Abstract
Most primates are social species whose reproduction is influenced by their social relationships. The cotton-top tamarin, Saguinus oedipus, and the common marmoset, Callithrix jacchus, are cooperative breeding species where the family structure alters reproductive function in many ways. While primates receive social effects on reproduction via all sensory stimuli, the marmosets and tamarins are particularly influenced by olfactory/chemosensory stimuli. The olfactory sensory processing is the ‘social glue’ that keeps the family together.
This review describes a number of studies using the marmosets and tamarins at the University of Wisconsin to demonstrate how odor cues are used for altering reproductive function and dysfunction. Several key studies will be discussed to show the role of odor signaling of the female reproductive state. The suppressive effects of odors are mediated by priming odors and can cause a suppressive influence on ovulation in young females via their mother’s scents. Additionally, odor cues from the infant function as priming odors to ensure that fathers and mothers are present and receptive to their parental care duties. Neural pathways occur via the processing of priming odors that consequently stimulate alterations in the behavioral and endocrine response to the stimuli. The dynamics of the cooperative breeding system ensure that offspring have essential needs met and that they develop in a family environment. Olfactory communication plays a key role in maintenance of the social system of Callitrichid monkeys.
INTRODUCTION
Social relationships are highly important in group-living primates and have influence over reproductive function. Who you spend time with and who you are related to influence your reproductive success. For instance, dominance status correlates with male [Duffy et al. 2007] and female reproductive success in species such as baboons and chimpanzees [Silk et al. 2009; Silk et al. 2010]. Social bonds between females can enhance infant survival in baboons [Silk et al. 2003], while social stressors can produce poor outcomes of reproductive success [Bethea et al. 2008].
Nonhuman primates use a variety of social stimuli to communicate their reproductive status among their social groups or to other groups where more long distance signals might prove best. Within a social group there are many means of communicating with other group members. Vocalizations can be used as social signals and to influence mating outcome [Clay et al. 2011; Pfefferle et al. 2008]. Visual signals such as facial expressions, movements or body posture encourage or discourage reproductive advances [Strier 2011]. Tactile stimulation indicates a close spatial relationship between two individuals and certainly can promote positive reproductive success. For example, grooming can strengthen social bonds and increase an individual’s chances of copulation [Tutin 1979]. Odors and taste can be combined to indicate signals that are chemical in nature and that are perceived by a combination of odors and taste. Sensory stimuli produce varying responses in primates depending upon the physiological state of an animal. For instance, sensory stimuli perceived as a stressor can result in altered biochemical status that changes the responsiveness of an individual to hormones and neuromodulators, affecting whole animal behavior in relations to sensory stimuli [Bethea et al., 2005].
Odor/chemical signals are important in many primate species for optimizing sex and reproductive outcome. Odor cues act as primer cues where receiving the odor signal alters the physiological condition of the recipient. Primer odors can have a longer-term physiological effect on the recipient of the odor signals [Wyatt 2003]. Signaling cues from odors often show an immediate effect, such as when the estrous state of a female is communicated. For instance, the timing of ovulation in humans and mice can be altered by odors of conspecifics [McClintock 1971; Schank and McClintock 1992]. Mate choice can be influenced by detecting the major histocompatibility complex (MHC) -dependent odors, which are highly polymorphic genes controlling immunological self/nonself recognition [Roberts et al. 2008; Wedekind et al. 1995]. This may be an important contribution to selecting males to ensure the fitness of their offspring by increasing genetic variability.
Like all primates, the callitrichid monkeys (marmosets and tamarins) rely on a variety of sensory stimuli to communicate and influence reproduction. However, the olfactory system is specially designed to communicate in these species, as it does in lemurs [Charpentier MJ 2008]. The following studies are presented to illustrate the use of olfactory/chemical cues for marmoset and tamarin reproduction. The scent signals cause both increased function and dysfunction of reproductive success. The following studies will demonstrate the reception of scent signals and their effect on inhibiting and/or delaying reproduction, odor processing, signaling of reproductive condition, kin recognition and paternal care. These studies, while not comprehensive for the Callitrichid species, do provide evidence that olfactory communication is the social glue that maintains the structure of the family cooperative breeding system.
The cooperative breeding marmosets and tamarins: models for family relationships
A cooperative breeding system as is seen in the Callitrichidae (marmosets and tamarins) is rare in primates. Cooperative breeders are usually monogamous (or sometimes polyandrous) and dispersal can be delayed until after puberty in offspring [Koening et al. 1992]. The small New World primates live in groups of 3–15 individuals in the wild that are comprised mainly of related individuals. Common to both wild and captivity, adult breeding pairs and their offspring form the family. While departure from the family group does occur in the wild, family groups are most common [Yamamoto et al. 2009]. These species have a high reproductive rate consisting of twin and triplet births as well as postpartum ovulations occurring within 10 to 20 days following birth [Lunn and McNeilly 1982; Ziegler et al. 1987a]. Conception is high, with more than 80% of ovulations ending in pregnancy [Ziegler et al. 1990]. Therefore, mothers are lactating and pregnant while infants are still dependant upon direct care. Gestational duration is variable in callitrichid species with the golden lion tamarin, Leontopithecus genera, lasting only 125–130 days [French & Stribley 1985] while the cotton-top tamarin gestation lasts around 184 days [Ziegler et al. 1987b]. High reproductive rate requires help from all family members for infant care [Snowdon & Ziegler 2007]. This family system creates a social bond where all individuals in a family are reacting to social stimuli from each other. The glue that holds these social bonds together appears to be the ability to provide and receive scent/chemical cues from one another.
Specialized olfactory system and reproduction
Males and females have specialized scent glands in the anogenital regions [Epple 1981; Epple 1982]. Females possess large circumgenital scent glands that appear to be under hormonal control where sebaceous secretions begin to be released at puberty in females [French and Cleveland 1984; Savage et al. 1988]. Scent marks produced by mixing gland secretions with urine and possibly vaginal discharge communicate the differences between species, subspecies, gender, individuals and dominance status [Belcher AM 1990] as well as ovulatory phase [Smith and Abbott 1998; Ziegler et al. 1993]. Both male and female marmosets and tamarins perform the behavior of scent marking although there are cross species differences in male scent marking frequencies. Marmosets have specialized jaw morphology and will gouge branches and other woody substrates in their environment to deposit their scent secretions [Coimbra-Filho & Mittermeier 1976]. Tamarin species lack this adaptation and do not gouge their environment. However, they do scent mark and leave their odors deposited for communication means. Both species can be trained (or encouraged) to deposit their scent secretions on a removable substrate that can be tested for valence to other individuals (Fig. 1) [Ziegler et al., 1993; Ziegler et al., 2005}.
