Abstract
Background
Rare but potentially life-threatening cutaneous adverse reactions have been associated with allopurinol, but population-based data on incidence and mortality of such reactions is scarce.
Methods
We conducted a propensity score-matched cohort study to evaluate incidence rate (IR) and in-hospital mortality of hospitalization for severe cutaneous adverse reactions (SCARs) in allopurinol initiators compared to non-allopurinol users, using data from five large Medicaid programs. The primary outcome was identified by the principal discharge diagnosis code 695.1. Cox proportional hazards model evaluated the relative risk of SCARs associated with use of allopurinol and determined the relative risk of SCARs associated with allopurinol dose.
Results
During a follow-up period of 65,625 person-years for allopurinol initiators, 45 were hospitalized with SCARs. The crude IR was 0.69 (95% CI 0.50–0.92) per 1,000 person-years. All 45 cases occurred within 365 days and 41 (91.1%) within 180 days after initiating treatment with allopurinol. Twelve (26.7%) patients died during the hospitalization. The crude IR in non-allopurinol users was 0.04 (95% CI 0.02–0.08) per 1,000 person-years. The risk of SCARs was increased in allopurinol initiators vs. non-users (HR 9.67, 95% CI 4.55–20.57). Among allopurinol initiators, the HR for the high- (>300mg/day) vs. low-dose allopurinol was 1.30 (95% CI 0.31–5.36) after adjusting for age, comorbidities and recent diuretic use.
Conclusions
Among allopurinol initiators, SCARs were found to be rare but often fatal and occurred mostly in the first 180 days of treatment. The risk of SCARs was ten times as high in allopurinol initiators compared to allopurinol non-users.
INTRODUCTION
Allopurinol is a xanthine oxidase inhibitor which reduces the production of uric acid. For past several decades, allopurinol has been commonly used to treat patients with gout or nephrolithiasis. Allopurinol is generally well-tolerated, but 2–5% of patients may develop side effects such as mild skin rash or gastrointestinal distress.(1–2) It can also cause severe hypersensitivity reactions characterized as a spectrum of clinical conditions ranging from a mild skin rash to life-threatening toxicity presenting as fever, hepatitis, vasculitis, eosinophilia, worsening renal function, and severe cutaneous adverse reactions (SCARs), including toxic epidermal necrolysis (TEN) and Stevens-Johnson syndrome (SJS).(3) Furthermore, allopurinol has been reported as one of the most common causes of SCARs.(4–5)
The precise mechanisms for the development of SCARs are still unknown, but several different factors have been postulated in its pathogenesis; mainly cell-mediated immunity directed toward allopurinol and its active metabolite, oxypurinol, genetic factors, and metabolic factors.(6) Recent studies reported a strong genetic association between HLA-B*5801 and SJS and TEN induced by allopurinol.(7–9) According to previous descriptive studies, mainly based on small hospital case series, fewer than 1% of the patients treated with allopurinol developed hypersensitivity reactions, but the mortality was as high as 27%.(3, 6) No population-based data, however, exist on the incidence or mortality from allopurinol hypersensitivity reactions including SCARs.
We examined the incidence and mortality of SCARs requiring hospitalization in patients starting allopurinol in a population-based propensity score-matched cohort to provide more accurate safety data necessary to inform clinical decision making for patients with hyperuricemia and gout.
METHODS
Study Design
We conducted a retrospective cohort study of allopurinol initiators using the US Medicaid claims data from California, Florida, New York, Ohio, and Pennsylvania (1999–2005). In total, these five states include approximately 13 million Medicaid enrollees, which account for about 35% of the Medicaid population. The database contains clinical, demographic, and death status information for their beneficiaries as well as the Medicaid claims for covered health care services including pharmacy benefits and hospitalizations from the time of a person’s Medicaid eligibility until death. As about 15–17% of Medicaid beneficiaries are also enrolled in Medicare, (21) Medicare data were obtained to assure complete data capture in dually-eligible. Quality of the data source was assured in previous research.(22)
Data use agreements were in place with the Centers for Medicare and Medicaid Services that supplied information for the study database. This study was approved by both the University of Pennsylvania and Brigham and Women’s Hospital’s Institutional Review Boards, which granted waivers of informed consent and Health Insurance Portability and Accountability Act authorization.
