Abstract
We investigated the relationship between nonphotochemical plastoquinone reduction and chlororespiration in leaves of growth-chamber-grown sunflower (Helianthus annuus L.). Following a short induction period, leaves of previously illuminated sunflower showed a substantially increased level of minimal fluorescence following a light-to-dark transition. This increase in minimal fluorescence was reversed by far-red illumination, inhibited by rotenone or photooxidative methyl viologen treatment, and stimulated by fumigation with CO. Using flash-induced electrochromic absorption-change measurements, we observed that the capacity of sunflower to reduce plastoquinone in the dark influenced the activation state of the chloroplast ATP synthase, although chlororespiratory transmembrane electrochemical potential formation alone does not fully explain our observations. We have added several important new observations to the work of others, forming, to our knowledge, the first strong experimental evidence that chlororespiratory, nonphotochemical plastoquinone reduction and plastoquinol oxidation occur in the chloroplasts of higher plants. We have introduced procedures for monitoring and manipulating chlorores-piratory activity in leaves that will be important in subsequent work aimed at defining the pathway and function of this dark electron flux in higher plant chloroplasts.
There are documented circumstances in which, contrary to expectation, the quinone acceptors of PSII in leaves become reduced rather than oxidized following a light-to-dark transition. For example, binary oscillations in the chlorophyll fluorescence yield of dark-adapted spinach (Spinacia oleracea) leaves could only be observed after far-red light was applied (Kramer et al., 1990). Dephasing of the binary oscillations originated from the accumulation of QB semiquinone on PSII during the dark-adaptation period (Rutherford et al., 1984; Kramer et al., 1990). Furthermore, analyses of the kinetics for single-turnover, flash-induced chlorophyll fluorescence, reflecting electron sharing among PSII quinone acceptors (i.e. QA, QB, and the PQ pool), showed that the net re-oxidation of QA− produced by a bright flash slowed because of the reduction of PQ in the dark (Groom et al., 1993).
Of the species examined for this phenomenon, the duration and net rate of PSII quinone acceptor dark reduction differed considerably. For example, in amaranth, a significantly reduced PQ pool was maintained in darkness for several hours following a light-to-dark transition, compared with only 20 min in sunflower (Helianthus annuus) leaves (Groom et al., 1993). In maize (Zea mays), no dark accumulation of PQH2 was detectable.
Both the mechanism and the function of nonphotochemical PQ reduction in plants are unclear, in part because the electron donors responsible for reducing the PQ pool in the dark have not been identified (Groom et al., 1993), although these reductants appear to be localized in the chloroplast stroma (Scherer, 1990). It is also not known whether the electrons derived from PQH2 formed in the dark drive proton uptake or simply terminate in the reduction of molecular oxygen without energy coupling. From several studies of dark reduction of PQ in leaves and algal cells, it appears that the dark electron flux proceeds at an exceedingly low rate (Peltier et al., 1987; Groom et al., 1993). In amaranth leaves, the maximum net rate of nonphotochemical PQ reduction was calculated to be 0.05% of the light-saturated rate of PQ reduction (Groom et al., 1993). This flux is insufficient to compete with photochemical oxidation of PQH2 by PSI. It follows that if this PQ reduction pathway were also to function under illumination, it would not be expected to cause any decrease in the steady-state quantum yield of photosynthesis (i.e. due to the accumulation of QA−), even at very low light intensities (Groom et al., 1993).
There have been numerous proposals for the activity of a respiratory electron transport chain in chloroplasts of green (Bennoun, 1982, 1994; Peltier et al., 1987) and brown algae (Ting and Owens, 1993), as well as for some plant cells (Garab et al., 1989; Gruszecki et al., 1994). This chlororespiratory pathway is thought to involve the reduction of PQ by NADPH or NADH, with the subsequent oxidation of PQH2 ultimately terminating with the reduction of molecular oxygen. Genes that could encode subunits homologous to the cyanobacterial NADH-PQ oxidoreductase complex are present in the plastid genome of numerous plant species (Berger et al., 1993; Guedeney et al., 1996; Kubicki et al., 1996; Sazanov et al., 1996). However, not all plants seem to have functional ndh genes, suggesting that chlororespiration may not function in all plants (Wakasugi et al., 1994). Molecular and biochemical evidence for a functional NADPH dehydrogenase complex as well as a chloroplast terminal oxidase is still largely indirect (Bennoun, 1982, 1994).