Figure 1.

A female common marmoset rubs scent secretions from the anogenital/suprapubic scent glands, mixed with urine, on a reagent stopper top. Once deposited these scent secretions can be diluted and transferred for storage and presentation to a recipient.
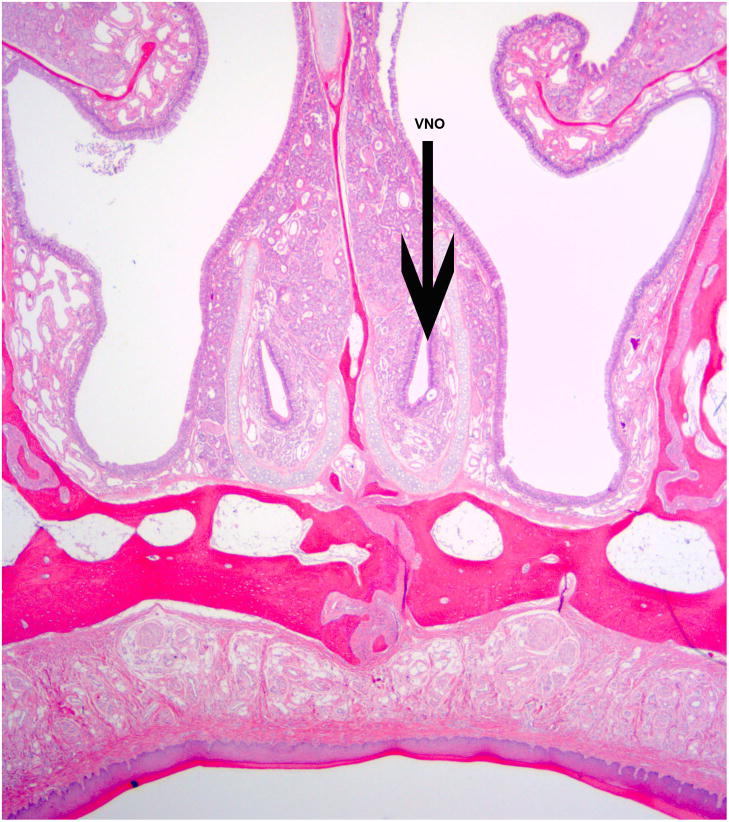
Callitrichid monkeys also have well-defined olfactory systems consisting of the main olfactory and the accessory olfactory system. The main olfactory system is important for processing all odors incoming into the brain while the accessory olfactory system has the peripheral receptor organ, the vomeronasal organ (VNO), involved in chemosensory signals and regulation of reproductive behavior [Taniguchi et al. 1992]. The functionality of the VNO has been debated extensively due to the residual VNOs described in apes, humans and Old World monkeys [Doty 2010]. Findings on the VNO and other olfactory structures indicate a complex pattern of reduction across the order for all primates [Smith and Rossie 2006]. Marmosets show a well-formed VNO and vomeronasal nerves (Fig. 2) while those of the Saguinus species are less developed [Smith et al. 2011]. However, both Callithrix and Saguinus demonstrate behavioral and hormonal responses to olfactory scents when tested as will be described below. Though further studies need to be performed to discern the source of the chemosensory input to the brain, it is evident in these species that it exists.
Figure 2.
Histological presentation of the nasal system in the common marmoset. Arrow points to the vomeronasal organ (VNO) embedded between the sinuses. The marmoset VNO has all the specific structures to suggest functionality.
The signal process
Processing sensory stimuli occurs through neural connections from sensory receptors in the nose. Airborne olfactory signals are received in the olfactory epithelium and processed through the olfactory bulbs where they relay signals to the higher olfactory centers. Primary olfactory cortex projections are sent to other parts of the forebrain, including the amygdala, the hippocampus, the hypothalamus, dorsal thalamus and the neocortex. The accessory olfactory system receives both airborne and liquid signals through the nasopalatine duct found in the roof of the mouth and sends inputs to the VNO embedded in the nasal sinuses. The olfactory nerve surrounds the VNO and extends to the accessory olfactory bulb to the medial nucleus of the amygdaloid complex and bed nucleus of stria terminalis.
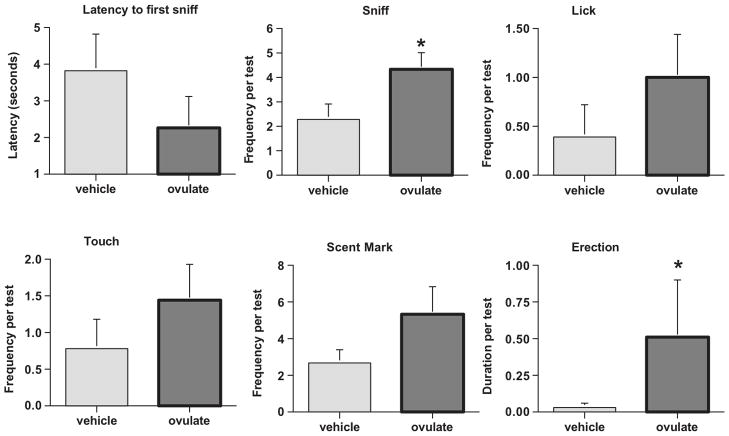
Odors act as primer signals where they modulate sexual behavior and alter hormone release. By examining the neuropathways through behavioral and hormonal outcomes we have been able to show the neural circuit that occurs in a male adult marmoset when he smells an ovulatory marmoset scent. A series of experiments were performed to show that the attractiveness of a female is transmitted by olfactory/chemical cues and results in an arousal signal producing hormonal and behavioral responses. In the first experiment four fully conscious male marmoset monkeys were imaged with functional magnetic resonance imaging (fMRI) [Ferris et al. 2004]. These monkeys were presented with the odors of a novel female collected when she was at or near ovulation as determined by a progesterone assay. A common responsive neural circuit was identified that comprised the temporal and cingulated cortices, putamen, hippocampus, medial preoptic area and cerebellum. The areas of the medial preoptic area/anterior hypothalamic continuum have been shown to produce male sexual behavior [Lloyd and Dixson 1988]. The study was repeated in the marmoset’s home cage where males were isolated from their families and presented with the novel ovulatory scent on a small wooden dowel [Ziegler et al. 2005]. Males were tested for their behavioral responses to the ovulatory odor and bled 20 minutes following the 10-minute interaction with the scent. Males showed significantly higher investigative and arousal behaviors to the ovulatory scent compared to a vehicle control scent (Fig. 3). Time sniffing the scent substrate and the duration of erections were significantly elevated in compared to the vehicle, but without differences in cortisol levels. Thirty minutes after presentation of an ovulatory scent, the males showed a significant increase in testosterone in response to the ovulatory scent but not to the vehicle scent. The study also indicated that depending upon the social status of the male, i.e. living with a family, paired with a mate and no offspring, or living alone for a few days prior to social housing, that single and paired males showed a significantly higher increase in testosterone following the scent presentation than the males who were living with their mate and offspring. Therefore, the neural circuit via the olfactory/chemical scents from novel ovulating marmosets causes a rapid increase in testosterone in response to the signal as well as behavioral arousal responses (Fig. 4). Additionally, the social condition upon which a male lives affects his response to the signal. This suggests a cortical assessment of the signal at the time of presentation. It appears that family males have invested more in their association with the breeding female and in assuring the successful rearing of their offspring.
