Abstract
Schizophrenia (SZ) is associated with high rates of smoking. We previously found that dorsal anterior cingulate (dACC) – striatum resting state functional connectivity (rsFC) is independently associated with nicotine addiction and psychiatric illness. Since the insula is implicated in nicotine dependence, we hypothesized that SZ smokers will have greater dysfunction in smoking-related insular and dACC circuits than normal control smokers (NC) independent of smoking severity, consistent with an inherent disease-related weakening of smoking-related circuits. Nicotine challenge was used to demonstrate that decreased rsFC in identified circuits reflects addiction trait and is not affected by pharmacological state. Twenty-four NC smokers and 20 smokers with SZ matched on nicotine addiction severity participated in a resting state fMRI study and were scanned during two separate sessions while receiving a placebo or nicotine patch, in a randomized, cross-over design. Using individualized, anatomically defined anterior and posterior insula and dACC as regions of interest (ROI), whole brain rsFC was performed using each ROI as a seed. Significant negative correlations between smoking severity and rsFC between insula, dACC and striatum were found for both groups. Furthermore, smokers with SZ demonstrated additive reductions in circuit strength between the dACC and insula compared to NC smokers independent of smoking severity. Nicotine challenge did not significantly alter rsFC in insula-dACC-striatal circuits. Reduced rsFC strength between the insula, dACC and striatum is associated with nicotine addiction severity in both non-psychiatrically ill and in SZ smokers. Decreased insula-dACC rsFC may index overlapping circuitry associated with smoking and SZ.
Keywords: resting state functional connectivity, schizophrenia, smoking, nicotine, insula, anterior cingulate
1. Introduction
The prevalence of smoking in schizophrenia (SZ) is over 60% (Chapman et al., 2009), three-fold higher than the general population (Grant et al., 2004). Patients with SZ extract more nicotine per cigarette (Williams et al., 2010) and have lower quit rates (de Leon and Diaz, 2005). Explanations for increased smoking in SZ include self-medication to improve cognitive function (Jacobsen et al., 2004), reduce antipsychotic medication side effects (Levin et al., 1996), or decrease negative symptoms (Freedman et al., 2008). Other hypotheses include shared neurobiology at the genetic or receptor level (Breese et al., 2000; Hong et al., 2011) or to improve decreased reward responsivity associated with anhedonia (Ahnallen et al., 2012).
These “self-medication” theories have supportive evidence, but do not easily explain other aspects of smoking in SZ. Increased smoking rates are noted years before psychosis onset (Diaz et al., 2008) and in non-psychotic relatives (Lyons et al., 2002). This study tests an alternative hypothesis that abnormalities in insular and dorsal anterior cingulate (dACC) circuits contribute to schizophrenia-smoking comorbidity.
Damage to the insula disrupts nicotine dependence (Naqvi et al., 2007). Both the ACC and insula are consistently activated in imaging studies of craving (Garavan et al., 2000; Janes et al., 2010). We previously described decreased resting state functional connectivity (rsFC) strength in circuits between dACC and ventral striatum (VS) in control smokers that correlated with nicotine addiction severity and were not affected by acute nicotine administration, suggesting that these circuits signify the trait of nicotine addiction rather than the state of acute nicotine exposure (Hong et al., 2009). Both the insula and dACC are strongly associated with SZ (Chan et al., 2011; Honey et al., 2005; Kerns et al., 2005; Minzenberg et al., 2009; White et al., 2010). The overlap of neuroanatomical areas related to SZ and to smoking led to the hypothesis that shared insular and/or dACC circuit abnormalities may contribute to smoking in SZ.
We chose rsFC fMRI as a means to study proposed functional “circuit” alterations in smoking and SZ. This tool employs task-independent functional connectivity between brain regions, as measured by temporally correlated, low frequency fluctuations in the blood oxygenation level-dependent (BOLD) signal (Raichle et al., 2001). We compared rsFC in normal control (NC) and SZ smokers matched on measures of nicotine addiction severity in the context of nicotine administration. Functional circuits significantly associated with nicotine addiction severity were extracted to test the hypotheses that (1) smoking in SZ is driven by a similar pattern of insular/dACC brain circuits that control smoking in NC, (2) SZ smokers will have greater impairment in rsFC in such circuits compared to NC smokers at all levels of smoking severity, and (3) these circuits reflect the trait of nicotine dependence regardless of pharmacological state (i.e., nicotine challenge).
2. Materials and methods
2.1 Subjects
Written informed consents approved by local IRBs were obtained from twenty smokers with SZ and 24 NC. Subjects were right-handed, 18 to 50 years of age, and had no major medical illnesses. They were recruited through media advertisements or from local outpatient clinics. Clinically stable subjects with SZ, all on antipsychotic medications (Supplementary Table 1), were diagnosed by the Structured Clinical Interview for DSM-IV. Chlorpromazine equivalent dose (CED) was calculated (Woods, 2003). Psychiatric symptoms were assessed by the Brief Psychiatric Rating Scale (BPRS). NC participants had no Axis I diagnoses (except nicotine dependence). Nicotine addiction severity was measured by the Fagerstrom Test for Nicotine Dependence (FTND) (Heatherton et al., 1991). Lifetime smoking was calculated as #packs per day × years of smoking.