Study Patients
We identified adult subjects who had at least 180 days of Medicaid plan eligibility and at least one outpatient or inpatient claim present before the first prescription of allopurinol. These criteria ensured their continuous eligibility for at least 180 days prior to the study entry, to permit us to identify new users of allopurinol, and to assess their comorbidities and other medication at baseline. For the allopurinol non-user group, Medicaid enrollees who had never received a prescription for allopurinol during the entire study period were identified. Subjects with any claims for a diagnosis of solid tumors, hematologic malignancies and myelodysplastic syndrome, or chemotherapy administration during the 180 days prior to cohort entry were excluded as these patients have very different morbidity and mortality from patients who were on allopurinol for gout, renal stones or asymptomatic hyperuricemia. Propensity score-matching methods were then used to create a group of allopurinol initiators and non-allopurinol users who were compatible with regard to the potential confounders described below (22).
Outcomes
The primary outcome of interest was hospitalization for SCARs defined with a principal ICD-9 code 695.1X after filling a prescription for allopurinol. The ICD code 695.1X is not specific to SJS or TEN and includes several conditions with different dermatopathology such as erythema multiforme (EM) major and minor, SJS, and TEN (see Table 1). The primary outcome in this study was intended to capture a wide range of severe cutaneous eruptions requiring hospitalization, not limited to a specific diagnosis of SJS or TEN. Previous validation studies reported that over 90% of the cases identified with the discharge 695.1 had a skin diagnosis including but not limited to EM, SJS and TEN. (23–26) Outcomes that occurred 100 days or more after filling the last prescription for allopurinol were excluded. If patients in the exposed group had multiple hospitalizations with the same diagnosis code, only the first hospitalization was counted. Our secondary outcome was the death during the hospitalization for principal diagnosis of SCARs.
Table 1.
Diagnosis codes for erythematous conditions
| ICD-9 CM code | Clinical condition |
|---|---|
| 695.1 | Erythema multiforme |
| 695.10 | Erythema multiforme, unspecified |
| 695.11 | Erythema multiforme minor |
| 695.12 | Erythema multiforme major |
| 695.13 | Stevens-Johnson syndrome |
| 695.14 | Stevens-Johnson syndrome-toxic epidermal necrolysis overlap syndrome |
| 695.15 | Toxic epidermal necrolysis |
| 695.19 | Other erythema multiforme |
Patients were censored at the earliest time of the following events during the follow-up period: 1) occurrence of the outcome, 2) no filling for allopurinol for 180 days, 3) end of the Medicaid eligibility, 4) end of the study period, or 5) death.
Covariates
Patient characteristics potentially related to SCARs were assessed using the data from the 180 days prior to the first prescription fill date defined as the index date. These characteristics included demographic factors (age, sex, race and state), health care utilization factors (acute care hospitalizations, emergency room visits, and number of physician visits and different medications), and other recorded comorbidities (hypertension, diabetes mellitus, chronic kidney disease (CKD), liver, pulmonary, and cardiovascular disease). To quantify patients’ comorbidities, we additionally calculated the Deyo-adapted Charlson Comorbidity Index based on ICD-9-CM from the 180 days prior to the first prescription fill date.(27–28) The Comorbidity Index is a summary score, based on 19 major medical conditions including myocardial infarction, pulmonary, renal, hepatic disease, diabetes, cancer, human immunodeficiency virus infection, etc. A score of 0 represents absence of comorbidity and a higher score indicates a greater number of comorbid conditions. Furthermore, data on the use of diuretics, the daily dose of allopurinol prescribed, and the duration of allopurinol treatment were obtained.
Statistical Analysis
To control confounding by indication to a large extent, we used the propensity score-matching method embedded in a new user cohort design. A propensity score is the estimated probability of starting treatment A versus treatment B, based on preexisting patient characteristics (18–19). Propensity score-matching has been increasingly used as an effective way to adjust a large number of confounders simultaneously even if the outcome is rare (18, 20). Logistic regression models were developed to calculate the propensity score of all the eligible subjects in the study cohort. The propensity score is the probability of initiating allopurinol versus no treatment as a function of a number of important potential confounders such as age, sex, race, use of lipid-lowering drug, diabetic medications, beta blockers, angiotensin I-converting enzyme inhibitors, angiotensin II receptor blockers, digoxin, antihypertensives, diuretics, anti-arrhythmics, oral steroids, Charlson Index, and number of prescriptions, emergency room visits, hospitalizations, and outpatient visits were included. Patients in each group (allopurinol initiators vs. non-users) were then matched with a 1:1 ratio using greedy matching techniques.