Figure 3.
Behavioral responses in male common marmosets to isolated periovulatory scents from a novel female versus a vehicle control scent. Frequence or latency was plotted against the scent condition received. Data from Ziegler et al., 2005, Hormones and Behavior 47, 56–64.
Figure 4.
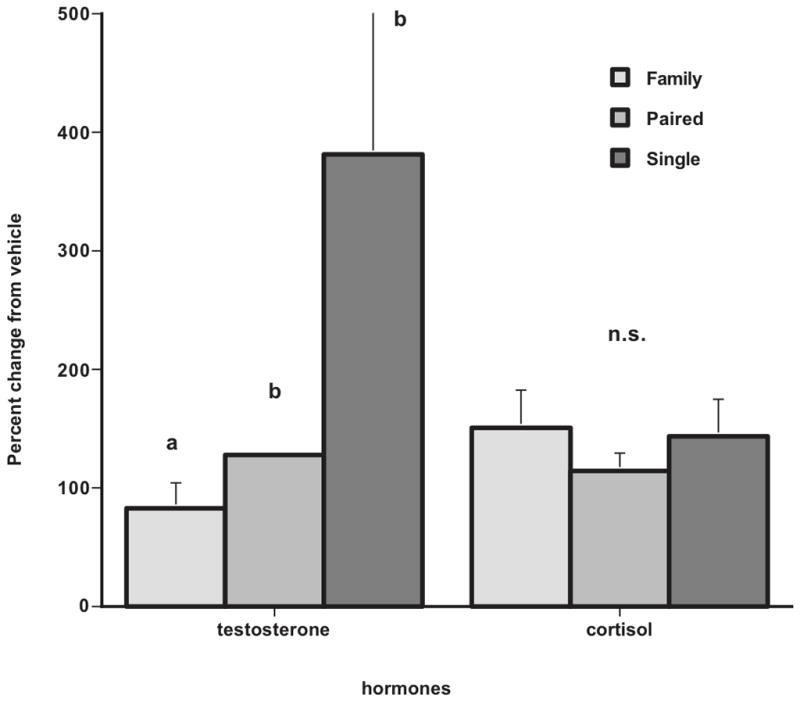
Significant differences in testosterone but not cortisol levels in male common marmosets following isolated scent exposure to a novel periovulatory scent relative to the vehicle control. Males showed significant differences in testosterone by social condition. Data from Ziegler et al., 2005, Hormones and Behavior 47, 56–64.
Olfactory signals can cause dysfunction of reproductive success
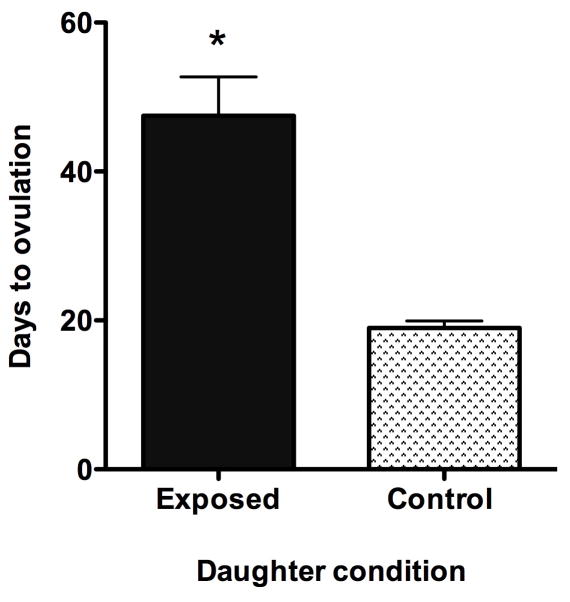
While callitrichid monkeys have a very high reproductive rate for primates, generally only the adult breeding female actually reproduces in a group. Under most conditions in the wild and in captivity, all genera display some levels of fertility control in daughter or subordinate females when they remain with a family past puberty, resulting in only one breeding female [Abbott et al. 1981; Arruda et al. 2005; Saltzman et al. 1997]. However, no suppression of ovarian cycling has been reported for daughter golden-lion tamarins [French and Stribley 1985]. The inhibition of reproduction has been shown to be a result of behavioral and hormonal control. In cotton-top tamarins, daughters live in family groups well past puberty without ovulating [French and Cleveland 1984; Ziegler et al. 1987a]. Daughters show levels of urinary estrogens and luteinizing hormone (LH) even up to 42 months of age without organized cycling [French and Cleveland 1984; Ziegler et al. 1987a]. While female offspring remain in their families, the normal feedback response between LH and estrogen does not occur (Fig. 5). As soon as 8 days after removal from the family and pairing with a reproductive-aged male, the hormones organize and the female ovulates and usually conceives on her first ovulation [Ziegler et al. 1987b]. The inhibition of ovulation appears to be due to olfactory signals received from their mothers [Savage et al. 1988]. When female daughters are removed from their families and placed with a male while receiving scent from the mother twice daily, days to ovulation for the daughter are delayed for up to 60 days (Fig. 6) and ovulation does not occur while a daughter receives her mother’s scent. These studies indicate that olfactory signals alone can suppress reproductive function in female offspring. Due to the high reproductive rate of breeding females who are often nursing and gestating the next twin births, older offspring are needed to help raise the young. A lack of ovulation and ability to conceive may present a clever means of delaying emigration in young to ensure the success of the breeding female’s young.
