Abstract
Obesity is a major risk factor for asthma, but the mechanisms for the development of asthma in the setting of obesity are not known. The purpose of this article is to review the effects of obesity on airway inflammation in patients with asthma, and to discuss the effects of obesity on airway reactivity in patients with asthma.
Obesity is particularly a risk factor for non-atopic asthma. Airway eosinophilic inflammation is not increased in obesity, in fact the preponderance of the evidence suggests that airway eosinophilia is decreased in obesity. There is some preliminary data suggesting that airway neutrophilia may be increased in obesity, and that this may be particularly related to dietary fats. Obesity also alters adaptive immunity, and may suppress lymphocyte function typically associated with asthmatic airway inflammation.
Population based studies are somewhat inconsistent on the relationship between airway reactivity and asthma, however, recent studies in bariatric surgery show that weight loss surgery in severely obese patients decreases airway reactivity. One study suggested that this was particularly the case for those with low IgE (a marker of the TH2 asthma phenotype), suggesting there may be some heterogeneity in asthma in obesity.
There are likely to be two phenotypes of asthma in the obese: one group with early onset disease and asthma complicated by obesity, and a 2nd group with late onset disease with asthma consequent to obesity. Obesity leads to profound changes in airway function, and adaptive and innate immune responses which alter the nature of pre-existing allergic airway disease, and also cause new onset asthmatic disease.
Keywords: Asthma, obesity, bariatric surgery, airway hyperreactivity
Introduction
Over the past 10 years, unequivocal evidence has emerged implicating obesity as a major risk factor for asthma. The reasons for this are still far from clear. During the same time period, intensive research efforts directed towards understanding the effects of obesity on human physiology and immunology have taken place, many of these studies are pertinent to our understanding of asthma in obesity. At the same time, data are emerging which suggest that asthma in obesity is not a single phenotype, but is likely to occur through the interaction of mechanical factors and altered immunology either interacting with pre-existing airway disease, or leading to de novo disease.
The purpose of this article is to briefly review the epidemiological data linking asthma and obesity, with a particular emphasis on the implications of this data for the phenotype of asthma in obesity. We will discuss the effects of obesity and weight loss on airway inflammation and physiology relevant to asthma, and discuss the relevance of these findings for our current understanding of asthma in obesity.
Epidemiology linking obesity and asthma
The prevalence of obesity has been increasing throughout the developed world, as has been discussed in detail in other articles in this issue.
A large number of publications have reported that obesity is a risk factor for asthma. This has been reported in adults and children, men and women, and publications have come from around the world suggesting that this is consistent across diverse ethnic populations (1–6). For example, one widely quoted meta-analysis published by Beuther and Sutherland in 2007 pooled data from seven studies, involving over 300 000 subjects: compared with normal weight, overweight and obesity status (body mass index, BMI ≥ 25 kg/m2) increased the odds of incident asthma by 1.51 (1). There was also a dose– response effect of elevated BMI on the incidence of asthma: there was a higher risk of developing incident asthma for subjects who were obese over and above the risk for those that were merely overweight. Some studies have reported an increased risk for the development of asthma in obese women but not men (7); in the meta-analysis by Beuther & Sutherland, the risk was elevated for both men and women, with an odds ratio of 1.46 in men, and 1.68 in women. While women may have a slightly higher risk of developing asthma in the setting of obesity, obesity is a risk factor for asthma in both men and women, indeed it is a risk factor for asthma among all demographic groups.
Elevated BMI is a risk factor particularly for non-atopic asthma
Most studies have reported on the overall risk of developing asthma in the setting of obesity. Some studies have reported on the risk of developing asthma in atopic versus non-atopic individuals (Table 1). An example of this was a cross-sectional survey study of 86 000 adult Canadians asthma published by Chen et al (3). These authors reported that non-allergic individuals had a higher risk of asthma in the setting of obesity than allergic individuals: the adjusted odds ratio of having asthma in the setting of obesity was 2.53 in non-allergic and only 1.57 in allergic women. Atopic status similarly affected the risk of asthma in the setting of obesity for men, though the overall risk of reporting asthma in the setting of obesity was a little lower for both allergic and non-allergic men. The preponderance of publications support the observation that obesity is a risk for asthma particularly among the non-allergic adults, and no publications have reported the reverse (8–12).