2.2 Study Design
Subjects underwent two fMRI sessions approximately one week apart, in the presence of nicotine or placebo patch, in a randomized, double-blind cross-over design. Patch dose was 35 mg for those smoking ≥ 15, or 21 mg for 10 – 15 cigarettes per day. Subjects maintained usual smoking patterns until patch administration and then abstained for 4.5 hours (2.5 hours before + 2 hours during imaging). This time window should avoid peak withdrawal symptoms, which occur ~6 – 12 hours after smoking cessation (Hughes et al., 1994). Carbon monoxide (CO) was measured prior to patch administration to assess recency of smoking. Nicotine blood levels were obtained post-scan. Withdrawal symptoms were assessed by a self-report symptom checklist before and after scanning. One session was excluded from analysis for two subjects due to unexplained signal interference (1 SZ and 1 NC, both placebo). Another four subjects (1 SZ and 3 NC, all nicotine) only completed one scan session (data included in group analyses). Some results from 19/24 NC were previously reported (Hong et al., 2009).
2.3 Region of Interest (ROI)
The anterior insula (aIns), posterior insula (pIns) and dACC were manually partitioned bilaterally for each subject. The central sulcus of the insula separates the aIns and pIns and is visible on anatomical MRI scans (Naidich et al., 2004). To parse the insula, the sagittal slice where the central sulcus was most prominent was first identified. The aIns and pIns were segregated and drawn on subsequent sagittal slices. The dACC was drawn on the coronal plane one slice anterior to the disappearance of the corpus callosum until the slice posterior to the anterior commissure (Hong et al., 2009).
2.4 MRI Data Acquisition
Scanning was performed on a 3-T MR Siemens Allegra scanner equipped with a quadrature volume head coil. Subjects were instructed to rest quietly (eyes open). Resting fMRI EPI scans were acquired in 39 axial, interleaved, 4-mm sections (150 volumes; echo time/repetition time 27/2000 milliseconds; flip angle 80°; field of view 220 × 220 mm2; image matrix 64 × 64). High resolution (1×1×1 mm3) T1-weighted MPRAGE images were acquired.
2.5 Statistical Analysis
Volumes were slice-timing aligned, motion corrected, spatially normalized and resampled to Talairach space at 3×3×3 mm3, spatially smoothed (full-width half maximum 6 mm) and temporally low-pass filtered (fcutoff = 0.1 Hz) using AFNI (Biswal et al., 1995; Cordes et al., 2001; Lowe et al., 1998; Oakes et al., 2005). Correlation coefficients between the average time course from each seed ROI and each voxel in the brain were calculated and Fisher-transformed to z-scores. White matter and cerebrospinal fluid ROI time courses and 6 rigid head-motion parameters were included as nuisance covariates to regress out activity unlikely related to neuronal activity.
To identify insula and dACC rsFC circuits associated with nicotine addiction severity (FTND), acute nicotine response (DRUG: nicotine vs. placebo) or both, we first assessed the FTND × DRUG interaction by regression analysis of the rsFC maps in all subjects. In the event of no FTND × DRUG interaction, each subject’s nicotine and placebo connectivity maps were averaged and regression analysis for FTND performed on averaged connectivity maps to identify circuits associated with nicotine addiction severity.
We next extracted mean rsFC for each subject’s session from these addiction-related circuits and entered them into a repeated measures linear mixed effects (LME) model with diagnosis as the GROUP factor, DRUG as repeated measure, FTND as a covariate, and rsFC as the dependent variable. Main effects of GROUP, DRUG, FTND and their interactions were included in the initial model; non-significant interactions were removed. We also performed a supportive analysis where we first identified rsFC circuits that differed significantly between SZ smokers and NC smokers for each seed ROI while controlling for FTND to isolate GROUP differences independent of nicotine addiction severity. Next, we overlapped these disease-related maps on to nicotine addiction severity maps obtained from FTND regression analysis. Candidate circuit(s) identified by overlapping disease and FTND maps supports our hypothesis of overlapping functional connectivity deficits in nicotine addiction and schizophrenia.
Paired t-tests identified circuits associated with acute nicotine versus placebo challenge. For all imaging analyses, lifetime cigarette use, nicotine plasma level and CO were included as additional covariates to investigate whether effects were secondary to chronic and/or acute nicotine exposure. Partial correlations controlled for gender, age, education, heart rate, blood pressure and withdrawal symptoms on rsFC. Control for multiple comparisons was performed by Monte Carlo simulations at puncorrected<0.001, requiring a minimal cluster size of 405 mm3 to obtain pcorrected<0.008 (corresponding to pcorrected<0.05 with Bonferroni correction for 6 seed regions: left and right aIns, pIns, and dACC).
Clinical characteristics associated with DRUG condition were compared using repeated measures ANOVA. Pearson correlations measured the relationship between FTND and BPRS scores/CED and between rsFC and these SZ-related variables.
3. Results
3.1 Clinical Characteristics
SZ and NC subjects did not significantly differ in age, gender or smoking parameters (Table 1). There was no significant correlation between FTND and BPRS total score (r=0.03, p=0.89) or CED (r=-0.28, p=0.24). Nicotine patch administration increased systolic blood pressure in both NC and SZ subjects (Table 2: t=-2.1, p=0.04). We found a significant group difference in post-scan HR (Table 2: t=-3.8, p<0.001), attributable to a trend towards decreased HR in NC subjects during the nicotine condition (t=-1.8, p=0.09).
Table 1.
Demographic and Clinical Characteristics
| Normal Control Smokers | Schizophrenia Smokers | ||||||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | t | df | p | |
| Age | 36.2 | 10.9 | 37.4 | 10.6 | -0.4 | 42 | 0.72 |
| Gender (Female/Male) | 6/18 | 2/18 | χ2 = 1.7 | 1 | 0.20 | ||
| Education (yrs) | 13.0 | 1.7 | 11.4 | 2.0 | 2.8 | 42 | 0.008* |
| Cigarettes per day | 18.9 | 6.7 | 19.3 | 9.4 | -0.2 | 42 | 0.86 |
| FTND | 4.1 | 2.4 | 4.5 | 2.0 | -0.6 | 42 | 0.58 |
| Lifetime cigarette use (Pack years) | 17.6 | 15.5 | 18.5 | 15.9 | -0.2 | 42 | 0.85 |
| BPRS total score | — | — | 33.3 | 7.0 | — | — | — |
| Chlorpromazine Equivalents (mg/day) | — | — | 319.5 | 336.8 | — | — | — |
Statistically significant
Abbreviations: Fagerstrom Test for Nicotine Dependence (FTND), Brief Psychiatric Rating Scale (BPRS)
Table 2.
