actually reduce overall oxygen delivery to vital tissues like the heart and brain, even despite supratherapeutic arterial oxygen concentrations. Although not addressed in our study, if the deleterious effects of hyperoxia are mediated via a mechanism other than ROS, such as vasoconstriction, it is not surprising that TH may fail to lessen these effects.
Our study has a number of limitations. First, this is a retrospective analysis of a prospectively collected, observational cohort that only allows us to suggest associations rather than causation. Non-invasive measurement of arterial oxygen saturation is inaccurate in the setting of hypotension and can be associated with the practitioner’s response of increasing the inspired oxygen, creating a situation where hyperoxia is a marker of shock rather than a true risk for poor outcomes. It is difficult to account for this aspect of bedside management in this analysis; however there was no difference in the incidence of shock between groups and shock was not significantly associated with either mortality or poor neurologic outcome in multivariable analysis, while maximum PaO2 remained significantly associated with both. Since acute physiology and chronic health evaluation (APACHE) scores have not been validated and may be inaccurate in cardiac arrest and therapeutic hypothermia populations (23) and have not been used in previous landmark studies (3, 4, 24–26), we chose not to control for APACHE score in our regression model. We tried to control for severity of illness by including shock in our model. However, omission of a more conventional severity of illness score such as APACHE allows the possibility that patients who were more ill were also more likely to receive higher FiO2. We did not have data on the FiO2 administered at the time of blood gas analysis to control for this additional, potential confounder. Finally, our analysis prohibits commentary on whether the associated outcomes are a result of one elevated PaO2 level or if the underlying pathogenesis involves a dose-response relationship.
Our results differ from a recent, large cohort study that found the association between hyperoxia and worse mortality was lost in multivariable analysis when severity of illness was included in the regression model (27). However, that study used worst alveolar-arterial (A-a) gradient and not PaO2. A-a gradient does not correlate in a linear fashion with the PaO2 as fraction of inspired oxygen increases (28, 29). Furthermore, the oxygen levels in their cohort were considerably lower than the hyperoxia seen in our and other studies. The mean PaO2 in their study was 112 mmHg compared to 248 mmHg in our study and the lowest end of their highest quartile of PaO2 starts around 187 mmHg while ours starts at 363 mmHg.
The implications of these findings are significant for the care of patients and generation of future research. First, hyperoxia is a potentially cost-free, modifiable risk factor that, in this analysis, seems to be detrimental to a therapy (TH) that is not only used to improve outcomes (3, 4), but is also expensive and labor intensive (30). The avoidance of hyperoxia after cardiac arrest promises to be a simple and attractive strategy in achieving better outcomes in patients treated with TH. Although current guidelines recommend use of 100% inspired oxygen during resuscitation (31), normoxic resuscitation or rapid titration of inspired oxygen after ROSC to a goal PaO2 of normoxia promises improvement in neurologic outcomes (12, 32) and warrant study in humans. Any future therapeutic interventions must also be weighed against the fact that hypoxia is clearly harmful in all patient populations and must be avoided. As our study is in agreement with past investigations (9) suggesting that the potential harm generated with exposure to increasing levels of PaO2 occurs above a level of 300 mmHg, it seems prudent and safe to avoid these high levels and, if encountered, to act with a sense of urgency to return the patient to normoxia.
Conclusions
Sudden cardiac arrest is associated with high hospital mortality and poor neurologic outcomes. Hyperoxia in the post-arrest setting is also a risk for the same outcomes. We found that with increasing levels of the maximum PaO2 measured in the 24 hours following cardiac arrest there is an association with increased hospital mortality and poor neurologic status on hospital discharge. Given the growing evidence suggesting the harm of hyperoxia and now the potential deleterious effects hyperoxia may have on the benefits of therapeutic hypothermia, large, randomized, controlled trials should be undertaken to confirm these findings. Additionally, more specific oxygenation goals are needed in the post-resuscitation care of victims of sudden cardiac arrest.
Acknowledgments
Role of funding: We thank the National Institutes of Health (HL81431) for financial support used in the analysis and interpretation of this data.
We would also like to thank Dr. John H Newman for his discussions and suggestions related to pulmonary physiology.
Footnotes
The authors have not disclosed any potential conflicts of interest
Contributors
DRJ, RDH, and TWR conceived the study design. DRJ, RDH, and JSP collected data and DRJ and TWR analyzed the data. All authors participated in interpretation of the results. DRJ drafted the manuscript, and all authors contributed to the critical review and revision of the manuscript. All authors have seen and approved the final version of the manuscript.
Conflict of Interest Statement
The authors declare that they have no conflicts of interest.