Table 1.
Summary of studies reporting relationship between risk of non-atopic versus atopic asthma in obesity
| AUTHORS | COUNTRY | POPULATION | n | Risk of allergic and non-allergic asthma in obese |
|---|---|---|---|---|
| Visness, 2010 | US, National Health and Nutrition Examination Survey | 2–19 years | 16.074 | Obese had increased risk of asthma in non-atopic vs. atopic (OR 2.46 vs 1.34) |
| Ma, 2010 | US, National Health and Nutrition Examination Survey | adult | 4773 | Obese had increased risk of asthma in non-atopic vs. atopic (OR 2.5 vs 2.0) |
| Chen, 2009 | Canada, population based | adults | 1997 | Obese non-atopic had increased risk of asthma OR, 2.0, atopic did not |
| Husemoen, 2008 | Denmark, population based study | adult | 3609 | Obese had similar risk of non-allergic and allergic asthma (OR 1.38 vs 1.31) |
| Braback, 2007 | Sweden (Military conscripts) | adult | 570 | Obese without allergic rhinoconjunctivitis had increased risk compared to those with allergic rhinoconjunctivitis (OR 1.53 vs 1.34) |
| Chen, 2006 | Canada | adult | 86, 144 | Obese had increased risk of asthma in non-atopic vs. atopic (OR 2.5 vs 1.6 in women, 1.3 vs 1.2 in men) |
Airway Inflammation in Obesity and Asthma
Typical early-onset allergic asthma is characterized by airway eosinophilia. Eosinophils release inflammatory mediators such as eosinophil cationic protein which cause airway inflammation in asthma (13). As reports of the increased rates of asthma in obesity were emerging, investigators started to report on the effects of obesity on markers of cellular inflammation. It seemed logical to assume that if obesity was a risk factor for asthma, it was likely increasing allergic inflammation in the airway. Airway eosinophilia can be quantified by the presence of eosinophilis in induced sputum or indirectly by detecting elevated nitric oxide in exhaled breath (14). A number of publications have reported on the relationship between airway eosinophilia and BMI. Veen et al reported on a group of severe asthmatics in a tertiary care setting, and found that BMI was inversely related to both sputum eosinophilia (r=−0..36, p=0.003) and exhaled nitric oxide (r=−0.30, p=0.005) (15). Similarly Lessard et al reported that airway eosinophilia was inversely related to waist circumference (16) (which some argue is a more sophisticated measure of obesity as it reflects accumulation of metabolically active visceral adipose tissue), and Komkula et al reported that BMI was inversely related to exhaled nitric oxide (17).
Most animal models of allergic asthma also support the concept that obesity increases airway reactivity through a mechanism dissociated from allergic airway inflammation. Shore et al showed that leptin (which is increased in obesity) increased airway reactivity, but did not affect markers of allergic airway inflammation (18), and Johnston et al showed in a number of different mouse models of obesity, that while obesity increased airway reactivity, this was not related to an increase in markers of allergic airway inflammation (19). One group has reported that tissue eosinophils are increased in a mouse model of allergic obese asthma (20); however, because of the lack of distinguishing characteristics of mouse eosinophils, this observation awaits confirmation with objective markers of tissue eosinophil infiltration by immunostaining or similar methodology (21, 22).
At the time of writing, we are unaware of any publications reporting an increase in markers of airway eosinophilic inflammation in obese asthmatics, though it should be acknowledged that some studies report no discernable relationship between BMI and markers of allergic airway inflammation (23, 24). Although obesity is a risk factor for asthma, it does not increase airway eosinophilic inflammation in asthmatics.