Oxygen uptake associated with chlororespiration appears to be catalyzed by an uncharacterized oxidase that is sensitive to the inhibitors KCN and CO in Chlamydomonas reinhardtii, whereas only SHAM effectively inhibits oxygen uptake by chloroplasts in Chlorella vulgaris (Bennoun, 1982); inhibitor sensitivity of oxygen uptake by chloroplasts has not been investigated in plants. Although chlororespiration is proposed to drive the formation of ΔμH+ across the thylakoid membrane in the dark (Bennoun, 1982; Peltier and Schmidt, 1991), the reactions involved in energy coupling by chlororespiration are still unknown. Moreover, the magnitude of ΔμH+ generated by chlororespiration may vary widely among the organisms performing chlororespiration (Bennoun, 1982, 1994; Garab et al., 1989; Ting and Owens, 1993; Buchel and Garab, 1995; Endo and Asada, 1996) and have implications for the physiological role of this enigmatic process in different organisms.
Although, like nonphotochemical PQ reduction, the net rates of chlororespiration are reported to be very low (Bennoun, 1982; Peltier et al., 1987; Garab et al., 1989; Buchel and Garab, 1995), the process may nevertheless have meaningful functions. Most of the roles suggested for chlororespiration center on the regulation of chloroplast metabolism in the dark. For example, some evidence indicates that chlororespiration supports the recycling of NADP+ during starch breakdown through the oxidation of NADPH, with the generation of a ΔpH (Bennoun, 1982; Gfeller and Gibbs, 1985; Peltier and Schmidt, 1991). In the diatom Phaeodactylum tricornatum (Ting and Owens, 1993) and the green alga C. reinhardtii, with acetate present (Endo and Asada, 1996), chlororespiration generated a ΔpH large enough to cause nonphotochemical quenching of PSII fluorescence in the dark, suggesting the intriguing possibility that chlororespiration may be able to generate a ΔμH+ large enough to maintain a catalytically active chloroplast ATP synthase.
Much of what is currently known about chlororespiration has been learned by investigating various species of single-celled algae. In the present study we investigated the relationship between nonphotochemical PQ reduction and chlororespiration in leaves of growth-chamber-grown sunflower.
MATERIALS AND METHODS
Plant Growth Conditions
Sunflower (Helianthus annuus L. cv IS894) plants were grown in a controlled-environment chamber with 14-h, 33°C days and 10-h, 33°C nights at 650 to 750 μmol quanta m−2 s−1 PPFD. Plants were grown from seed in 40-cm pots with greenhouse-blended soil (3 parts soil, 1 part vermiculite, and 1 part peat) supplemented with 12N/6P/3K fertilizer. Plants were watered with one-fifth-strength Hoagland solution twice a week.
Measurement of Minimal Chlorophyll Fluorescence Yield
The chlorophyll fluorescence emission responses of sunflower leaves were measured with a fluorimeter (model FL-100, Photon Systems Instruments, Brno, Czech Republic) using the method of Nedbal and Trtilek (1995) modified for work with intact leaf tissue. A randomized, trifurcated light guide was used to merge the excitation beam, photodiode detector, and far-red (approximately 730 nm) light source at the leaf surface. The excitation beam for fluorescence measurements was created by nonperiodic, probing flashes of adjustable energy and duration from four red-light-emitting diodes (peak at 654 nm; model HLMP 8104, Hewlett-Packard). The energy, duration (10 μs), and frequency (0.2 Hz) of the probing flashes were adjusted to ensure that there was no actinic effect on the minimal fluorescence yield (i.e. less than a 1% reaction center turnover per flash).