References
- 1.Peberdy MA, Kaye W, Ornato JP, et al. Cardiopulmonary resuscitation of adults in the hospital: a report of 14720 cardiac arrests from the national registry of cardiopulmonary resuscitation. Resuscitation. 2003;58:297–308. doi: 10.1016/s0300-9572(03)00215-6. [DOI] [PubMed] [Google Scholar]
- 2.Stiel IG, Wells GA, Field B, et al. Advanced cardiac life support in out-of-hospital cardiac arrest. N Engl J Med. 2004;351:647–656. doi: 10.1056/NEJMoa040325. [DOI] [PubMed] [Google Scholar]
- 3.Bernard SA, Gray TW, Busted, et al. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N Engl J Med. 2002;346:557–563. doi: 10.1056/NEJMoa003289. [DOI] [PubMed] [Google Scholar]
- 4.Hypothermia After Cardiac Arrest Study Group. Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N Engl J Med. 2002;346:549–556. doi: 10.1056/NEJMoa012689. [DOI] [PubMed] [Google Scholar]
- 5.Nolan JP, Morley TL, Vanden Hoek RW, et al. Therapeutic hypothermia after cardiac arrest: an advisory statement by the advanced life support task force of the international liaison committee on resuscitation. Circulation. 2003;108:118–121. doi: 10.1161/01.CIR.0000079019.02601.90. [DOI] [PubMed] [Google Scholar]
- 6.Hazinski MF, Nolan JP, Billi JE, et al. Part1: executive summary: 2010 international consensus on cardiopulmonary resuscitation and emergency cardiovascular care science with treatment recommendations. Circulation. 2010;122:S250–275. doi: 10.1161/CIRCULATIONAHA.110.970897. [DOI] [PubMed] [Google Scholar]
- 7.Stegman BM, Newby LK, Hochman JS, et al. Post-myocardial infarction cardiogenic shock is a systemic illness in need of a systemic treatment. J Am Col Card. 2012;59:644–647. doi: 10.1016/j.jacc.2011.11.010. [DOI] [PubMed] [Google Scholar]
- 8.Nolan JP, Neumar RW, Adrie C, et al. Post-cardiac arrest syndrome: epidemiology, pathophysiology, treatment, and prognostication. Resuscitation. 2008;79:350–379. doi: 10.1016/j.resuscitation.2008.09.017. [DOI] [PubMed] [Google Scholar]
- 9.Kilgannon JH, Jones AE, Shapiro NI, et al. Association between arterial hyperoxia following resuscitation from cardiac arrest and in-hospital mortality. JAMA. 2010;303:2165–2171. doi: 10.1001/jama.2010.707. [DOI] [PubMed] [Google Scholar]
- 10.Backer LB. New concepts in reactive oxygen species and cardiovascular reperfusion physiology. Cardvasc Res. 2004;61:461–470. doi: 10.1016/j.cardiores.2003.10.025. [DOI] [PubMed] [Google Scholar]
- 11.Douzinas EE, Patsouris E, Kypriades EM, et al. Hypoxaemic reperfusion ameliorates the histopathological changes in the pig brain after a severe global cerebral ischaemic insult. Inten Care Med. 2001;27:905–910. doi: 10.1007/s001340100932. [DOI] [PubMed] [Google Scholar]
- 12.Liu Y, Rosenthal RE, Haywood Y, et al. Normoxic ventilation after cardiac arrest reduces oxidation of brain lipids and improves neurological outcome. Stroke. 1998;29:1679–1686. doi: 10.1161/01.str.29.8.1679. [DOI] [PubMed] [Google Scholar]
- 13.Richards EM, Fiskum G, Rosenthal RE, et al. Hyperoxic reperfusion after global ischemia decreases hippocampal energy metabolism. Stroke. 2007;38:1578–1584. doi: 10.1161/STROKEAHA.106.473967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johnston AJ, Steine LA, Gupta AK, et al. Cerebral oxygen vasoreactivity and cerebral tissue oxygen reactivity. Br J Anaesth. 2003;90:774–786. doi: 10.1093/bja/aeg104. [DOI] [PubMed] [Google Scholar]
- 15.Floyd TF, Clark M, Gelfand R, et al. Independent cerebreal vasoconstrictive effects of hyperoxia and accompanying arterial hypocapnia at 1 ATA. J Appl Physiol. 2003;95:2453–2461. doi: 10.1152/japplphysiol.00303.2003. [DOI] [PubMed] [Google Scholar]
- 16.Polderman KH. Mechanisms of action, physiological effects, and complications of hypothermia. Crit Care Med. 2009;37:S186–202. doi: 10.1097/CCM.0b013e3181aa5241. [DOI] [PubMed] [Google Scholar]