Airway neutrophilia in obesity and asthma
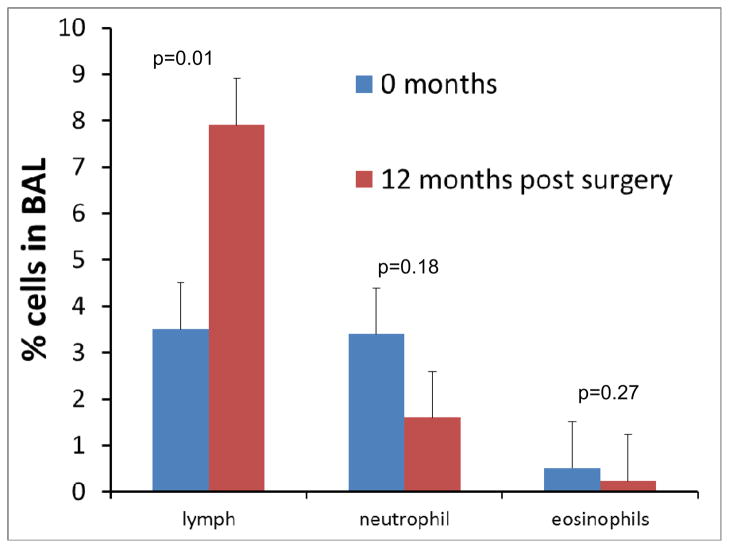
Airway neutrophilia may also occur in individuals with asthma, and has been reported to be particularly characteristic of those with severe asthma (25, 26). A few studies have reported on the presence of airway neutrophilia in obese asthmatics. Some have not found any significant relationship between airway neutrophilia and BMI (24), though a recent report from Scott et al found that airway neutrophilia was Increased in obese asthmatic women (27). There was no increased airway neutrophilia in obese asthmatic men, but airway neutrophilia in men was positively related to the level of total plasma saturated acids and negatively with the level of monosaturated acids. Related to this, was another report from the same group, which found that a high fat diet, particularly a diet rich in trans fats, increased airway neutrophilia in both lean and obese asthmatics (28). Dietary fats, acting through toll-like receptors can lead to signaling events that increase nuclear translocation of NFκB leading to increased pro-inflammatory cytokine production and so enhancing inflammation through the innate immune system (29–31). In our own studies, we found a trend towards decreased neutrophilia following bariatric surgery in obese asthmatics, supporting the concept that airway neutrophilia may be increased in obese asthmatics (Figure 1). The studies by Scott et al and Wood et al also illustrate that it may not simply be the effects of adipose tissue on immune function, but dietary fats (which are likely to be different in lean and obese individuals) that need to be considered in the etiology of airway inflammation occurring in asthma in obesity. Certainly, given the pleotropic effects of obesity on innate immunity, there is a great deal of interest in the potential role of neutrophils in asthma in obesity, though at present, there is insufficient data to draw definitive conclusions.
Figure 1.
Differential cell counts in bronchoalveolar lavage fluid of asthmatics at the time of, and 12 months after bariatric surgery.
Airway lymphocytic inflammation in obesity and asthma
CD4 positive T helper cells are central effector cells in promoting adaptive immunity (32). CD4 cells elaborate cytokines such as interleukin 5 which is chemotactic for eosinophils, interleukin 17 which can promote neutrophilic inflammation, or TH1 cytokines such as interferon-γ. TH2 cells elaborating IL4 and IL5 are implicated in allergic asthma (32).
Obesity leads to profound changes in immune cell function, and the study of “immunometabolism” is an exciting new field of investigation (33). Changes in lymphocyte function occur in the obese state, and this is likely to be an important consideration in obesity related asthma (33).
There have been few studies of lymphocyte function in obese asthmatics. We investigated how lymphocyte function was affected by bariatric surgery in obese asthmatics. We also included a group of non-asthmatic control patients undergoing bariatric surgery. Cells were isolated and stimulated for 48 hours in the presence of CD3 and CD28. We found significant increases in cytokine production from cells. We measured cytokines that were markers of Th1, Th2 and Th17 inflammation. We found that cytokine production was similar in asthmatics and controls at baseline, prior to bariatric surgery. To our surprise, we found that cytokine production from cells was significantly increased 12 months after surgery. We also saw a significant increase in airway lymphocytes (Figure 1) (34). Because the decrease in airway lymphocytes may have been related to change in medication use, we repeated the analysis limited to those patients who did not change in their use of inhaled corticosteroids, and saw similar results.
There is little other data on lymphocyte function in obese asthmatics, though there was an interesting recent publication in a mouse model of allergic asthma; de Vries et al showed that lymphocytes from lung draining lymph nodes of mice fed a high fat diet showed reduced pro-inflammatory cytokine response to both antigen specific and non-antigen specific challenge (35). As high fat diet is common in obesity, this may be one mechanism by which lymphocyte function is reduced in obesity.