Figure 5.
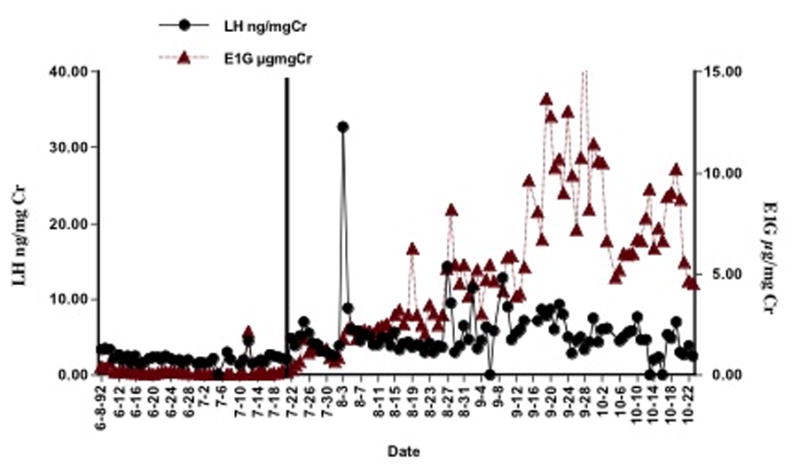
Inhibition of ovulation occurs in female cotton-top tamarin daughters well after puberty. Levels of urinary luteinizing hormone (LH) and estrogens (E1G) while the daughter lived with the family and when she was paired with a male (date 7–22). An LH peak can be seen on day 8–3 and chorionic gonadotropin levels were elevated by day 8–27 indicating pregnancy.
Figure 6.
Time to ovulation from pairing with a male in female cotton-top tamarins. Exposed daughters were exposed twice daily to wooden dowls containing their mother’s scents while control females had no scent transfer. No female who received mother’s scent ovulated during that time. Time to ovulation was significantly longer for the females received their mother’s scent. Data presented in Savage et al., 1988, American J Primatol. 14, 345–359.
Signaling reproductive condition by odors
Mammals have evolved many different types of cues to indicate reproductive status. Communicating the timing of the physiological event of ovulation and coordinating sexual behavior is important to ensure successful fertilization. The signals of ovulation include proceptive and receptive sexual behavior. Female callitrichids do not display overt signals of their reproductive state [Ziegler et al. 1993]. There are no visual signs of the periovulatory condition such as sexual behaviors or sexual skin colorations as occur in many primate species. Marmosets and tamarins will mate throughout the ovarian cycle and so sexual behaviors do not determine the precise timing of ovulation. Olfactory signals, however, do relay the females’ reproductive condition to their mates. Male cotton-top tamarins have shown reproductive arousal behaviors when given isolated scent marks from a novel cycling female [Ziegler et al. 1993]. When males are given freshly collected daily scents from an unknown, periovulatory female while the males are paired with a pregnant female, the males show a significant increase in erections, elevated testosterone, and increased mounts to their own mate after sniffing and licking the scent [Ziegler et al. 2005]. Without any other sensory cues, the males are responding directly to olfactory signals. Cotton-top tamarins also show increased androgen response to the female’s periovulatory period (Fig. 7). Male tamarins were followed with daily urine samples from the time their mate gave birth through the timing of the postpartum ovulation and subsequent pregnancy. The male’s urinary hormones indicated a rise in the androgens, testosterone and dihydrotestosterone (DHT) five days prior to her ovulatory LH peak [Ziegler et al. 2004a]. The androgens continued to remain elevated for the five days following ovulation as well. The onset of the male’s increased androgens occurred at the onset of the female’s follicular phase as indicated by rising estrogens. It appears that sexual communication between the male and female ensures mating at the time of optimum fertility. Although males were also participating in infant care at a high rate during the time females were ovulating, their hormonal system appears to be responsive to both their mate and their offspring.
Figure 7.
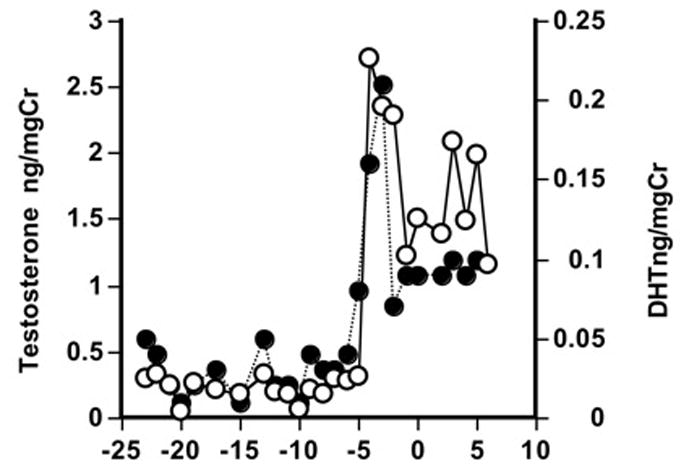
Cotton-top tamarin males show increased androgen excretion (testosterone and DHT) in the five days pre- and post-ovulation in their mate during the postpartum period. Zero indicates the day of ovulation in the mate (x-axis). Males were caring for infants during this time. Data from Ziegler et al., 2004 Amer J Primatol 64, 57–69.