Far-red illumination (approximately 730 nm at 10 μmol quanta m−2 s−1), used to oxidize the PSII quinone acceptors, was produced by filtering light from a tungsten-halogen lamp through a 730-nm interference filter (5-nm bandwidth, Corion, Franklin, MA). Green actinic light (approximately 540 nm) was produced from a tungsten-halogen lamp filtered by a heat-reflecting mirror (model 03 MHG 007, Melles-Griot, Irvine, CA) and an interference filter (DT Gruen, Blazers, Frankfurt, Germany) and delivered to the side of the leaf opposite the trifurcated light guide.
Measurements of the Flash-Induced Electrochromic Change in Leaves
A kinetic spectrophotometer similar to that described by Chylla et al. (1987) was used to measure the ΔA518 induced by saturating, single-turnover flashes. Red actinic flashes were produced by a xenon-tube flash lamp (6-μs duration at half-peak width; model FX-193, EG&G, Salem, MA) filtered by a heat-reflecting mirror (Melles-Griot) and a red cutoff filter (model CS 2–58, Corning, Inc., Corning, NY). A monochrometer (Instrument SA, Inc., Metuchen, NJ) was used to produce a 518-nm measuring beam (2-nm half-bandwidth) at an intensity of <2 μmol quanta m−2 s−1. Actinic flashes and the measuring beam were brought to the leaf surface through a randomized, bifurcated fiber-optic guide positioned perpendicularly to the leaf surface within an aluminum chamber. A second light guide was positioned directly below the leaf abaxial surface to direct the measuring beam passing through the leaf to a photomultiplier tube (model R268, Hamamatsu, Bridgewater, NJ), which was protected by a red-light-blocking filter (model 2–58, Corning).
The decay kinetics for ΔA518 were analyzed as the sum of two first-order exponential functions (Oxborough and Ort, 1995):
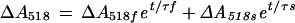 |
where f and s denote the fast and slow components of ΔA518 decay, respectively, t represents time in milliseconds, and τ represents the relaxation time constant (also in milliseconds). For these analyses, the ΔA518 following a single-turnover flash was followed for 400 ms so that the effect of ATP synthase activity on thylakoid ionic conductance could be observed (Kramer and Crofts, 1989; Ort and Oxborough, 1992; Oxborough and Ort, 1995).
Quenching Analysis of Chlorophyll Fluorescence in Leaves
Chlorophyll a fluorescence measurements were made with a pulse-amplitude-modulated fluorimeter (PAM-2000, Walz, Effeltrich, Germany). Saturation pulses (>4000 μmol quanta m−2 s−1) were used to separate photochemical and nonphotochemical quenching components of the fluorescence emission (Genty et al., 1989). The fiber-optic light guide was positioned 90° relative to the leaf surface within an aluminum chamber. The initial fluorescence yield was measured with a nonactinic excitation beam (0.7 μmol quanta m−2 s−1). A single 400-ms saturation pulse was given to determine the Fm. Dark-adapted values for Fm and Fo were determined from sunflower plants held in the dark for a minimum of 10 h.
Inhibitor Additions and CO Fumigation of Leaf Discs
A 10% CO (balanced with nitrogen) gas stream was used to fumigate sunflower leaf discs for 65 s under a fume hood in an airtight flask. Leaf discs were floated on top of distilled water during this fumigation period. Photooxidative MV treatment was performed on sunflower leaf discs floated on a 100 μm MV solution for 300 s in the light (1000 μmol quanta m−2 s−1). Rotenone was introduced by floating leaf discs on a 200 μm aqueous solution of the inhibitor under illumination (1000 μmol quanta m−2 s−1). The treated leaf discs remained in this solution during dark adaptation as fluorescence yield measurements were taken. Rotenone stock solutions were made by dissolving rotenone into a 50:50 (v/v) solution of methanol:ethylene glycol, but the organic solvent was held below 1% during the experimental treatment.