Though certainly more studies are needed, the data at present suggest that lymphocyte function is altered in obesity, and it is altered in such a way that one would anticipate a dampening of adaptive immune responses that are driven by CD4 positive lymphocytes. This has important implications for the pathogenesis of a disease which in lean individuals is thought to depend on lymphocyte function: as these effector cells produce cytokines promoting airway eosinophilia and mucus hypersecretion in allergic asthma, dampening of this function could dampen allergic airway inflammation. It also has important implications for host defense against respiratory infections as discussed in detail in the article by Mancuso in this issue of the journal (36).
Effect of obesity on airway physiology in asthma
The effects of obesity on airway physiology have been reviewed in detail by Salome et al. This section will briefly review the effects of obesity on airway physiology relevant to asthma, and the results of recent studies in humans. There are certainly biomechanical explanations for airway hyperreactivity (AHR) to occur in obesity. The obese breathe at low lung volumes, with a reduced functional residual capacity. This is associated with airway narrowing, and loss of parenchymal tethering. Both of these effects (smooth muscle shortening, and loss of airway parenchymal attachments that tether the airway open, and tend to resist airway narrowing), would increase airway narrowing in response to a provovative stimulus – or more simply to increase airway hyperreactivity. Indeed, even normal subjects will develop airway hyperreactivity if they breathe at low lung volumes (37).
The data on AHR in obese asthmatics is somewhat contradictory; this is perhaps related to different populations studied. Some authors have reported a clear relationship between BMI and airway hyperreactivity (38, 39), others that no such relationship exists (40). In our own studies, we have found that bariatric surgery decreased airway hyperreactivity in obese asthmatics, but there was a significant interaction between the presence of elevated IgE and the change in AHR with weight loss, such that only those with low IgE (who tended to be much older when first diagnosed with asthma) had a significant improvement in AHR (34). We do not know why AHR improved only in those with low IgE, though we speculate that AHR in those with low IgE is more directly related to obesity, whereas in those with high IgE it is dependent on the dynamic interaction between allergic airway inflammation (which may be dampened in obesity) and obesity itself. Boulet et al similarly found that bariatric surgery improved AHR, (these investigators did not measure IgE, but found no effect of atopic status measured by skin testing) (41). The preponderance of the evidence suggests that obesity is likely related to airway reactivity, and in those individuals with severe obesity, bariatric surgery improves airway responsiveness. Perhaps some of the previous studies which have shown no relationship between obesity and AHR related to differences in baseline demographics such as TH2 phenotype of which IgE level is a marker.
Asthma as a syndrome
Asthma is a complex syndrome, with many definable phenotypes, as highlighted by recent cluster analysis studies (42, 43). These cluster analysis studies have identified a unique phenotype of asthma, characterized by an older, female predominat population, with little evidence of airway eosinophilia. This is entirely consistent with the data that we have presented on airway inflammation in obesity earlier in this paper. However, there are likely to be at least two distinct phenotypes of asthma in obesity. Holguin et al showed that there were two distinct phenotypes of asthma in obesity that could be distinguished based on the age of onset of disease: one group with early onset disease, and a higher prevalence of atopy, and a second group with late onset disease, lower prevalence of atopy, lower IgE levels (less evidence of a TH2 phenotype). This is also consistent with our findings pertaining to the effects of bariatric surgery: we found two groups of asthmatics, one with early onset disease and high IgE, with AHR that did not improve with surgery, and a 2nd group with late onset disease, low IgE, with AHR that improved with surgery (34).
It is likely that there is one group of obese asthmatics with childhood onset asthma subsequently complicated by the development of obesity, and that there is another group with late onset disease that develops consequent to obesity. The pathogenesis of disease and response to treatment may differ in these two phenotypes of obesity related asthma.
Conclusions
Obesity is a major risk factor for the development of asthma, particularly for non-atopic asthma. Obesity is associated with changes in immune cell function and airway physiology, which likely lead to new onset disease in individuals without pre-existing asthma, and profoundly modify disease in those with pre-existing asthma.