In addition to detecting the ovulatory signals from their mates and novel females, expectant fathers appear to be detecting olfactory/chemical cues of pregnancy in their pair-bonded mates and responding with the onset of reproductive hormones [Ziegler et al. 2004a] and weight gain during their mate’s pregnancy [Ziegler et al. 2006]. Cotton-top tamarin expectant fathers have an increase in prolactin levels mid-gestation and again in the last month of the pregnancy [Ziegler et al. 2004b]. Levels of testosterone, DHT, estradiol, and estrone are also significantly higher at the end of pregnancy in the expectant fathers. Significant differences in the levels of these hormones are found between experienced fathers and first-time fathers, where levels are higher in the experienced males. Both marmoset and tamarin fathers show weight gain during their mate’s pregnancy: beginning about mid-pregnancy the weight of the expectant fathers increases and is significantly higher in the last month of the pregnancy [Ziegler et al. 2006]. Tamarin studies during the gestational period have shown that female tamarins have a mid-gestation increase in cortisol that remains elevated through to the end of pregnancy [Ziegler et al. 1995]. This profile is typical for pregnant primates and is seen in both urine and blood samples. Through the interaction of the fetal-placental-maternal unit, large amounts of cortisol are excreted into the urine. Experienced expectant tamarin fathers also have elevated levels of glucocorticoids during their mate’s pregnancy. Fathers show a peak of glucocorticoid excretion generally the week following their mate’s onset of cortisol elevation at mid-pregnancy [Ziegler et al. 2004b]. As glucocorticoids are known to be involved in regulating pheromones in mice, with corticosterone influencing both the release of the signal and response of the recipient [Marchlewska-Koj and Zacharczuk-Kakietek 1990], it may be that the fetal-placental-maternal urinary glucocorticoid increase is acting as an endocrine/pheromone signal that initiates a glucocorticoid response in the expectant father. Thus fathers are ready to take on a major role in infant care at the time of birth and during the postpartum period to ensure the survival of their offspring.
Endocrine responses to Infant Odor
Perhaps one of the most striking behaviors found in marmosets and tamarins are their paternal care behaviors. Fathers of both the common marmoset and cotton-top tamarin will spend more than half the first day postpartum carrying the newborns. Experienced male cotton-top tamarins will spend an average of 80% of their time caring for infants during the first week and mothers in large families will generally only take infants for nursing [Ziegler et al. 1990]. The interaction of fathers with their infants has a profound effect on their hormones. Common marmoset fathers who are sampled following infant carrying show increased prolactin over fathers who were not recently carrying their infants [Dixson and George 1982; Mota and Sousa 2000]. Speculations have been that the contact of the infant causes the elevation of prolactin shortly after. However, olfactory communication cannot be ruled out, as recent studies in common marmosets indicate that infant scent alone can cause alterations in reproductive steroids in fathers. Marmoset fathers and parentally naïve males were tested with isolated scents from their two to three week offspring for fathers and a novel, unrelated infant for naïve males in a room located away from the olfactory odors of the marmoset colony [Prudom et al. 2008]. Fathers received ten minutes of direct odor contact with the infant scents or a vehicle control and then a blood sample was taken 20 minutes following the scent exposure. Testosterone was significantly lower in fathers after exposure to their infant’s scent compared with vehicle, but no differences in cortisol levels occurred. The naïve males showed no change in testosterone or cortisol between the novel infant scent and the vehicle. These data indicate that fathers are responsive to their infant’s scent but parentally naïve males are not hormonally responsive to an infant scent. The lowering of testosterone in fathers could be a mechanism initiated by the infant to ensure tolerance from fathers and ensure fewer aggressive behaviors.
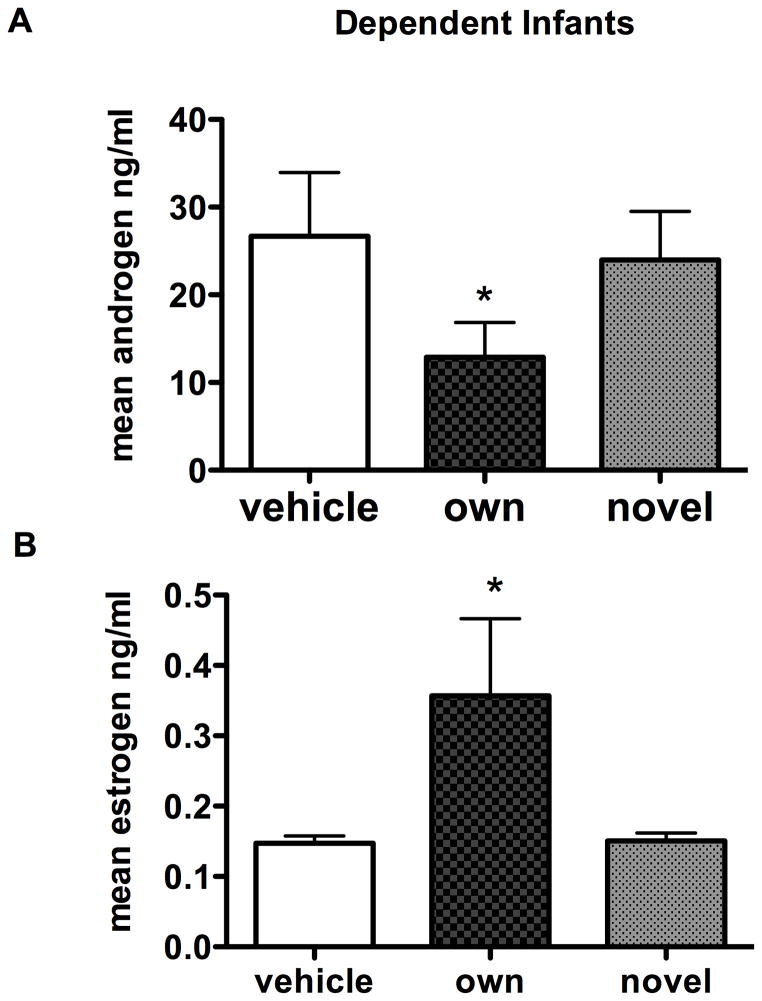
Fathers are known to be very responsive to infant stimuli and in fact fathers will respond to infant distress cries from unknown infants as rapidly as they do to their own infants [Zahed et al. 2008]. However, it seems unlikely that fathers will show the same endocrine response to novel infant scent as they do to their own. Additionally, fathers may only show an endocrine response when the infants are totally reliant on them. In a recent study, fathers were tested with infant scents as previously with scent marks from five to ten day old infants that were either the male’s own infant or a novel infant of the same age (Fig. 8) [Ziegler et al. 2011]. Males were tested again when these same infants were three months of age and independent of being carried. Males were bled 30 minutes following the beginning of the exposure and samples were tested for androgens (testosterone and DHT) and estrogens (estradiol and estrone). When tested against a vehicle and a novel infant, males only showed a decrease in androgens when the infant was his own and at the dependent age. The decrease in androgens was also associated with increased estrogens in the fathers. Fathers showed no endocrine response to independent, older infant scent, whether the scent belonged to his own or a novel infant [Ziegler et al. 2011]. This suggests that males actually perceive the scent as more relevant when the infants are young. Hormonally primed fathers may be more receptive to the odors of their own young infants. There may also be a change in the odor signature of older infants. The olfactory-hormonal system in parents may be highly tuned or flexible to show hormonal responses to the odor of dependent offspring but less to the odors of independent or non-related offspring.