Nicotine versus Placebo Patch Measurements
| Normal Control Smokers | Schizophrenia Smokers | GROUP main effect | DRUG main effect | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||||
| Placebo | Nicotine | Placebo | Nicotine | |||||||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | t | df | p | t | df | p | |
| CO levels before patch (ppm) | 25.5 | 13.1 | 24.2 | 13.6 | 31.5 | 21.5 | 30.7 | 22.2 | 1.6 | 42 | 0.11 | -0.3 | 36b | 0.78 |
|
| ||||||||||||||
| Nicotine level at end of scan (ng/mL) | 3.1 | 4.7 | 32.8 | 15.0 | 4.5 | 4.7 | 34.1 | 9.4 | 0.9 | 34b | 0.38 | 14.1 | 30b | <0.001* |
|
| ||||||||||||||
| Change in pulse rate (beats/min)a | 10.6 | 8.4 | 4.3 | 11.9 | 1.1 | 7.6 | -0.1 | 7.3 | -3.8 | 42 | <0.001* | -1.9 | 36b | 0.05 |
|
| ||||||||||||||
| Change in systolic blood pressure (mm Hg)a | -1.3 | 11.7 | -7.6 | 12.1 | -7.8 | 12.9 | -10.8 | 12.6 | -1.7 | 42 | 0.10 | -2.1 | 36b | 0.04* |
|
| ||||||||||||||
| Change in diastolic blood pressure (mm Hg)a | -5.1 | 8.6 | -5.3 | 9.2 | -2.9 | 10 | -2.3 | 10.8 | 1.1 | 42 | 0.26 | 0.02 | 36b | 0.98 |
Based on repeated measures ANOVA of nicotine versus placebo-related measurements with DRUG as a repeated measure and GROUP (diagnosis) as a main effect. No significant interactions were found.
Changes in vital signs from prior to patch application to 4.5 hours later at end of scanning session
Degrees of freedom reduced because 6 subjects only had one scan. Nicotine levels were not available on an additional 9 subjects on at least one scan, due to laboratory assay failure, venipuncture difficulty, or other technical issues.
Statistically significant
There was a significant GROUP × DRUG interaction in which SZ patients had a greater increase in withdrawal symptoms on placebo (Kruskal-Wallis H=6.1, p=0.01). Post-hoc analyses revealed that 80% of SZ subjects during the nicotine condition and 66.7% during the placebo condition (DRUG effect, χ2 =0.87, p=0.35) had identical withdrawal scores vs. 77.3% of the NC during nicotine condition and 81.0% during the placebo condition (DRUG effect, χ2=0.09, p=0.77), suggesting no change in withdrawal across DRUG or GROUP for most subjects. Further exploration revealed that 6 SZ subjects (33.3%) reported an increase in withdrawal symptoms after the placebo scan compared with 2 NC (9.5%).
3.2 Nicotine Addiction Severity and rsFC
There were no FTND × DRUG interactions for any seed. Therefore, each subject’s rsFC z-maps from nicotine and placebo conditions were averaged. Regression analysis with FTND identified 13 circuits significantly associated with nicotine addiction. All remained significant after controlling for lifetime use, ruling out chronic nicotine exposure as a primary contributor. Two circuits were no longer significant after controlling for age and education (left dACC-premotor and right pIns-somatosensory/precuneus) and were excluded from further analysis. Controlling for age, gender, education, CO, nicotine plasma level, vital signs or withdrawal symptoms did not alter the significance of FTND-rsFC relationships in the remaining 11 circuits (Table 3).
Table 3.
Nicotine addiction severity (FTND) and resting state functional connectivity: dACC and insula seeds
| FTNDa, c | GROUP main effect on rsFCb | DRUG main effect on rsFCb | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|||||||||||||||||||
| Seed ROI | Co-activating cluster | Volume (mm3) |
Peak activity: Talairach coordinates (x y z) |
All subjects | SZ Smokers | NC Smokers | SZ Smokers | NC Smokers | |||||||||||
| r | p | Mean z-score | SE | Mean z-score | SE | t | p | Mean z-score | SE | Mean z-score | SE | t | p | ||||||
| Left pIns | Left caudate/NA | 2214 | 13.5 | -4.5 | 11.5 | -0.63 | <0.001 | 0.16 | 0.03 | 0.17 | 0.03 | -0.22 | 0.83 | 0.16 | 0.03 | 0.17 | 0.03 | -0.29 | 0.77 |
| Left DLPFC | 594 | 40.5 | -31.5 | 26.5 | -0.59 | <0.001 | 0.08 | 0.03 | 0.16 | 0.02 | -2.14 | 0.04e | 0.15 | 0.02 | 0.09 | 0.03 | 1.52 | 0.13 | |
|
| |||||||||||||||||||