The number of primary outcomes was assessed in the first 30 days, days 31 to 180, and days 181 to 365 days after initiating allopurinol therapy. The incidence rate (IR) and 95 % confidence interval (CI) of hospitalization for SCARs was calculated in both allopurinol initiators and non-users. A Cox proportional hazards model (29) was used to examine the relative risk of hospitalization with SCARs in allopurinol initiators compared with non-users. Among allopurinol initiators, separate unadjusted and multivariable Cox proportional hazards models (29) were used to determine the relative risk of SCARs associated with high dosage (> 300mg/day) of allopurinol, after adjusting for age, comorbidities, and recent diuretic use The allopurinol dosage was analyzed as a time-dependent covariate. In order to avoid overfitting of the regression model, presence of CKD was not included in the multivariable analysis. The reporting of this study follows the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines.(30)
RESULTS
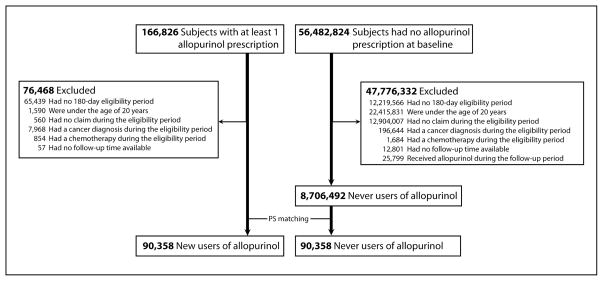
We identified 166,826 patients with at least one prescription filled for allopurinol and 56,482,824 Medicaid enrollees who never had a prescription for allopurinol during the entire study period. After applying the exclusion criteria, 90,358 allopurinol initiators and 8,706,492 allopurinol non-users were identified. After 1:1 propensity score-matching, our final cohort consisted of 90,358 allopurinol initiators and 90,358 non-users. Figure 1 illustrates our cohort selection process.
Figure 1. Study cohort selection.
PS: propensity score
The baseline characteristics of our propensity score-matched study population are listed in Table 2. Overall, the two groups were similar. The mean age was 65.5 years (standard deviation (SD) 15.6) in the allopurinol initiators and 65.6 years (SD 15.5) in the non-allopurinol group. 52% of the allopurinol initiators and non-users were men. The mean number of prescription drugs was 6.9 (SD 4.9) among the allopurinol initiators and 5.7 (SD 4.8) in the allopurinol non-user group. Approximately 12 % of allopurinol initiators had at least one diagnosis of gout or renal stones before starting treatment with allopurinol and about one-fourth of allopurinol initiators received at least one diagnosis of gout or renal stones during the follow-up period. Among the allopurinol initiators, 45 were hospitalized with SCARs over a follow-up period of 65,625 person-years. The crude IR of hospitalization with SCARs was 0.69 (95 % CI 0.50–0.92) per 1,000 person-years. All 45 cases occurred within the first 365 days and 41 (91.1%) within 180 days (see Table 3). Of those, twelve (26.7%) patients died during the hospitalization.
Table 2.
Baseline characteristics of the propensity score-matched cohort in 180 days before the index date
| Allopurinol initiators (N= 90,358) | Allopurinol non-users (N= 90,358) | |
|---|---|---|
|
Demographic characteristics
| ||
| Age (year), mean (SD) | 65.5 (15.6) | 65.6 (15.5) |
| Male, n (%) | 47,278 (52.3) | 47,278 (52.3) |
| White, n (%) | 37,403 (41.4) | 37,108 (41.1) |
|
| ||
|
Health care utilization
| ||
| No. of outpatient visit, mean (SD) | 27.0 (32.4) | 22.9 (30.6) |
| 1 or more ER visits, n (%) | 32,827 (36.3) | 31,000 (34.3) |
| No. of all prescription drugs, mean (SD) | 6.9 (4.9) | 5.7 (4.8) |
| 1 or more hospitalization, n (%) | 22,919 (25.4) | 20,607 (22.8) |
|
| ||
|
Comorbidities
| ||
| Comorbidity Index, mean (SD) | 1.9 (2.1) | 1.5 (1.9) |
| Diabetes mellitus, n (%) | 16,942 (18.8) | 14,727 (16.3) |
| Gout, n (%) | 9,095 (10.1) | 355 (0.4) |
| Liver disease, n (%) | 1,581 (1.8) | 1,760 (2.0) |
| Kidney disease, n (%) | 5,356 (5.9) | 3,117 (3.5) |
| Nephrolithiasis, n (%) | 2,024 (2.2) | 4,309 (4.8) |
| Hypertension, n (%) | 29,419 (32.6) | 23,970 (26.5) |
| Heart failure, n (%) | 12,209 (13.5) | 8,017 (8.9) |
| Coronary artery disease, n (%) | 10,445 (11.6) | 9,447 (10.5) |
| COPD, n (%) | 9,843 (10.9) | 9,449 (10.5) |
SD: standard deviation, ER: emergency room, COPD: chronic obstructive pulmonary disease
Table 3.