Figure 8.
Common marmoset fathers show decreased circulating androgen and increased estrogen production 30 minutes following smelling their own infant scent compared to a novel infant’s scent of the same age. The scent was collected from infants 5–10 days of age and presented to the males in an isolated room. The x-axis indicates the vehicle, own, or novel treatment the males received during the scent presentation. Data from Ziegler et al. 2011, Hormones and Behavior 59, 265–270.
This study provides a powerful indicator of the olfactory/chemical communication that occurs in this species. As we have seen when males respond to isolated scents of ovulating females [Ziegler et al. 2005], males have a rapid response of altering androgen production. The chemical/olfactory communication in marmosets provides an important mechanism for maintaining the bonds between individuals with their family groups. Olfactory cues play a primary role in social bonding in small-brained mammals such as rodents but are thought to play a lesser role in large-brained primates [Broad et al. 2006]. Odor recognition of offspring in rodents and other small-brained mammals that are regulated by odor cues is short lived compared to essential long-lasting cues in a primate with a long birth to weaning period and continued parental care up to offspring reproduction or longer. While primate species may use all sensory cues to form and maintain parent – infant bonds, odor cues may still be essential for many aspects of parental care. Human mothers have been shown to recognize their newborn infants by olfactory cues [Kaitz et al. 1987]. Human fathers are more affectionate toward children whose smell they can identify than towards children’s smells that they do not recognize [Dubas et al. 2009]. As our data indicate, marmoset fathers are highly responsive to infant scent cues and hormonal responses are strongest when the infants are related, pre-weaned and dependent upon paternal care for survival.
The hormonal response to odor stimuli from a male’s own infant also demonstrates that male marmosets have a neuroplastic response to infant stimuli as do mothers. While males of bi-parental species do not gestate infants, they do have hormonal changes occurring prior to and following their offspring’s birth. In mothers these hormonal changes stimulate neural growth, neurogenisis and remodeling in many brain areas. In father marmosets first-time and experienced males have increased density of dendritic spines on pyramidal neurons in the prefrontal cortex during the active parenting stage indicating that males also experience neural growth as mothers do [Kozorovitskiy etal., 2006]. Furthermore, the neuroplasticity of the system is indicated by the reduction of dendritic spine density in their brain the further away from the birth of the infants and their need to show active parenting.
Synthesis
Marmosets and tamarins are excellent model species for examining olfactory/chemical communication and its influence on reproductive functioning. Due to the cooperative breeding system of these species, it is important to maintain a close communication in the entire family. The social bonds that are formed in families help keep the members together, providing the social glue needed to ensure the success of the entire group. Scent communication can influence the ability of a female daughter to remain in her family much longer since mother’s scent can inhibit ovulation and thus, emigration. Thus female marmosets and tamarins remain in their families beyond puberty and help ensure the survival of their younger siblings by caring for them. During this time they are able to learn the skills they will need to become successful parents. Delayed dispersal allows the adult offspring, both male and female, to remain with their natal family until breeding opportunity arises.
Signaling ovulation and pregnancy by odors is an ideal method for small arboreal primates such as marmosets and tamarins. Living in trees where visual signals can be obscured and auditory calls may compete with the surrounding birdcalls, scent marks are easily placed on substrate where they can remain for long periods of time. Sexual communication between a paired male and female ensures mating at the time of optimum fertility. Callitrichid monkeys have a high fertility rate, with multiple births from each pregnancy, and a high rate of postpartum conception. If a high reproductive rate is important for this species due to high mortality of offspring, then a clear signaling process to indicate fertility is essential. Breeding males participate in infant care at high rates during the periovulatory period since infants are only two to three weeks of age at this time. Males will be actively involved in infant carrying and staying close to the female where he can receive the ovulatory signals.
Cooperative breeding species remain in close social communication to ensure the cooperative environment for raising offspring. Olfactory communication and other chemical signals are effective in optimizing reproductive outcomes. The perception of the reproductive signal allows these species the ability to maintain their strong pair bond and ensure that males are available for the demands of infant care. The dynamics of the bi-parental species ensures that offspring have essential needs met and that they develop in a family environment. Determining the fundamentals of communication of reproductive function may help our understanding of the needs of the individual species of Callitrichids in their native environment and therefore aid in the process of conservation of some of the highly endangered species in this family.
Acknowledgments
I would like to thank all of my collaborators for the studies referenced here, particularly Professor Charles T. Snowdon. Two members of the Assay Services of the Wisconsin National Primate Research Center (WNPRC) were instrumental in almost every study: Frederick Wegner and Daniel Wittwer. The National Institute of Health grants supported these studies: MH 35215 to CTS, MH56413 to TEZ, HD057684 to TEZ, the WNPRC base grant RR000167, and the Institute for Clinical & Translational Research 1U1RR025011. Information presented in this review adhered to the American Society of Primatologists’ Principles for the Ethical Treatment of Primates.