| Right pIns | Left caudate/NA/ putamen | 1917 | 19.5 | -4.5 | 5.5 | -0.59 | <0.001 | 0.16 | 0.04 | 0.22 | 0.03 | -1.15 | 0.26 | 0.17 | 0.03 | 0.21 | 0.03 | -1.24 | 0.23 |
| Bilateral dACC | 1296 | 7.5 | 1.5 | 38.5 | -0.59 | <0.001 | 0.26 | 0.03 | 0.41 | 0.03 | -3.86 | <0.001d | 0.32 | 0.03 | 0.35 | 0.03 | -0.55 | 0.59 | |
| Left precentral gyrus (premotor) | 1107 | 13.5 | 16.5 | 65.5 | -0.62 | <0.001 | 0.14 | 0.03 | 0.15 | 0.03 | -0.10 | 0.92 | 0.16 | 0.03 | 0.13 | 0.03 | 0.69 | 0.49 | |
| Left inferior frontal gyrus | 675 | 28.5 | -31.5 | -6.5 | -0.6 | <0.001 | 0.05 | 0.03 | 0.09 | 0.03 | -1.28 | 0.21 | 0.06 | 0.02 | 0.08 | 0.03 | -0.83 | 0.41 | |
|
| |||||||||||||||||||
| Left dACC | Right putamen/SI | 1242 | -28.5 | 10.5 | -0.5 | -0.59 | <0.001 | 0.18 | 0.03 | 0.27 | 0.03 | -1.87 | 0.07 | 0.23 | 0.03 | 0.22 | 0.04 | 0.13 | 0.89 |
| Left aIns | 594 | 34.5 | -1.5 | -12.5 | -0.6 | <0.001 | 0.14 | 0.03 | 0.29 | 0.03 | -3.57 | <0.001d | 0.23 | 0.03 | 0.20 | 0.03 | 0.79 | 0.43 | |
| Left putamen/SI/pIns | 567 | 31.5 | 16.5 | 5.5 | -0.54 | <0.001 | 0.21 | 0.04 | 0.27 | 0.04 | -1.09 | 0.28 | 0.24 | 0.03 | 0.24 | 0.04 | 0.01 | 0.99 | |
|
| |||||||||||||||||||
| Right dACC | R putamen/SI/ pIns | 783 | -28.5 | 13.5 | -0.5 | -0.63 | <0.001 | 0.22 | 0.03 | 0.29 | 0.03 | -1.89 | 0.07 | 0.26 | 0.03 | 0.24 | 0.03 | 0.50 | 0.62 |
| Left putamen | 729 | 25.5 | 1.5 | 5.5 | -0.55 | <0.001 | 0.18 | 0.03 | 0.25 | 0.03 | -1.60 | 0.12 | 0.20 | 0.03 | 0.22 | 0.04 | -0.35 | 0.72 | |
Correlation of resting state functional connectivity with FTND obtained from averaged nicotine and placebo sessions, as there was no FTND x DRUG interaction.
Linear mixed effects model with DRUG as repeated measure, GROUP as main effect, and FTND as covariate. There were no significant interactions for any of the circuits.
Correlations significant at p<0.001 with correction for multiple comparisons corresponding to pcorrected<0.008 (p<0.05 with Bonferroni correction for 6 seed regions)
Significant after Bonferroni correction for 6 regions.
Nominally significant at p<0.05
Abbreviations: aIns = anterior insula, dACC = dorsal anterior cingulate cortex, DLPFC = dorsolateral prefrontal cortex, FTND = Fagerstrom Test for Nicotine Dependence, NA = nucleus accumbens, pIns = posterior insula, rsFC = resting state functional connectivity SE = standard error, SI = substantia innominata, SS = somatosensory association cortex
Circuits identified using right and left pIns seeds targeted the caudate/nucleus accumbens (NA; Figure 1A-B). The right pIns seed identified a circuit that involved bilateral dACC/middle cingulate cortex (Figure 1B). We also identified a circuit between left dACC (seed) and left aIns (Figure 1C). Both dACC seeds revealed decreased rsFC with the putamen that negatively correlated with nicotine addiction severity in SZ patients, and as previously reported (Hong et al., 2009), in NC subjects (Figure 1C-D). Importantly, all circuits demonstrated reduced rsFC as a function of nicotine addiction severity. These rsFC-FTND relationships did not significantly differ between SZ and NC (i.e., no significant FTND × GROUP interactions, p=0.23-0.97).
Figure 1.
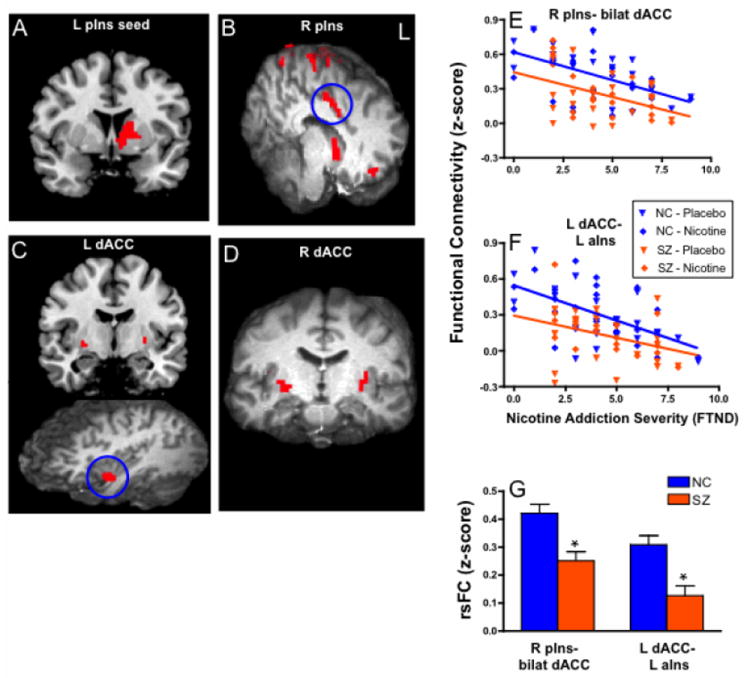
Decreased resting state functional connectivity (rsFC) in insular and dorsal anterior cingulate (dACC) circuits across all subjects associated with more severe nicotine addiction. Significant correlations with FTND were found between both right and left posterior insula (pIns) regions and caudate/nucleus accumbens (A-B). Right pIns seed also targets dACC and frontal/parietal sites (B). Left (C) and right (D) dACC seeds target putamen. Left dACC circuit with anterior insula also identified (C). In addition, the rsFC of two circuits (blue circles), right pIns – bilateral dACC (E) and left dACC – left anterior insula (aIns) (F), inversely correlated with nicotine addiction severity and had significant decreases in rsFC in schizophrenia patients at all levels of smoking severity compared with control smokers; mean ± SE rsFC shown in G.