Incidence rates (IR) of hospitalization for severe cutaneous adverse reactions in allopurinol initiators (N=90,358)
| Number of events | Follow-up period (1,000 person-years) | IR (95% CI) per 1,000 person-years | |
|---|---|---|---|
| Total | 45 | 65.6 | 0.69 (0.50–0.92) |
| First 30 days | 20 | 7.2 | 2.77 (1.69–4.28) |
| 31–180 days | 21 | 19.9 | 1.05 (0.65–1.61) |
| 181–365 days | 4 | 12.8 | 0.31 (0.85–0.80) |
CI: confidence interval
Among allopurinol non-users, 10 hospitalizations with SCARS occurred over a period of 237,025 person-years and only one patient (10%) died during the hospitalization. The crude IR of hospitalization with SCARs in non-allopurinol users was 0.04 (95% CI 0.02–0.08) per 1,000 person-years. The HR of hospitalization with SCARs compared to allopurinol non-users was 9.68 (95% CI 4.55–20.57).
The results from our crude and multivariable Cox regression analyses among allopurinol initiators were presented in Table 4. Age and the Comorbidity index score, but neither high dosage nor presence of chronic kidney disease (CKD), were significantly associated with an increased risk of hospitalization with SCARs. In our multivariable analysis adjusting for age, comorbidities and recent use of any diuretic, the HR of hospitalization with SCARs was 1.30 (95% CI 0.31–5.36) for the high-dose allopurinol initiators compared to the low-dose allopurinol initiators. Table 5 shows baseline demographic characteristics, health care utilization pattern, and comorbidities of allopurinol initiators who developed SCARs and those who did not.
Table 4.
Unadjusted and multivariable hazard ratios of hospitalization for severe cutaneous adverse reactions in allopurinol initiators (N=90,358)
| Unadjusted HR (95% CI) | Multivariable HR (95% CI) | |
|---|---|---|
| Age* | 1.04 (1.02–1.06) | 1.03 (1.01–1.06) |
| Comorbidity Index* | 1.22 (1.09–1.35) | 1.18 (1.05–1.33) |
| High dose allopurinol (>300mg/day) | 1.13 (0.27–4.68) | 1.30 (0.31–5.36) |
| Chronic kidney disease | 0.72 (0.18–2.97) | - |
| Recent diuretic use | 2.10 (1.10–4.01) | 1.49 (0.77–2.90) |
CI: confidence interval,
: modeled as continuous variables
Table 5.
Baseline characteristics of allopurinol initiators who developed severe cutaneous adverse reactions (SCARs) vs. those who did not.