RESULTS
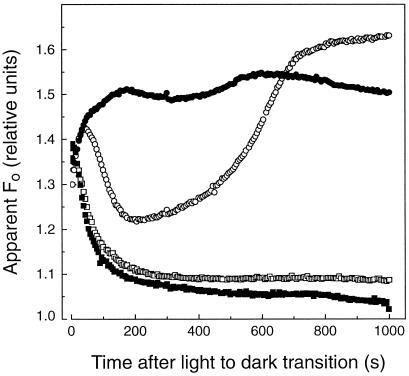
Minimal Fluorescence Emission Increases during Dark Adaptation in Light-Acclimated Sunflower Leaves
The behavior of the apparent Fo from sunflower leaves following a light-to-dark transition depended on the preillumination history of the plant. Following 4 h of growth-chamber illumination (Fig. 1), the apparent Fo increased and quickly relaxed to a steady level. The initial increase in apparent Fo emission during the first 100 s of dark adaptation was almost certainly the result of the relaxation of energization-dependent nonphotochemical quenching of apparent Fo as ΔμH+ formed during the previous illumination period was discharged. The dip in fluorescence yield after the initial increase can most consistently be accounted for as a combination of PQH2 reoxidation (Vernotte et al., 1979), QA− reoxidation (Krause and Weis, 1991), and S2-S3/QB2− state recombination (Joliot and Joliot, 1980).
Figure 1.
Changes in apparent Fo emission after a light-to-dark transition in sunflower leaves with different light-acclimation treatments. In all cases, fluorescence emission was measured from the leaves following 5 min of preillumination under 1000 μmol quanta m−2 s−1 green light. The sunflower plants had been light acclimated in a growth chamber at 650 μmol quanta m−2 s−1 PPFD for 4 h (○), light acclimated for 4 h and then treated with 10% CO for 65 s (•), or dark adapted for 10 h in the presence (▪) or absence (□) of CO.
These transient changes were followed by a slower increase in the apparent Fo that reached a maximum level after approximately 10 min in darkness (Fig. 1). Evidence will be presented below that this gradual increase in the apparent Fo beginning about 400 s after the light-to-dark transition resulted from an accumulation of QA− as a consequence of PQ reduction in the dark, which is also expected to result in an equilibrium distribution of electrons among the PSII quinone acceptors, leading to a partial reduction of QA (Velthuys and Amesz, 1974). An increasing fraction of QA in the reduced state would explain the postillumination increase in apparent Fo yield because QA− is a highly fluorescent state. Reduction of PQ to PQH2 should further contribute to the increase in apparent Fo emission because oxidized PQ quenches chlorophyll fluorescence more than does PQH2 (Vernotte et al., 1979).
Figure 1 also shows that fully dark-adapted sunflower leaves (10 h), which were then preilluminated for 300 s under 1000 μmol quanta m−2 s−1, did not exhibit a postillumination increase in apparent Fo. Preillumination treatments as long as 30 min did not induce any increase in the minimal fluorescence during dark adaptation (data not shown).