References
- Abbott DH, McNeilly AS, Lunn SF, Hulme MJ, Burden FJ. Inhibition of ovarian function in subordinate female marmoset monkeys (Callithrix jacchus jacchus) J Reprod Fertil. 1981;63(2):335–45. doi: 10.1530/jrf.0.0630335. [DOI] [PubMed] [Google Scholar]
- Arruda MF, Araujo A, Sousa MB, Albuquerque FS, Albuquerque AC, Yamamoto ME. Two breeding females within free-living groups may not always indicate polygyny: alternative subordinate female strategies in common marmosets (Callithrix jacchus) Folia Primatol (Basel) 2005;76(1):10–20. doi: 10.1159/000082451. [DOI] [PubMed] [Google Scholar]
- Belcher AMEG, Greenfield KL, Richards LE, Kuerling I, Smith AB., III Proteins: biologically relevant components of the scent marks of a primate (Saguinus fuscicollis) Chemical Senses. 1990;15:431–446. [Google Scholar]
- Bethea C, Centeno M, Cameron J. Neurobiology of stress-induced reporductive dysfunction in female macaques. Molecular Neurobiology. 2008;38(3):199–230. doi: 10.1007/s12035-008-8042-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broad KD, Curley JP, Keverne EB. Mother-infant bonding and the evolution of mammalian social relationships. Philos Trans R Soc Lond B Biol Sci. 2006;361(1476):2199–214. doi: 10.1098/rstb.2006.1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charpentier BM, Drea CM. Smelling right: the scent of male lemurs advertises genetic quality and realtedness. Molecular Ecology. 2008;17(14):3225–3233. doi: 10.1111/j.1365-294X.2008.03831.x. [DOI] [PubMed] [Google Scholar]
- Clay Z, Pika S, Gruber T, Zuberbuhler K. Female bonobos use copulation calls as social signals. Biol Lett. 2011;7(4):513–6. doi: 10.1098/rsbl.2010.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixson AF, George L. Prolactin and parental behaviour in a male New World primate. Nature. 1982;299(5883):551–3. doi: 10.1038/299551a0. [DOI] [PubMed] [Google Scholar]
- Doty R. The great pheromone myth. Baltimore: Johns Hopkins University Press; 2010. [Google Scholar]
- Dubas J, Heijkoop M, van Aken M. A preliminary investigation of parent-progeny olfactory recognition and parental investment. Human Nature. 2009;20:80–92. [Google Scholar]
- Duffy KG, Wrangham RW, Silk JB. Male chimpanzees exchange political support for mating opportunities. Curr Biol. 2007;17(15):R586–7. doi: 10.1016/j.cub.2007.06.001. [DOI] [PubMed] [Google Scholar]
- Epple G. Effects of prepubertal castration on the development of the scent glands, scent marking, and aggression in the saddle back tamarin (Saguinus fuscicollis, Callitrichidae, primates) Horm Behav. 1981;15(1):54–67. doi: 10.1016/0018-506x(81)90034-9. [DOI] [PubMed] [Google Scholar]
- Epple G. Effects of prepubertal ovariectomy on the development of scent glands, scent marking, and aggressive behaviors of female tamarin monkeys (Saguinus fuscicollis) Horm Behav. 1982;16(3):330–42. doi: 10.1016/0018-506x(82)90031-9. [DOI] [PubMed] [Google Scholar]
- Ferris CF, Snowdon CT, King JA, Sullivan JM, Jr, Ziegler TE, Olson DP, Schultz-Darken NJ, Tannenbaum PL, Ludwig R, Wu Z, et al. Activation of neural pathways associated with sexual arousal in non-human primates. J Magn Reson Imaging. 2004;19(2):168–75. doi: 10.1002/jmri.10456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French J, Cleveland J. Scent-marking in the tamarin, Saguinus oedipus: sex differences and ontogeny. Animal Behavior. 1984;32:615–623. [Google Scholar]
- French JA, Stribley JA. Patterns of urinary oestrogen excretion in female golden lion tamarins (Leontopithecus rosalia) J Reprod Fertil. 1985;75(2):537–46. doi: 10.1530/jrf.0.0750537. [DOI] [PubMed] [Google Scholar]
- Kaitz M, Good A, Rokem AM, Eidelman AI. Mothers’ recognition of their newborns by olfactory cues. Dev Psychobiol. 1987;20(6):587–91. doi: 10.1002/dev.420200604. [DOI] [PubMed] [Google Scholar]
- Lloyd S, Dixson A. Effects of hypothalamic lesions upon the sexual and social behaviour of the male common marmoset (callithrix jacchus) Brain Research. 1988;463:317–329. doi: 10.1016/0006-8993(88)90405-2. [DOI] [PubMed] [Google Scholar]
- Lunn SF, McNeilly AS. Failure of lactation to have a consistent effect on interbirth interval in the common marmoset, Callithrix jacchus jacchus. Folia Primatol (Basel) 1982;37(1–2):99–105. doi: 10.1159/000156023. [DOI] [PubMed] [Google Scholar]
- Marchlewska-Koj A, Zacharczuk-Kakietek M. Acute increase in plasma corticosterone level in female mice evoked by pheromones. Physiol Behav. 1990;48(5):577–80. doi: 10.1016/0031-9384(90)90194-9. [DOI] [PubMed] [Google Scholar]
- McClintock MK. Menstrual synchorony and suppression. Nature. 1971;229(5282):244–5. doi: 10.1038/229244a0. [DOI] [PubMed] [Google Scholar]
- Mota MT, Sousa MB. Prolactin levels of fathers and helpers related to alloparental care in common marmosets, Callithrix jacchus. Folia Primatol (Basel) 2000;71(1–2):22–6. doi: 10.1159/000021727. [DOI] [PubMed] [Google Scholar]
- Pfefferle D, Brauch K, Heistermann M, Hodges JK, Fischer J. Female Barbary macaque (Macaca sylvanus) copulation calls do not reveal the fertile phase but influence mating outcome. Proc Biol Sci. 2008;275(1634):571–8. doi: 10.1098/rspb.2007.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prudom SL, Broz CA, Schultz-Darken N, Ferris CT, Snowdon C, Ziegler TE. Exposure to infant scent lowers serum testosterone in father common marmosets (Callithrix jacchus) Biol Lett. 2008;4(6):603–5. doi: 10.1098/rsbl.2008.0358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts S, Gosling L, Carter V, MP MHC-correlated odour preferences in humans and the use of oral contraceptives. Proceedings of the Royal Academy of Sciences. 2008;275:2715–2722. doi: 10.1098/rspb.2008.0825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saltzman W, Schultz-Darken NJ, Severin JM, Abbott DH. Escape from social suppression of sexual behavior and of ovulation in female common marmosets. Ann N Y Acad Sci. 1997;807:567–70. doi: 10.1111/j.1749-6632.1997.tb51970.x. [DOI] [PubMed] [Google Scholar]