Therefore, the most consistent ‘targets’ detected by pIns and dACC seeds anatomically converged onto striatal destinations, suggesting that functional circuits between these three areas are critically linked to nicotine addiction severity. Furthermore, the similarity of slopes (i.e., no FTND × GROUP interaction) between NC and SZ groups for rsFC-FTND relationships suggests that the neural substrates of nicotine addiction are similar in NC and SZ smokers. Exploratory stepwise linear regression revealed that 2 circuits combined, left pIns – left caudate/NA and right dACC – right putamen explained 50.0% of the variance in nicotine addiction severity (F(2,41)=22.5, p<0.001). There was no significant correlation between rsFC and clinical symptoms, not surprising given the near zero correlation between FTND and BPRS (R2=0.001).
3.3 Disease-related alterations of rsFC
Two of 11 rsFC circuits that predicted nicotine addiction severity were also associated with reduced rsFC in SZ compared with NC: right pIns-bilateral dACC (GROUP main effect, t=-3.86, p<0.001), and left dACC- left aIns (t=-3.57, p<0.001) (Figure 1E-G, Table 3).
Neuroleptic medication exposure may have contributed to decreased rsFC in SZ. However, we found positive correlations between CED and rsFC in several circuits: right pIns-left caudate/NA (r= 0.74, p<0.001); right pIns-left premotor (r=0.69, p=0.002); left pIns-caudate/NA (r=0.62, p=0.007), left pIns-left inferior frontal gyrus (r=0.53, p=0.02), and left pIns-right parietal (r=0.58, p=0.01). This finding suggests that antipsychotic medication is not driving the strong negative rsFC-nicotine addiction correlations, and may have ameliorated some SZ-rsFC relationships.
Because SZ subjects had a greater increase in withdrawal during the placebo condition, we performed Spearman’s correlations between withdrawal symptoms and rsFC. There was a significant negative correlation between withdrawal symptoms and rsFC in the left dACC – left aIns circuit in the SZ group during placebo (ρ=-0.59, p=0.01), suggesting that withdrawal symptoms may have contributed to decreased circuit strength. Therefore, we repeated the analysis on the subgroup of subjects with no increase in withdrawal symptoms after scanning (n= 14 SZ, n=20 NC) and still found significant FTND (t=-5.2, p<0.001) and GROUP main effects (t=-3.2, p=0.003).
As an alternate approach to test the hypothesis that SZ-related and nicotine addiction-related circuits overlap, we identified rsFC circuits that differed between SZ and NC while controlling for nicotine addiction severity to isolate group effects. Multiple circuits with decreased rsFC were identified in SZ smokers (Figure 2). We overlapped these SZ-specific circuits with nicotine addiction severity circuits and identified one circuit between right pIns and dACC (cluster size 459 mm3) (Figure 3). The significant GROUP difference in rsFC for this circuit remained significant after controlling for potential confounding variables (i.e., age, gender, education, withdrawal symptoms, CO, nicotine level, vital signs, pack year). Therefore, reduced pIns-dACC rsFC is associated with nicotine addiction and is further weakened in smokers with SZ at all levels of addiction severity. This was reciprocally demonstrated using either FTND or diagnosis while controlling for FTND as a regressor in whole brain analyses, suggesting that nicotine addiction severity and disease have a shared circuit in smokers with SZ captured by the reduced pIns-dACC rsFC fMRI assay.
Figure 2.
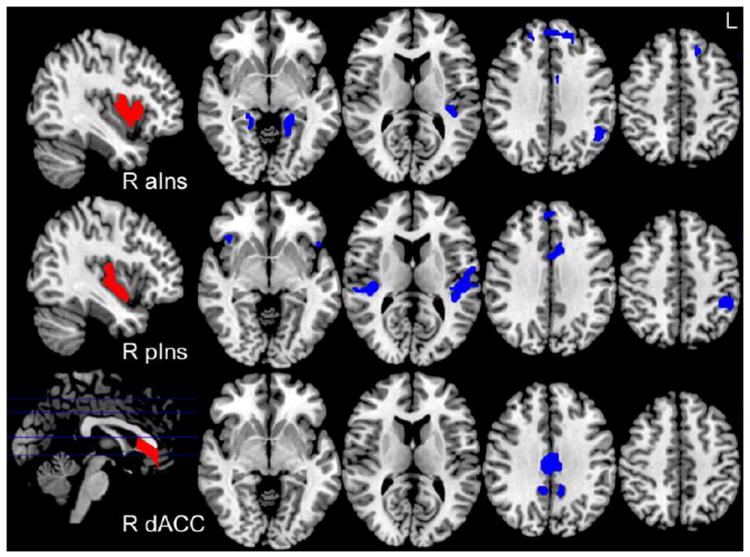
Areas of decreased resting state functional connectivity (rsFC) in schizophrenia (SZ) compared to control smokers (NC) while controlling for nicotine addiction severity (GROUP effect, p<0.001, results from right hemisphere seeds are depicted above). Manual parcellation of seeds depicted in red on sagittal sections: right anterior insula (R aIns), posterior insula (R pIns) and dorsal anterior cingulate (R dACC). Blue clusters represent diagnosis effect.