Acknowledgments
Supported by NIH grants: P20 RR15557, RR019965, P30 GM 103532
Abbreviations
- AHR
airway hyperreactivity
- BMI
body mass index
- IgE
immunoglobulin E
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Beuther DA, Sutherland ER. Overweight, obesity, and incident asthma: A meta-analysis of prospective epidemiologic studies. Am J Respir Crit Care Med. 2007;175:661–666. doi: 10.1164/rccm.200611-1717OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Camargo CA, Jr, Weiss ST, Zhang S, Willett WC, Speizer FE. Prospective study of body mass index, weight change, and risk of adult-onset asthma in women. Arch Intern Med. 1999;159:2582–2588. doi: 10.1001/archinte.159.21.2582. [DOI] [PubMed] [Google Scholar]
- 3.Chen Y, Dales R, Jiang Y. The association between obesity and asthma is stronger in nonallergic than allergic adults. Chest. 2006;130:890–895. doi: 10.1378/chest.130.3.890. [DOI] [PubMed] [Google Scholar]
- 4.Chinn S, Downs SH, Anto JM, Gerbase MW, Leynaert B, de Marco R, Janson C, Jarvis D, Kunzli N, Sunyer J, et al. Incidence of asthma and net change in symptoms in relation to changes in obesity. Eur Respir J. 2006;28:763–771. doi: 10.1183/09031936.06.00150505. [DOI] [PubMed] [Google Scholar]
- 5.Okabe Y, Itazawa T, Adachi Y, Yoshida K, Ohya Y, Odajima H, Akasawa A, Miyawaki T. Association of overweight with asthma symptoms in japanese school children. Pediatr Int. 2011;53:192–198. doi: 10.1111/j.1442-200X.2010.03197.x. [DOI] [PubMed] [Google Scholar]
- 6.Kajbaf TZ, Asar S, Alipoor MR. Relationship between obesity and asthma symptoms among children in ahvaz, iran: A cross sectional study. Ital J Pediatr. 2011;37:1. doi: 10.1186/1824-7288-37-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen Y, Dales R, Tang M, Krewski D. Obesity may increase the incidence of asthma in women but not in men: Longitudinal observations from the canadian national population health surveys. Am J Epidemiol. 2002;155:191–197. doi: 10.1093/aje/155.3.191. [DOI] [PubMed] [Google Scholar]
- 8.Visness CM, London SJ, Daniels JL, Kaufman JS, Yeatts KB, Siega-Riz AM, Calatroni A, Zeldin DC. Association of childhood obesity with atopic and nonatopic asthma: Results from the national health and nutrition examination survey 1999–2006. J Asthma. 2010;47:822–829. doi: 10.3109/02770903.2010.489388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ma J, Xiao L, Knowles SB. Obesity, insulin resistance and the prevalence of atopy and asthma in us adults. Allergy. 2010;65:1455–1463. doi: 10.1111/j.1398-9995.2010.02402.x. [DOI] [PubMed] [Google Scholar]
- 10.Chen Y, Rennie D, Cormier Y, Dosman J. Atopy, obesity, and asthma in adults: The humboldt study. J Agromedicine. 2009;14:222–227. doi: 10.1080/10599240902724051. [DOI] [PubMed] [Google Scholar]
- 11.Husemoen LL, Glumer C, Lau C, Pisinger C, Morch LS, Linneberg A. Association of obesity and insulin resistance with asthma and aeroallergen sensitization. Allergy. 2008;63:575–582. doi: 10.1111/j.1398-9995.2007.01613.x. [DOI] [PubMed] [Google Scholar]
- 12.Braback L, Hjern A, Rasmussen F. Body mass index, asthma and allergic rhinoconjunctivitis in swedish conscripts-a national cohort study over three decades. Respir Med. 2005;99:1010–1014. doi: 10.1016/j.rmed.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 13.Jacobsen EA, Ochkur SI, Lee NA, Lee JJ. Eosinophils and asthma. Curr Allergy Asthma Rep. 2007;7:18–26. doi: 10.1007/s11882-007-0026-y. [DOI] [PubMed] [Google Scholar]
- 14.Dweik RA, Boggs PB, Erzurum SC, Irvin CG, Leigh MW, Lundberg JO, Olin AC, Plummer AL, Taylor DR. An official ats clinical practice guideline: Interpretation of exhaled nitric oxide levels (feno) for clinical applications. Am J Respir Crit Care Med. 2011;184:602–615. doi: 10.1164/rccm.9120-11ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van Veen IH, Ten Brinke A, Sterk PJ, Rabe KF, Bel EH. Airway inflammation in obese and nonobese patients with difficult-to-treat asthma. Allergy. 2008;63:570–574. doi: 10.1111/j.1398-9995.2007.01597.x. [DOI] [PubMed] [Google Scholar]