- Savage A, Ziegler T, Snowdon C. Sociosexual development, pair bond bormation, and mechanisms of fertility suppression in female cotton-top tamarins (Saguinus oedipus oedipus) American Journal of Primatology. 1988;14:345–359. doi: 10.1002/ajp.1350140404. [DOI] [PubMed] [Google Scholar]
- Schank JC, McClintock MK. A coupled-oscillator model of ovarian-cycle synchrony among female rats. J Theor Biol. 1992;157(3):317–62. doi: 10.1016/s0022-5193(05)80614-9. [DOI] [PubMed] [Google Scholar]
- Silk JB, Alberts SC, Altmann J. Social bonds of female baboons enhance infant survival. Science. 2003;302(5648):1231–4. doi: 10.1126/science.1088580. [DOI] [PubMed] [Google Scholar]
- Silk JB, Beehner JC, Bergman TJ, Crockford C, Engh AL, Moscovice LR, Wittig RM, Seyfarth RM, Cheney DL. The benefits of social capital: close social bonds among female baboons enhance offspring survival. Proc Biol Sci. 2009;276(1670):3099–104. doi: 10.1098/rspb.2009.0681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silk JB, Beehner JC, Bergman TJ, Crockford C, Engh AL, Moscovice LR, Wittig RM, Seyfarth RM, Cheney DL. Female chacma baboons form strong, equitable, and enduring social bonds. Behav Ecol Sociobiol. 2010;64(11):1733–1747. doi: 10.1007/s00265-010-0986-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith T, Garrett E, Bhatnagar K, Bonar C, Breuening A, Dennis J, Kinznger J, Johnson E, Morrison E. The vomeronasal organ of New World monkeys (Platyrrhini) The anatomical record. 2011;294:2158–2178. doi: 10.1002/ar.21509. [DOI] [PubMed] [Google Scholar]
- Smith T, Rossie J. Primate olfaction: Anatomy and evolution. In: Brewer WDC, Pantelis C, editors. Olfaction and the brain: window to the mind. Cambridge: Cambridge University Press; 2006. pp. 135–166. [Google Scholar]
- Smith TE, Abbott DH. Behavioral discrimination between circumgenital odor from peri-ovulatory dominant and anovulatory female common marmosets (Callithrix jacchus) Am J Primatol. 1998;46(4):265–84. doi: 10.1002/(SICI)1098-2345(1998)46:4<265::AID-AJP1>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Strier K. Chapter 10: Communication and Cognition. Boston: Prentice Hall; 2011. [Google Scholar]
- Taniguchi K, Matsusaki Y, Ogawa K, TRS Fine structure of the vomeronasal organ in the common marmoset (Callithrix jacchus) Folia Primatologica. 1992;59:169–176. doi: 10.1159/000156655. [DOI] [PubMed] [Google Scholar]
- Tutin G. Mating patterns and reproductive strategies in a community of wild chimpanzees (Pan troglodytes schweinfurthii) Behav Ecol Sociobiol. 1979;6:29–38. [Google Scholar]
- Wedekind C, Seebeck T, Bettens F, Paepke A. MHC-Dependent mate preferences in humans. Proceedings of the Royal Academy of Sciences. 1995;260:245–249. doi: 10.1098/rspb.1995.0087. [DOI] [PubMed] [Google Scholar]
- Wyatt T. Pheromones and Animal Behaviour: Communication by smell and taste. Cambridge Press; Cambridge, UK: 2003. [Google Scholar]
- Yamamoto M, Arruda M, Alencar A, de Sousa M, Araujo A. Mating systems and female-female competition in the common marmoset, Callithrix jacchus. In: Susan M, Ford LMPaLCD, editors. The Smallest Anthropoids, The Marmoset/Callimico Radiation. New York: Springer; 2009. [Google Scholar]
- Zahed SR, Prudom SL, Snowdon CT, Ziegler TE. Male parenting and response to infant stimuli in the common marmoset (Callithrix jacchus) Am J Primatol. 2008;70(1):84–92. doi: 10.1002/ajp.20460. [DOI] [PubMed] [Google Scholar]
- Ziegler T, Epple G, Snowdon C, Porter T, Belcher A, Kuderling I. Detection of the chemical signals of ovulation in the cotton-top tamarin, Saguinus oedipus. Animal Behavior. 1993;45:313–322. [Google Scholar]
- Ziegler T, Savage A, Scheffler G, Snowdon C. The endocrinology of puberty and reproductive functioning in female cotton-top tamarins (Saguinus oedipus) under varying social conditions. Biol Reprod. 1987a;37(3):618–27. doi: 10.1095/biolreprod37.3.618. [DOI] [PubMed] [Google Scholar]
- Ziegler TE, Bridson W, CTS, SE Urinary gonadotropin and estrogen excretion during the postpartum estrous, conception and pregnancy in the cotton-to tamarin (Saguinus oedipus oedipus) American Journal of Primatology. 1987b;12:127–140. doi: 10.1002/ajp.1350120202. [DOI] [PubMed] [Google Scholar]
- Ziegler TE, Jacoris S, Snowdon CT. Sexual communication between breeding male and female cotton-top tamarins (Saguinus oedipus), and its relationship to infant care. Am J Primatol. 2004a;64(1):57–69. doi: 10.1002/ajp.20061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler TE, Peterson LJ, Sosa ME, Barnard AM. Differential endocrine responses to infant odors in common marmoset (Callithrix jacchus) fathers. Horm Behav. 2011;59(2):265–70. doi: 10.1016/j.yhbeh.2010.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler TE, Prudom SL, Schultz-Darken NJ, Kurian AV, Snowdon CT. Pregnancy weight gain: marmoset and tamarin dads show it too. Biol Lett. 2006;2(2):181–3. doi: 10.1098/rsbl.2005.0426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler TE, Scheffler G, Snowdon CT. The relationship of cortisol levels to social environment and reproductive functioning in female cotton-top tamarins, Saguinus oedipus. Horm Behav. 1995;29(3):407–24. doi: 10.1006/hbeh.1995.1028. [DOI] [PubMed] [Google Scholar]
- Ziegler TE, Schultz-Darken NJ, Scott JJ, Snowdon CT, Ferris CF. Neuroendocrine response to female ovulatory odors depends upon social condition in male common marmosets, Callithrix jacchus. Horm Behav. 2005;47(1):56–64. doi: 10.1016/j.yhbeh.2004.08.009. [DOI] [PubMed] [Google Scholar]
- Ziegler TE, Washabaugh KF, Snowdon CT. Responsiveness of expectant male cotton-top tamarins, Saguinus oedipus, to mate’s pregnancy. Horm Behav. 2004b;45(2):84–92. doi: 10.1016/j.yhbeh.2003.09.003. [DOI] [PubMed] [Google Scholar]
- Ziegler TE, Widowski TM, Larson ML, Snowdon CT. Nursing does affect the duration of the post-partum to ovulation interval in cotton-top tamarins (Saguinus oedipus) J Reprod Fertil. 1990;90(2):563–70. doi: 10.1530/jrf.0.0900563. [DOI] [PubMed] [Google Scholar]