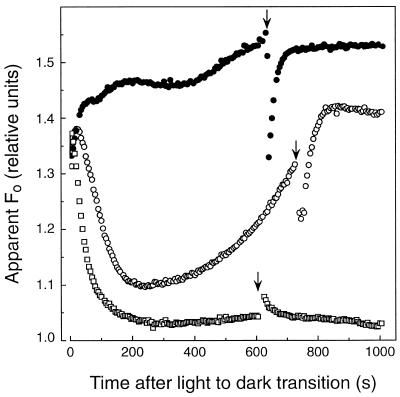
Far-Red Excitation Reverses the Postillumination Increase in Minimal Fluorescence Yield
To further investigate the basis for the increase in apparent Fo during dark adaptation in light-acclimated sunflower leaves, far-red light (approximately 730 nm, 10 μmol quanta m−2 s−1) was applied for 10 s after leaves had been in darkness for 600 s (Fig. 2). Far-red light treatment preferentially energized PSI and thereby oxidized the intersystem electron carriers, including PQ, causing a reversal of the increase in apparent Fo. Following far-red illumination, the apparent Fo recovered quickly (t½ = 30 s) and thereafter continued to increase gradually. The rate of recovery of the apparent Fo should approximate the net rate of PQ reduction in the dark in these leaves. Consistent with our interpretation, far-red illumination of fully dark-adapted sunflower leaves (10 h) did not induce a decrease in apparent Fo. The slight increase in apparent Fo observed in dark-adapted leaves implies that a small, steady-state population of QA− was formed during the far-red illumination period, which then relaxed in the dark via aerobic oxidation of PQH2. Although the far-red illumination would induce ΔpH-dependent fluorescence quenching, the effect would be too small and rapidly relaxing to observe in the experiments depicted in Figure 2.
Figure 2.
Reversal of the increase in the apparent Fo in light-acclimated sunflower leaves by far-red illumination. Leaves were exposed to growth chamber light (650 μmol quanta m−2 s−1 PPFD) for 4 h, and the apparent Fo was measured following a preillumination treatment with (•) or without (○) CO. CO and preillumination conditions are the same as described in Figure 1. The effects of far-red light on a dark-adapted leaf (10 h) that was preilluminated for 5 min with 1000 μmol quanta m−2 s−1 green light are also depicted (□). The beginning of the 10-s far-red light pulses (approximately 730 nm, 10 μmol quanta m−2 s−1) is indicated by arrows.
CO Fumigation Accelerates the Rate of PQH2 Accumulation in the Dark
CO is a well-known inhibitor of hemoprotein oxidases such as Cyt oxidase, in which CO exerts its inhibitory effect by competing with oxygen for the sixth coordinate of the heme a iron. We investigated the potential involvement of a terminal hemoprotein oxidase in chloroplasts of light-acclimated sunflower leaves by examining the effect of CO fumigation on the increase in apparent Fo during dark adaptation. We anticipated that if a pathway exists that couples the nonphotochemical reduction of PQ to such a terminal oxidase, then elimination of this activity should accelerate the rate of PQH2 accumulation and thus the rate of apparent Fo increase in the dark.
Figure 1 shows the anticipated behavior of CO on the apparent Fo in sunflower after leaf discs were fumigated with 10% CO for 65 s. Following CO treatment, the apparent Fo increased to a maximum level (about 200 s after a light-to-dark transition) faster than the untreated control (approximately 700 s). These effects of CO on apparent Fo were not observed in dark-adapted leaves (Fig. 1) or in leaves exposed to less than several hours of growth-chamber light (data not shown).
The increase in apparent Fo following CO fumigation was affected by far-red illumination in a manner similar to the control leaves (Fig. 2). It is noteworthy, however, that the recovery rate in apparent Fo following far-red illumination was more rapid (t½ = 15 s) in leaves pretreated with CO compared with untreated leaves. Collectively, these data indicate that the nonphotochemical reduction of PQ is largely, if not entirely, insensitive to CO, but dark PQH2 oxidation is mediated by a chloroplast-localized, CO-sensitive hemoprotein oxidase.
Anaerobic conditions qualitatively mimicked the effects of CO fumigation, although the acceleration of the increase in the apparent Fo was less dramatic, and anaerobic conditions enhanced the final apparent Fo level more than CO fumigation (data not shown). Harris and Heber (1993) also found that anaerobic conditions resulted in an increase in the apparent Fo in the dark in spinach (Spinacia oleracea) leaves. However, in contrast to our results with light-acclimated sunflower leaves, they observed the dark increase in apparent Fo only under anaerobic conditions. It is not known what the differences between spinach and sunflower may be with respect to the operation of chlororespiration, but this difference could be accounted for by a lower capacity for dark PQ reduction in the spinach leaves used in the Harris and Heber (1993) study.