- 16.Lessard A, Turcotte H, Cormier Y, Boulet LP. Obesity and asthma: A specific phenotype? Chest. 2008;134:317–323. doi: 10.1378/chest.07-2959. [DOI] [PubMed] [Google Scholar]
- 17.Komakula S, Khatri S, Mermis J, Savill S, Haque S, Rojas M, Brown L, Teague GW, Holguin F. Body mass index is associated with reduced exhaled nitric oxide and higher exhaled 8-isoprostanes in asthmatics. Respir Res. 2007;8:32. doi: 10.1186/1465-9921-8-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shore SA, Schwartzman IN, Mellema MS, Flynt L, Imrich A, Johnston RA. Effect of leptin on allergic airway responses in mice. J Allergy Clin Immunol. 2005;115:103–109. doi: 10.1016/j.jaci.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 19.Johnston RA, Zhu M, Rivera-Sanchez YM, Lu FL, Theman TA, Flynt L, Shore SA. Allergic airway responses in obese mice. Am J Respir Crit Care Med. 2007;176:650–658. doi: 10.1164/rccm.200702-323OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Calixto MC, Lintomen L, Schenka A, Saad MJ, Zanesco A, Antunes E. Obesity enhances eosinophilic inflammation in a murine model of allergic asthma. Br J Pharmacol. 2010;159:617–625. doi: 10.1111/j.1476-5381.2009.00560.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meyerholz DK, Griffin MA, Castilow EM, Varga SM. Comparison of histochemical methods for murine eosinophil detection in an rsv vaccine-enhanced inflammation model. Toxicol Pathol. 2009;37:249–255. doi: 10.1177/0192623308329342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stevens WW, Kim TS, Pujanauski LM, Hao X, Braciale TJ. Detection and quantitation of eosinophils in the murine respiratory tract by flow cytometry. J Immunol Methods. 2007;327:63–74. doi: 10.1016/j.jim.2007.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Todd DC, Armstrong S, D’Silva L, Allen CJ, Hargreave FE, Parameswaran K. Effect of obesity on airway inflammation: A cross-sectional analysis of body mass index and sputum cell counts. Clin Exp Allergy. 2007;37:1049–1054. doi: 10.1111/j.1365-2222.2007.02748.x. [DOI] [PubMed] [Google Scholar]
- 24.Sutherland TJ, Cowan JO, Young S, Goulding A, Grant AM, Williamson A, Brassett K, Herbison GP, Taylor DR. The association between obesity and asthma: Interactions between systemic and airway inflammation. Am J Respir Crit Care Med. 2008;178:469–475. doi: 10.1164/rccm.200802-301OC. [DOI] [PubMed] [Google Scholar]
- 25.Fahy JV, Kim KW, Liu J, Boushey HA. Prominent neutrophilic inflammation in sputum from subjects with asthma exacerbation. J Allergy Clin Immunol. 1995;95:843–852. doi: 10.1016/s0091-6749(95)70128-1. [DOI] [PubMed] [Google Scholar]
- 26.Ordonez CL, Shaughnessy TE, Matthay MA, Fahy JV. Increased neutrophil numbers and il-8 levels in airway secretions in acute severe asthma: Clinical and biologic significance. Am J Respir Crit Care Med. 2000;161:1185–1190. doi: 10.1164/ajrccm.161.4.9812061. [DOI] [PubMed] [Google Scholar]
- 27.Scott HA, Gibson PG, Garg ML, Wood LG. Airway inflammation is augmented by obesity and fatty acids in asthma. Eur Respir J. 2011;38:594–602. doi: 10.1183/09031936.00139810. [DOI] [PubMed] [Google Scholar]
- 28.Wood LG, Garg ML, Gibson PG. A high-fat challenge increases airway inflammation and impairs bronchodilator recovery in asthma. J Allergy Clin Immunol. 2011;127:1133–1140. doi: 10.1016/j.jaci.2011.01.036. [DOI] [PubMed] [Google Scholar]