- 17.Liu L, Yenari MA. Therapeutic hypothermia: neuroprotective mechanisms. Front in Biosc. 2007;12:816–825. doi: 10.2741/2104. [DOI] [PubMed] [Google Scholar]
- 18.Becker LB, Aufderheide TP, Romergryke GG, et al. Primary outcomes for resuscitation science studies: a consensus statement from the American Heart Association. Circulation. 2011;124:2158–2177. doi: 10.1161/CIR.0b013e3182340239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kilgannon JH, Jones AE, Parrillo JE, et al. Relationship between supranormal oxygen tension and outcome after resuscitation from cardiac arrest. Circulation. 2011;123:2717–2722. doi: 10.1161/CIRCULATIONAHA.110.001016. [DOI] [PubMed] [Google Scholar]
- 20.Austin MA, Wills KE, Blizzard L, et al. Effect of high flow oxygen on mortality in chronic obstructive pulmonary disease patients in prehospital setting: randomized controlled trial. BMJ. 2010;341:5462. doi: 10.1136/bmj.c5462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wijesinghe M, Perrin K, Healy B, et al. Pre-hospital oxygen therapy in acute exacerbations of chronic obstructive pulmonary disease. Intern Med J. 2011;41:618–622. doi: 10.1111/j.1445-5994.2010.02207.x. [DOI] [PubMed] [Google Scholar]
- 22.Rawles JM, Kenmure AC. Controlled trial of oxygen in uncomplicated myocardial infarction. BMJ. 1976;1:1121–1123. doi: 10.1136/bmj.1.6018.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Niskanen M, Kari A, Nikki P, et al. Acute physiology and chronic health evaluation (APACHE II) and Glasgow coma scores as predictors of outcome from intensive care after cardiac arrest. Crit Care Med. 1991;19:1465–1473. doi: 10.1097/00003246-199112000-00005. [DOI] [PubMed] [Google Scholar]
- 24.Trzeciak S, Jones AE, Kilgannon JH, et al. Significance of arterial hypotension after resuscitation from cardiac arrest. Crit Care Med. 2009;37:2895–2903. doi: 10.1097/ccm.0b013e3181b01d8c. [DOI] [PubMed] [Google Scholar]
- 25.Mongardon N, Perbet S, Lemiale V, et al. Infectious complications in out-of-hospital cardiac arrest patients in the therapeutic hypothermia era. Crit Care Med. 2011;39:1359–1364. doi: 10.1097/CCM.0b013e3182120b56. [DOI] [PubMed] [Google Scholar]
- 26.Oddo M, Ribordy V, Feihl F, et al. Early predictors of outcome in comatose survivors of ventricular fibrillation and non-ventricular fibrillation cardiac arrest treated with hypothermia: a prospective study. Crit Care Med. 2008;36:2296–2301. doi: 10.1097/CCM.0b013e3181802599. [DOI] [PubMed] [Google Scholar]
- 27.Eastwood G, Bellomo R, Bailey M, et al. Arterial oxygen tension and mortality in mechanically ventilated patients. Int Care Med. 2012;38:91–98. doi: 10.1007/s00134-011-2419-6. [DOI] [PubMed] [Google Scholar]
- 28.Gilbert R, Auchincloss JH, Kuppinger M, Thomas MV. Stability of the alveolar/arterial oxygen partial pressure ratio: effects of low ventilation/perfusion regions. Crit Care Med. 1979;7:267–272. doi: 10.1097/00003246-197906000-00003. [DOI] [PubMed] [Google Scholar]
- 29.Wagner PD, Laravuso RB, Uhl RR, West JB. Continuous distributions of ventilation-perfusion ratios in normal subjects breathing air and 100% O2. J Clin Invest. 1974;54:54–68. doi: 10.1172/JCI107750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Merchant RM, Becker LB, Abella BS, et al. Cost-effectiveness of therapeutic hypothermia after cardiac arrest. Circ Cardiovasc Qual Outcomes. 2009;2:421–428. doi: 10.1161/CIRCOUTCOMES.108.839605. [DOI] [PubMed] [Google Scholar]
- 31.Advanced cardiac life support guidelines: adjuncts for airway control and ventilation. Circulation. 2005;112:S51–57. [Google Scholar]
- 32.Balan IS, Fiskum G, Hazelton J, et al. Oximetry-guided reoxygenation improves neurological outcome after experimental cardiac arrest. Stroke. 2006;37:3008–3013. doi: 10.1161/01.STR.0000248455.73785.b1. [DOI] [PMC free article] [PubMed] [Google Scholar]