| SCARs (N= 45) | No SCARs (N= 90,313) | |
|---|---|---|
|
Demographic characteristics
| ||
| Age (year), mean (SD) | 73.7 (11.8) | 65.5 (15.6) |
| Male, n (%) | 11 (24.4) | 47,267 (52.3) |
| White, n (%) | 11 (24.4) | 37,392 (41.4) |
|
| ||
|
Health care utilization
| ||
| No. of outpatient visit, mean (SD) | 43.1 (45.8) | 27.0 (32.4) |
| 1 or more ER visits, n (%) | 22 (48.9) | 32,805 (36.3) |
| No. of all prescription drugs, mean (SD) | 8.5 (4.1) | 6.9 (4.9) |
| 1 or more hospitalization, n (%) | 22 (48.9) | 22,897 (25.4) |
|
| ||
|
Comorbidities
| ||
| Comorbidity Index, mean (SD) | 3.0 (1.8) | 1.9 (2.1) |
| Diabetes mellitus, n (%) | 11 (24.4) | 16,931 (18.8) |
| Hypertension, n (%) | 23 (51.1) | 29,396 (32.6) |
| Heart failure, n (%) | 11 (24.4) | 12,198 (13.5) |
SD: standard deviation, ER: emergency room,
DISCUSSION
The spectrum of cutaneous and systemic manifestations of drug reactions is diverse ranging from self-limited exanthematous drug eruptions to severe drug hypersensitivity syndromes, EM major, SJS, or TEN. The pathophysiologic mechanisms of these SCARs are not well established. More than 100 drugs including allopurinol, trimethoprim-sulfamethoxazole, carbamazepine, phenobarbital, cephalosporins, antifungal agents, and non-steroidal anti-inflammatory drugs have been implicated as causes of these conditions.(24) The incidence of TEN and SJS in the general population is reported to be generally low and estimated at 0.4 to 1.2 and 1 to 6 cases per million person-years, respectively.(24) Allopurinol has been repeatedly reported to have one of the highest relative risks for SCARs in the literature,(4, 31) but few studies examined its incidence and mortality in a population-based cohort. To date, little data is available to examine the risk of SCARs associated with use of newer xanthine oxidase inhibitors.
Our study has important clinical implications for management of hyperuricemia. We evaluated the incidence and mortality of SCARs in allopurinol initiators using the health care utilization data from five large Medicaid programs. Our study showed that the incidence of hospitalization with SCARs among allopurinol initiators was less than one per 1,000 person-years and was highest in the first 30 days of use. The risk of SCARs associated with initiation of allopurinol treatment was ten times greater compared to non-users of allopurinol in the propensity score matched cohort. The number needed to harm would be approximately 1540. In other words, there would be one additional hospitalization for SCARs for each 1540 patients newly treated with allopurinol. Although the risk seems small, the in-hospital mortality was high at around 27%. While prior literature suggests that high dosage of allopurinol, diuretic use, and CKD increase the risk of SCARs, our study, even with this large size, did not show a statistically significant association with these variables, although the point estimate for high vs. low dose was elevated.
It is known that hyperuricemia is closely associated with cardiovascular disease, metabolic syndrome, and CKD. As a result, allopurinol is frequently prescribed to patients with other comorbidities and used with other drugs such as anti-hypertensive drugs, diuretics, anti-neoplastic agents and antibiotics. It is important to examine whether the concomitant use of certain drugs increases the risk of serious adverse events in patients receiving allopurinol. Although we had extensive data on comorbidities and use of other drugs, we could not conduct multivariable analyses examining the effects of many comorbid conditions and concomitant use of individual drugs or classes of drug because of the small number of outcomes.
Other potential limitations include misclassification of exposures and outcomes. While we used pharmacy claims data which are considered as one of the best data sources for the drug exposure to identify the exposure in this study (32), the actual patient adherence to the medication is unknown. The main outcomes in the present study were identified by a principal ICD-9 code 695.1, which includes a wide range of severe cutaneous eruptions requiring hospitalization, not limited to specific diagnosis of SJS or TEN.(23–25) Thus, the incidence of true SJS or TEN associated with use of allopurinol is probably even lower. Our study cohort was identified as new users of allopurinol, not necessarily limited to patients with gout. Although there is currently no guideline that supports use of allopurinol in asymptomatic hyperuricemia, some of the patients in our study cohort might have had asymptomatic hyperuricemia.(33–34) Whether the risk of SCARs would differ between patients with and without gout is unknown. Lastly, our study did not find an association between CKD and SCARs. Although we used a comprehensive list of diagnosis codes to define CKD and the validity of such codes in claims data was acceptable in a prior study,(35) some subjects with mild CKD might have not been captured in our study. As true in most epidemiologic studies, patients were not randomly exposed to the drug in our study. Hence, it is possible that patients with moderate to severe CKD did not receive allopurinol or were monitored more carefully before they could develop a SCAR.