Figure 3.
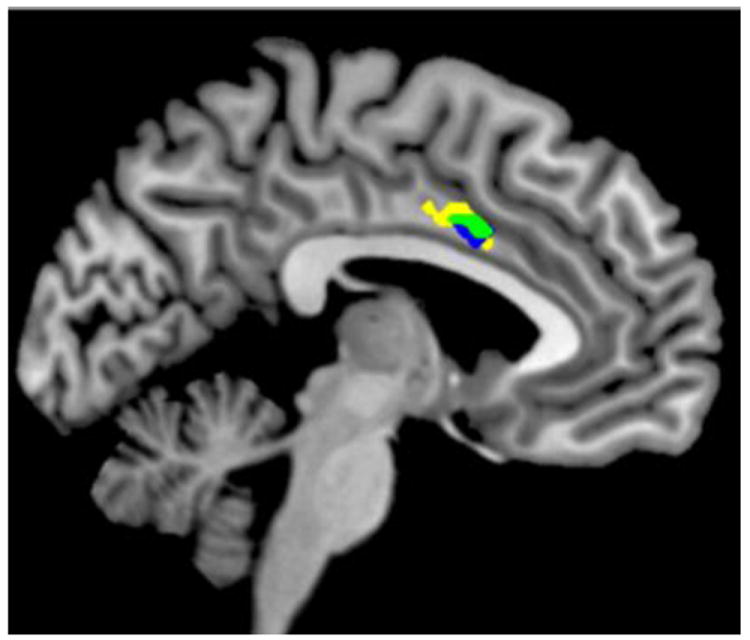
Using right posterior insula (pIns) as seed region, decreased resting state functional connectivity (rsFC) between right pIns and dorsal anterior cingulate (dACC) was associated with nicotine addiction severity (yellow: FTND) and with schizophrenia (blue: GROUP effect, control vs schizophrenia while controlling for FTND). Area of overlap (green) indicates right pIns – dACC circuit common to schizophrenia and nicotine addiction severity.
3.4 Acute Nicotine Effects
There was no significant DRUG main effect on any circuit identified above using either FTND regressions or SZ vs. NC contrast (Table 3). However, nicotine administration was associated with increased rsFC in circuits between dACC seeds and somatosensory-parietal regions (Supplemental Fig. 1 A-B, DRUG main effect, all p<0.001) and between left aIns and occipital cortex (Suppl. Fig. 1 C: DRUG main effect, t=5.6, p<0.001) in both SZ and NC (Suppl. Fig. 1D-E).
3.5 “Reversed” Analysis – Striatal Seeds
Using manually partitioned dorsal (DS) and ventral (VS) striatal seeds (Hong et al., 2010), we identified reduced rsFC in VS-ACC and VS-pIns circuits associated with increasing FTND, providing further confirmation of our findings (Supplemental Table 2, Suppl. Fig. 2).
4. Discussion
We report evidence that functional connectivity between the insula, dACC and striatum is associated with nicotine addiction (FTND) in a cohort of SZ and NC smokers. Indeed, pIns - striatum and dACC - striatum circuits combined explained half of the variance in nicotine addiction severity. Thus, low frequency synchronization among these three critical regions is robustly associated with nicotine addiction regardless of diagnosis, suggesting that neural correlates of nicotine dependence are fundamentally similar in SZ and non-SZ smokers. Nicotine challenge did not significantly alter the relationship between addiction severity and rsFC, confirming the hypothesis that these circuits reflect addiction trait and not pharmacological state.
Our hypothesis that SZ smokers have additive weakening in smoking-related circuits was supported by reduced insular-dACC rsFC associated with both nicotine addiction and SZ. Because nicotine addiction severity was matched in SZ and NC subjects, and higher addiction severity was associated with lower rsFC in these circuits, the additively reduced circuit strength in SZ at all levels of smoking and while controlling for smoking severity is consistent with an inherent reduction of resting coherence in smokers with SZ.
Our finding of decreased rsFC strength between dACC and insula associated with addiction severity parallels results from a study where smoking cue-induced reactivity was associated with decreased functional connectivity in a network that included dACC and insula, and which also predicted relapse (Janes et al., 2010). Network models of brain function support the existence of a “salience network” that involves dACC, insula and VS and modulates processing between large-scale brain networks based on the current homeostatic salience of external or internal stimuli (Seeley et al., 2007). Thus, circuits identified in this study may be related to enhanced salience attributed to external drug-related stimuli or internal physiological states (e.g., withdrawal) by addicted individuals (Robinson and Berridge, 1993). In addition, the critical role of both DS and VS is supported by preclinical models and imaging studies that implicate VS in reward learning and DS in compulsive drug-seeking behavior (Koob and Volkow, 2010).
We found positive correlations between antipsychotic medications and rsFC in two circuits between pIns and caudate/nucleus accumbens. Studies to date are inconsistent with some suggesting that acute administration of antipsychotics leads to widespread decreases in rsFC (Lui et al., 2010) while others show focal increases in functional connectivity (Honey et al., 2003). Consistent with our findings, one study found robust increases in functional connectivity in circuits involving the caudate with antipsychotic medication (Honey et al., 2003), suggesting that alterations in dopaminergic neurotransmission can lead to changes in functional connectivity.