- 29.Holland WL, Bikman BT, Wang LP, Yuguang G, Sargent KM, Bulchand S, Knotts TA, Shui G, Clegg DJ, Wenk MR, et al. Lipid-induced insulin resistance mediated by the proinflammatory receptor tlr4 requires saturated fatty acid-induced ceramide biosynthesis in mice. J Clin Invest. 2011;121:1858–1870. doi: 10.1172/JCI43378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shi H, Kokoeva MV, Inouye K, Tzameli I, Yin H, Flier JS. Tlr4 links innate immunity and fatty acid-induced insulin resistance. J Clin Invest. 2006;116:3015–3025. doi: 10.1172/JCI28898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nguyen MT, Favelyukis S, Nguyen AK, Reichart D, Scott PA, Jenn A, Liu-Bryan R, Glass CK, Neels JG, Olefsky JM. A subpopulation of macrophages infiltrates hypertrophic adipose tissue and is activated by free fatty acids via toll-like receptors 2 and 4 and jnk-dependent pathways. J Biol Chem. 2007;282:35279–35292. doi: 10.1074/jbc.M706762200. [DOI] [PubMed] [Google Scholar]
- 32.Hamid Q, Tulic M. Immunobiology of asthma. Annu Rev Physiol. 2009;71:489–507. doi: 10.1146/annurev.physiol.010908.163200. [DOI] [PubMed] [Google Scholar]
- 33.Mathis D, Shoelson SE. Immunometabolism: An emerging frontier. Nat Rev Immunol. 2011;11:81. doi: 10.1038/nri2922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dixon AE, Pratley RE, Forgione PM, Kaminsky DA, Whittaker-Leclair LA, Griffes LA, Garudathri J, Raymond D, Poynter ME, Bunn JY, et al. Effects of obesity and bariatric surgery on airway hyperresponsiveness, asthma control, and inflammation. J Allergy Clin Immunol. 2011;128:508–515. e501–502. doi: 10.1016/j.jaci.2011.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.de Vries A, Hazlewood L, Fitch PM, Seckl JR, Foster P, Howie SE. High-fat feeding redirects cytokine responses and decreases allergic airway eosinophilia. Clin Exp Allergy. 2009;39:731–739. doi: 10.1111/j.1365-2222.2008.03179.x. [DOI] [PubMed] [Google Scholar]
- 36.Mancuso P. Obesity and respiratory infections: Does adiposity weigh down host defense? Pulmonary Pharmacology and Therapeutics. 2012 doi: 10.1016/j.pupt.2012.04.006. accepted for publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Skloot G, Permutt S, Togias A. Airway hyperresponsiveness in asthma: A problem of limited smooth muscle relaxation with inspiration. J Clin Invest. 1995;96:2393–2403. doi: 10.1172/JCI118296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chinn S, Jarvis D, Burney P. Relation of bronchial responsiveness to body mass index in the ecrhs. European community respiratory health survey. Thorax. 2002;57:1028–1033. doi: 10.1136/thorax.57.12.1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Litonjua AA, Sparrow D, Celedon JC, DeMolles D, Weiss ST. Association of body mass index with the development of methacholine airway hyperresponsiveness in men: The normative aging study. Thorax. 2002;57:581–585. doi: 10.1136/thorax.57.7.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schachter LM, Salome CM, Peat JK, Woolcock AJ. Obesity is a risk for asthma and wheeze but not airway hyperresponsiveness. Thorax. 2001;56:4–8. doi: 10.1136/thorax.56.1.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Boulet LP, Turcotte H, Martin J, Poirier P. Effect of bariatric surgery on airway response and lung function in obese subjects with asthma. Respir Med. 2012 doi: 10.1016/j.rmed.2011.12.012. [DOI] [PubMed] [Google Scholar]
- 42.Haldar P, Pavord ID, Shaw DE, Berry MA, Thomas M, Brightling CE, Wardlaw AJ, Green RH. Cluster analysis and clinical asthma phenotypes. Am J Respir Crit Care Med. 2008;178:218–224. doi: 10.1164/rccm.200711-1754OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moore WC, Meyers DA, Wenzel SE, Teague WG, Li H, Li X, D’Agostino R, Jr, Castro M, Curran-Everett D, Fitzpatrick AM, et al. Identification of asthma phenotypes using cluster analysis in the severe asthma research program. Am J Respir Crit Care Med. 2010;181:315–323. doi: 10.1164/rccm.200906-0896OC. [DOI] [PMC free article] [PubMed] [Google Scholar]