Confounding bias is another important issue in observational studies for pharmacoepidemiologic research. We conducted a large population-based cohort study and attempted to minimize this bias by selecting new users of allopurinol and matching them to non-allopurinol users based on a propensity score that included many potentially important confounders, resulting in the well-balanced cohorts with respect to measured variables in the database. It is, however, possible that differences still exist between the groups resulting in residual confounding; due to unmeasured confounders (e.g., history of drug reaction, mildly elevated serum creatinine, and genetic susceptibility) not included in the propensity score calculation. With regard to genetic susceptibility, a number of recent studies examined the relationship between several specific HLA-B alleles and severe allopurinol hypersensitivity reactions and suggested that HLA-B*5801 can be a useful tool for assessing individual susceptibility to SCARs, particularly in the Asian populations.(7–9, 36–37) In a recent cohort study of Korean patients with CKD, allopurinol-induced SCARs occurred in 18% of subjects with HLA-B*5801.(37) However, because of the different frequency of the HLA-B*5801 allele in different racial and ethnic backgrounds, the role of HLA-B*5801 as a screening tool has been limited.(38–39)
In summary, the incidence of hospitalization for SCARs was 0.69 per 1000 person-years in the first year of use among new users of allopurinol, often fatal, and occurred mostly in the first 180 days of treatment. Our study suggests that the risk of SCARs was ten times as high in allopurinol initiators compared to non-allopurinol users. Future studies using a large detailed clinical dataset from a large prospective inception cohort of allopurinol users is needed for a better understanding of other risk factors of SCARs such as impaired renal function and concomitant medication use.
Significance & Innovation.
In a large population-based cohort, SCARs requiring hospitalization in patients starting allopurinol were rare with an incidence rate less than one in 1,000 person-years, but fatal.
To date, this is the first and largest study to examine the safety of allopurinol using population-based data and rigorous methodologies, such as the new-user design and propensity score-matched analysis.
Acknowledgments
This study was supported by an investigator-initiated grant from Takeda Pharmaceuticals North America, Inc. The study was conducted by the authors independent of the sponsor. The sponsor was given the opportunity to make non-binding comments on a draft of the manuscript, but the authors retained right of publication and to determine the final wording.
Kim is supported by the NIH grant K23 AR059677 and received research support from Takeda Pharmaceuticals North America and Pfizer and tuition support for the Pharmacoepidemiology Program at the Harvard School of Public Health funded by Pfizer and Asisa.
Hennessy has consulted for Millenium, a subsidiary of Takeda Pharmaceutical Company Limited, unrelated to this therapeutic area.
Roy has received research funding from Roche and Amgen.
The authors acknowledge Qufei Wu for his data programming and statistical analysis and thank Cristin Freeman for her help in managing this research project. Dr. Kim is supported by the NIH grant K23 AR059677.
Footnotes
Disclosures:
Newcomb and Margolis have nothing to disclose.
Disclosure/Competing interests
This study was funded by an Investigator-initiated research grant from Takeda Pharmaceuticals North America.
Dr. Kim received research support from Takeda Pharmaceuticals North America and Pfizer and tuition support for the Pharmacoepidemiology Program at the Harvard School of Public Health funded by Pfizer and Asisa. Dr. Hennessy consulted for Millenium, a subsidiary of Takeda Pharmaceutical Company Limited, unrelated to this therapeutic area. Dr. Roy received research funding from Roche and Amgen.
References
- 1.Chan H, Ku G, Khoo O. Allopurinol associated hypersensitivity reactions: cutaneous and renal manifestations. Aust N Z J Med. 1977;7(5):518–22. doi: 10.1111/j.1445-5994.1977.tb03375.x. [DOI] [PubMed] [Google Scholar]