There are several potential limitations of this study. We did not include non-smokers because our primary goal was to identify circuits associated with nicotine addiction severity. Therefore, our results are specific to smokers with SZ and do not generalize to non-smoking individuals with SZ. Group differences in rsFC could be due to factors other than disease such as increased smoking severity in SZ and/or chronic exposure to neuroleptics. However, SZ and NC subjects were matched on addiction severity, and medication dose positively correlated with rsFC, suggesting a partial amelioration of circuit impairments. SZ-related reductions in rsFC in the left dACC – left aIns circuit may have been due to increased withdrawal symptoms in SZ subjects on placebo. However, controlling for withdrawal symptoms or removing subjects who experienced any withdrawal from the analysis did not alter the significance of disease-related reduction of rsFC. Reproducing findings of reduced strength of insular-dACC circuits in SZ non-smokers is required to investigate whether there is an inherent circuit deficit in non-smokers as well or whether this finding is specific to SZ smokers.
Supplementary Material
Acknowledgments
Funding source: Financial support was received from National Institutes of Health grants R01DA027680, R01MH085646, T32MH067533, and a grant from the Maryland Cigarette Restitution Fund Program. This research was also supported by the NIDA Intramural Research Program, NIH.
Footnotes
Contributors: L.V. Moran: data analysis, wrote manuscript. H. Sampath: assisted with data analysis. E.A. Stein and L.E. Hong: study design, manuscript preparation. All authors contributed to and have approved the final manuscript.
Conflict of Interest: All authors declare no conflicts of interest
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahnallen CG, Liverant GI, Gregor KL, Kamholz BW, Levitt JJ, Gulliver SB, Pizzagalli DA, Koneru VK, Kaplan GB. The relationship between reward-based learning and nicotine dependence in smokers with schizophrenia. Psychiatry Res. 2012;196(1):9–14. doi: 10.1016/j.psychres.2011.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 1995;34(4):537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- Breese CR, Lee MJ, Adams CE, Sullivan B, Logel J, Gillen KM, Marks MJ, Collins AC, Leonard S. Abnormal regulation of high affinity nicotinic receptors in subjects with schizophrenia. Neuropsychopharmacology. 2000;23(4):351–364. doi: 10.1016/S0893-133X(00)00121-4. [DOI] [PubMed] [Google Scholar]
- Chan RC, Di X, McAlonan GM, Gong QY. Brain anatomical abnormalities in high-risk individuals, first-episode, and chronic schizophrenia: an activation likelihood estimation meta-analysis of illness progression. Schizophr Bull. 2011;37(1):177–188. doi: 10.1093/schbul/sbp073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman S, Ragg M, McGeechan K. Citation bias in reported smoking prevalence in people with schizophrenia. Aust N Z J Psychiatry. 2009;43(3):277–282. doi: 10.1080/00048670802653372. [DOI] [PubMed] [Google Scholar]
- Cordes D, Haughton VM, Arfanakis K, Carew JD, Turski PA, Moritz CH, Quigley MA, Meyerand ME. Frequencies contributing to functional connectivity in the cerebral cortex in “resting-state” data. AJNR Am J Neuroradiol. 2001;22(7):1326–1333. [PMC free article] [PubMed] [Google Scholar]
- de Leon J, Diaz FJ. A meta-analysis of worldwide studies demonstrates an association between schizophrenia and tobacco smoking behaviors. Schizophr Res. 2005;76(2-3):135–157. doi: 10.1016/j.schres.2005.02.010. [DOI] [PubMed] [Google Scholar]
- Diaz FJ, Velasquez DM, Susce MT, de Leon J. The association between schizophrenia and smoking: unexplained by either the illness or the prodromal period. Schizophr Res. 2008;104(1-3):214–219. doi: 10.1016/j.schres.2008.06.004. [DOI] [PubMed] [Google Scholar]
- Freedman R, Olincy A, Buchanan RW, Harris JG, Gold JM, Johnson L, Allensworth D, Guzman-Bonilla A, Clement B, Ball MP, Kutnick J, Pender V, Martin LF, Stevens KE, Wagner BD, Zerbe GO, Soti F, Kem WR. Initial phase 2 trial of a nicotinic agonist in schizophrenia. Am J Psychiatry. 2008;165(8):1040–1047. doi: 10.1176/appi.ajp.2008.07071135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garavan H, Pankiewicz J, Bloom A, Cho JK, Sperry L, Ross TJ, Salmeron BJ, Risinger R, Kelley D, Stein EA. Cue-induced cocaine craving: neuroanatomical specificity for drug users and drug stimuli. Am J Psychiatry. 2000;157(11):1789–1798. doi: 10.1176/appi.ajp.157.11.1789. [DOI] [PubMed] [Google Scholar]
- Grant BF, Hasin DS, Chou SP, Stinson FS, Dawson DA. Nicotine dependence and psychiatric disorders in the United States: results from the national epidemiologic survey on alcohol and related conditions. Arch Gen Psychiatry. 2004;61(11):1107–1115. doi: 10.1001/archpsyc.61.11.1107. [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. Br J Addict. 1991;86(9):1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Honey GD, Pomarol-Clotet E, Corlett PR, Honey RA, McKenna PJ, Bullmore ET, Fletcher PC. Functional dysconnectivity in schizophrenia associated with attentional modulation of motor function. Brain. 2005;128(Pt 11):2597–2611. doi: 10.1093/brain/awh632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honey GD, Suckling J, Zelaya F, Long C, Routledge C, Jackson S, Ng V, Fletcher PC, Williams SC, Brown J, Bullmore ET. Dopaminergic drug effects on physiological connectivity in a human cortico-striato-thalamic system. Brain. 