- 2.McInnes G, Lawson D, Jick H. Acute adverse reactions attributed to allopurinol in hospitalised patients. Ann Rheum Dis. 1981;40(3):245–9. doi: 10.1136/ard.40.3.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Singer J, Wallace S. The allopurinol hypersensitivity syndrome. Unnecessary morbidity and mortality. Arthritis Rheum. 1986;29(1):82–7. doi: 10.1002/art.1780290111. [DOI] [PubMed] [Google Scholar]
- 4.Halevy S, Ghislain P, Mockenhaupt M, et al. Allopurinol is the most common cause of Stevens-Johnson syndrome and toxic epidermal necrolysis in Europe and Israel. J Am Acad Dermatol. 2008;58(1):25–32. doi: 10.1016/j.jaad.2007.08.036. [DOI] [PubMed] [Google Scholar]
- 5.Roujeau J, Kelly J, Naldi L, et al. Medication use and the risk of Stevens-Johnson syndrome or toxic epidermal necrolysis. N Engl J Med. 1995;333(24):1600–7. doi: 10.1056/NEJM199512143332404. [DOI] [PubMed] [Google Scholar]
- 6.Arellano F, Sacristan J. Allopurinol hypersensitivity syndrome: a review. Ann Pharmacother. 1993;27:337–43. doi: 10.1177/106002809302700317. [DOI] [PubMed] [Google Scholar]
- 7.Hung S, Chung W, Liou L, et al. HLA-B*5801 allele as a genetic marker for severe cutaneous adverse reactions caused by allopurinol. Proc Natl Acad Sci U S A. 2005;102(11):4134–9. doi: 10.1073/pnas.0409500102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaniwa N, Saito Y, Aihara M, et al. HLA-B locus in Japanese patients with anti-epileptics and allopurinol-related Stevens-Johnson syndrome and toxic epidermal necrolysis. Pharmacogenomics. 2008;9(11):1617–22. doi: 10.2217/14622416.9.11.1617. [DOI] [PubMed] [Google Scholar]
- 9.Tassaneeyakul W, Jantararoungtong T, Chen P, et al. Strong association between HLA-B*5801 and allopurinol-induced Stevens-Johnson syndrome and toxic epidermal necrolysis in a Thai population. Pharmacogenet Genomics. 2009;19(9):704–9. doi: 10.1097/FPC.0b013e328330a3b8. [DOI] [PubMed] [Google Scholar]
- 10.Grayson P, Kim S, LaValley M, et al. Hyperuricemia and incident hypertension: a systematic review and meta-analysis. Arthritis Care Res (Hoboken) 2011;63(1):102–10. doi: 10.1002/acr.20344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim S, Guevara J, Kim K, et al. Hyperuricemia and risk of stroke: a systematic review and meta-analysis. Arthritis Rheum. 2009;61(7):885–92. doi: 10.1002/art.24612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim S, Guevara J, Kim K, et al. Hyperuricemia and coronary heart disease: a systematic review and meta-analysis. Arthritis Care Res (Hoboken) 2010;62(2):170–80. doi: 10.1002/acr.20065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mazzali M, Kanellis J, Han L, et al. Hyperuricemia induces a primary arteriolopathy in rats by a blood pressure-independent mechanism. Am J Physiol Renal Physiol. 2002;282:F991–7. doi: 10.1152/ajprenal.00283.2001. [DOI] [PubMed] [Google Scholar]
- 14.Watanabe S, Kang D, Feng L, et al. Uric acid, hominoid evolution, and the pathogenesis salt-sensitivity. Hypertension. 2002;40:355–6. doi: 10.1161/01.hyp.0000028589.66335.aa. [DOI] [PubMed] [Google Scholar]
- 15.Mazzali M, Hughes J, Kim Y, et al. Elevated uric acid increases blood pressure in the rat by a novel crystal-independent mechanism. Hypertension. 2001;38:1101–6. doi: 10.1161/hy1101.092839. [DOI] [PubMed] [Google Scholar]
- 16.Sanchez-Lozada L, Tapia E, Avila-Casado C, et al. Mild hyperuricemia induces glomerular hypertension in normal rats. Am J Physiol Renal Physiol. 2002;283:F1105–10. doi: 10.1152/ajprenal.00170.2002. [DOI] [PubMed] [Google Scholar]
- 17.Feig D, Soletsky B, Johnson R. Effect of Allopurinol on Blood Pressure of Adolescents With Newly Diagnosed Essential Hypertension. JAMA. 2008;300(8):924–32. doi: 10.1001/jama.300.8.924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schneeweiss S. A basic study design for expedited safety signal evaluation based on electronic healthcare data. Pharmacoepidemiol Drug Saf. 2010;19(8):858–68. doi: 10.1002/pds.1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ray W. Evaluating medication effects outside of clinical trials: new-user designs. Am J Epidemiol. 2003;158(9):915–20. doi: 10.1093/aje/kwg231. [DOI] [PubMed] [Google Scholar]