2003;126(Pt 8):1767–1781. doi: 10.1093/brain/awg184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong LE, Gu H, Yang Y, Ross TJ, Salmeron BJ, Buchholz B, Thaker GK, Stein EA. Association of nicotine addiction and nicotine’s actions with separate cingulate cortex functional circuits. Arch Gen Psychiatry. 2009;66(4):431–441. doi: 10.1001/archgenpsychiatry.2009.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong LE, Hodgkinson CA, Yang Y, Sampath H, Ross TJ, Buchholz B, Salmeron BJ, Srivastava V, Thaker GK, Goldman D, Stein EA. A genetically modulated, intrinsic cingulate circuit supports human nicotine addiction. Proc Natl Acad Sci U S A. 2010;107(30):13509–13514. doi: 10.1073/pnas.1004745107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong LE, Yang X, Wonodi I, Hodgkinson CA, Goldman D, Stine OC, Stein ES, Thaker GK. A CHRNA5 allele related to nicotine addiction and schizophrenia. Genes Brain Behav. 2011;10(5):530–535. doi: 10.1111/j.1601-183X.2011.00689.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes JR, Higgins ST, Bickel WK. Nicotine withdrawal versus other drug withdrawal syndromes: similarities and dissimilarities. Addiction. 1994;89(11):1461–1470. doi: 10.1111/j.1360-0443.1994.tb03744.x. [DOI] [PubMed] [Google Scholar]
- Jacobsen LK, D’Souza DC, Mencl WE, Pugh KR, Skudlarski P, Krystal JH. Nicotine effects on brain function and functional connectivity in schizophrenia. Biol Psychiatry. 2004;55(8):850–858. doi: 10.1016/j.biopsych.2003.12.023. [DOI] [PubMed] [Google Scholar]
- Janes AC, Pizzagalli DA, Richardt S, de BFB, Chuzi S, Pachas G, Culhane MA, Holmes AJ, Fava M, Evins AE, Kaufman MJ. Brain reactivity to smoking cues prior to smoking cessation predicts ability to maintain tobacco abstinence. Biol Psychiatry. 2010;67(8):722–729. doi: 10.1016/j.biopsych.2009.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerns JG, Cohen JD, MacDonald AW, 3rd, Johnson MK, Stenger VA, Aizenstein H, Carter CS. Decreased conflict- and error-related activity in the anterior cingulate cortex in subjects with schizophrenia. Am J Psychiatry. 2005;162(10):1833–1839. doi: 10.1176/appi.ajp.162.10.1833. [DOI] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35(1):217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin ED, Wilson W, Rose JE, McEvoy J. Nicotine-haloperidol interactions and cognitive performance in schizophrenics. Neuropsychopharmacology. 1996;15(5):429–436. doi: 10.1016/S0893-133X(96)00018-8. [DOI] [PubMed] [Google Scholar]
- Lowe MJ, Mock BJ, Sorenson JA. Functional connectivity in single and multislice echoplanar imaging using resting-state fluctuations. Neuroimage. 1998;7(2):119–132. doi: 10.1006/nimg.1997.0315. [DOI] [PubMed] [Google Scholar]
- Lui S, Li T, Deng W, Jiang L, Wu Q, Tang H, Yue Q, Huang X, Chan RC, Collier DA, Meda SA, Pearlson G, Mechelli A, Sweeney JA, Gong Q. Short-term effects of antipsychotic treatment on cerebral function in drug-naive first-episode schizophrenia revealed by “resting state” functional magnetic resonance imaging. Arch Gen Psychiatry. 2010;67(8):783–792. doi: 10.1001/archgenpsychiatry.2010.84. [DOI] [PubMed] [Google Scholar]
- Lyons MJ, Bar JL, Kremen WS, Toomey R, Eisen SA, Goldberg J, Faraone SV, Tsuang M. Nicotine and familial vulnerability to schizophrenia: a discordant twin study. J Abnorm Psychol. 2002;111(4):687–693. doi: 10.1037//0021-843x.111.4.687. [DOI] [PubMed] [Google Scholar]
- Minzenberg MJ, Laird AR, Thelen S, Carter CS, Glahn DC. Meta-analysis of 41 functional neuroimaging studies of executive function in schizophrenia. Arch Gen Psychiatry. 2009;66(8):811–822. doi: 10.1001/archgenpsychiatry.2009.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naidich TP, Kang E, Fatterpekar GM, Delman BN, Gultekin SH, Wolfe D, Ortiz O, Yousry I, Weismann M, Yousry TA. The insula: anatomic study and MR imaging display at 1.5 T. AJNR Am J Neuroradiol. 2004;25(2):222–232. [PMC free article] [PubMed] [Google Scholar]
- Naqvi NH, Rudrauf D, Damasio H, Bechara A. Damage to the insula disrupts addiction to cigarette smoking. Science. 2007;315(5811):531–534. doi: 10.1126/science.1135926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakes TR, Johnstone T, Ores Walsh KS, Greischar LL, Alexander AL, Fox AS, Davidson RJ. Comparison of fMRI motion correction software tools. Neuroimage. 2005;28(3):529–543. doi: 10.1016/j.neuroimage.2005.05.058. [DOI] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci U S A. 2001;98(2):676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Brain Res Rev. 1993;18(3):247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Reiss AL, Greicius MD. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 2007;27(9):2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White TP, Joseph V, Francis ST, Liddle PF. Aberrant salience network (bilateral insula and anterior cingulate cortex) connectivity during information processing in schizophrenia. Schizophr Res. 2010;123(2-3):105–115. doi: 10.1016/j.schres.2010.07.020. [DOI] [PubMed] [Google Scholar]
- Williams JM, Gandhi KK, Lu SE, Kumar S, Shen J, Foulds J, Kipen H, Benowitz NL. Higher nicotine levels in schizophrenia compared with controls after smoking a single cigarette. Nicotine Tob Res. 2010;12(8):855–859. doi: 10.1093/ntr/ntq102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods SW. Chlorpromazine equivalent doses for the newer atypical antipsychotics. J Clin Psychiatry. 2003;64(6):663–667. doi: 10.4088/jcp.v64n0607. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