- 20.D’Agostino RJ. Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med. 1998;17:2265–81. doi: 10.1002/(sici)1097-0258(19981015)17:19<2265::aid-sim918>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 21.Clark W, Hulbert M. Research issues: Dually eligible Medicare and Medicaid beneficiaries, challenges and opportunities. Health Care Financing Review. 1998;20:1–10. [PMC free article] [PubMed] [Google Scholar]
- 22.Hennessy S, Leonard C, Palumbo C, et al. Quality of Medicaid and Medicare data obtained through Centers for Medicare and Medicaid Services (CMS) Med Care. 2007;45(12):1216–20. doi: 10.1097/MLR.0b013e318148435a. [DOI] [PubMed] [Google Scholar]
- 23.Strom B, Carson J, Halpern A, et al. Using a claims database to investigate drug-induced Stevens-Johnson syndrome. Stat Med. 1991;10(4):565–76. doi: 10.1002/sim.4780100408. [DOI] [PubMed] [Google Scholar]
- 24.Chan H, Stern R, Arndt K, et al. The incidence of erythema multiforme, Stevens-Johnson syndrome, and toxic epidermal necrolysis. A population-based study with particular reference to reactions caused by drugs among outpatients. Arch Dermatol. 1990;126(1):43–7. [PubMed] [Google Scholar]
- 25.Strom B, Carson J, Halpern A, et al. A population-based study of Stevens-Johnson syndrome. Incidence and antecedent drug exposures. Arch Dermatol. 1991;127(6):831–8. [PubMed] [Google Scholar]
- 26.Schneider G, Kachroo S, Jones N, et al. Mini-Sentinel systematic evaluation of health outcome of interest definitions for studies using administrative data: Mini-Sentinel. 2010. Erythema multiforme major/minor/not otherwise specified, Stevens-Johnson syndrome, or toxic epidermal necrolysis report. [Google Scholar]
- 27.Deyo R, Cherkin D, Ciol M. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613–9. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 28.Schneeweiss S, Seeger J, Maclure M, et al. Performance of comorbidity scores to control for confounding in epidemiologic studies using claims data. Am J Epidemiol. 2001;154(9):854–64. doi: 10.1093/aje/154.9.854. [DOI] [PubMed] [Google Scholar]
- 29.Cox D. Regression models and life-tables. J R Stat Soc. 1972;34:187–220. [Google Scholar]
- 30.von Elm E, Altman D, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370(9596):1453–7. doi: 10.1016/S0140-6736(07)61602-X. [DOI] [PubMed] [Google Scholar]
- 31.Fritsch P. European Dermatology Forum: skin diseases in Europe. Skin diseases with a high public health impact: toxic epidermal necrolysis and Stevens-Johnson syndrome. Eur J Dermatol. 2008;18(2):216–7. doi: 10.1684/ejd.2008.0382. [DOI] [PubMed] [Google Scholar]
- 32.Strom B. Overview of automated databases in pharmacoepidemiology. In: Strom B, Kimmel S, editors. Textbook of Pharmacoepidemiology. John Wiley & Sons, Ltd; 2006. pp. 167–71. [Google Scholar]
- 33.Dincer H, Dincer A, Levinson D. Asymptomatic hyperuricemia: To treat or not to treat. Cleve Clin J Med. 2002;69(8):594–608. doi: 10.3949/ccjm.69.8.594. [DOI] [PubMed] [Google Scholar]
- 34.Sloan R. Hyperuricemia and gout. J Fam Pract. 1982;14(5):923–6. [PubMed] [Google Scholar]
- 35.Winkelmayer W, Schneeweiss S, Mogun H, et al. Identification of individuals with CKD from Medicare claims data: a validation study. Am J Kidney Dis. 2005;46(2):225–32. doi: 10.1053/j.ajkd.2005.04.029. [DOI] [PubMed] [Google Scholar]
- 36.Chan S, Tan T. HLA and allopurinol drug eruption. Dermatologica. 1989;179(1):32–3. doi: 10.1159/000248097. [DOI] [PubMed] [Google Scholar]
- 37.Jung J, Song W, Kim Y, et al. HLA-B58 can help the clinical decision on starting allopurinol in patients with chronic renal insufficiency. Nephrol Dial Transplant. 2011 doi: 10.1093/ndt/gfr060. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 38.Chung W, Hung S, Chen Y. Human leukocyte antigens and drug hypersensitivity. Curr Opin Allergy Clin Immunol. 2007;7(4):317–23. doi: 10.1097/ACI.0b013e3282370c5f. [DOI] [PubMed] [Google Scholar]
- 39.Lonjou C, Borot N, Sekula P, et al. A European study of HLA-B in Stevens-Johnson syndrome and toxic epidermal necrolysis related to five high-risk drugs. Pharmacogenet Genomics. 2008;18(2):99–107. doi: 10.1097/FPC.0b013e3282f3ef9c. [DOI] [PubMed] [Google Scholar]