MV and Rotenone Inhibit Dark Reduction of the PQ Pool
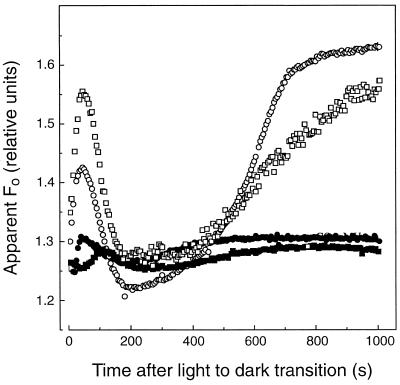
It is well known that MV can catalyze a rapid, light-dependent depletion of chloroplast stromal reductants, including NADPH and ascorbate (Ort and Izawa, 1973; Aristarkhov et al., 1987). Following a 5-min incubation of light-acclimated sunflower leaf discs in 100 μm MV under 1000 μmol quanta m−2 s−1 green light, the increase in apparent Fo during dark adaptation was almost completely abolished (Fig. 3). Even in leaf discs treated with CO, no postillumination increase in apparent Fo was detectable in leaf discs following the photooxidative MV treatment (Fig. 3), indicating that the nonphotochemical reduction of PQ was completely abolished. The increase in apparent Fo following a light-to-dark transition was not inhibited by MV when it was added to the leaf disc during darkness (Fig. 3).
Figure 3.
Photooxidative MV treatment prevents the increase in the apparent Fo following a light-to-dark transition in sunflower leaf discs. Leaves were light acclimated under 4 h of growth-chamber light (650 μmol quanta m−2 s−1 PPFD) before sampling. The control (○) and MV-treated (▪) leaf discs were preilluminated for 5 min under 1000 μmol quanta m−2 s−1 green actinic light as before. The leaf discs were floated in an aqueous 100 μm MV solution during the preillumination period. CO fumigation had virtually no effect in MV-treated leaf discs (•). MV added in the dark (i.e. no photooxidative preillumination) had very little effect on the postillumination increase in apparent Fo (□).
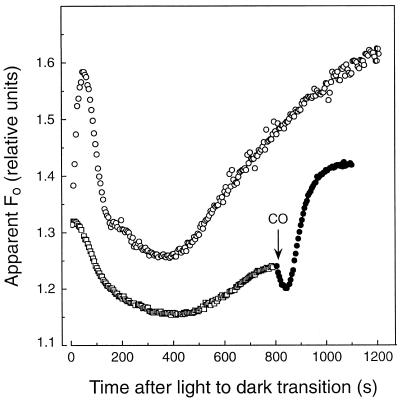
Rotenone is an inhibitor of the type-I primary oxidoreductase of mitochondria that catalyzes electron transfer from NADH to the quinone pool and is coupled to transmembrane proton translocation. Following a 5-min incubation of light-acclimated sunflower leaf discs in 200 μm rotenone (Fig. 4), the increase in apparent Fo was substantially inhibited relative to the control leaf (i.e. incubated for 5 min in the carrier solution minus rotenone). Sunflower leaf discs treated with 200 μm rotenone showed more quantitative variability among samples than we observed with other treatments and inhibitors, but the increase in apparent Fo was always greatly diminished relative to the control.
Figure 4.
Rotenone inhibits the postillumination increase of apparent Fo in sunflower leaf discs. Leaves used for experiments were exposed to 4 h of growth-chamber light (650 μmol quanta m−2 s−1 PPFD) prior to the introduction of the inhibitor. The leaf discs were floated on distilled water (○) or on a 200 μm rotenone solution (□) during the preillumination period (i.e. 5 min at 1000 μmol quanta m−2 s−1). At 800 s after the light-to-dark transition, the rotenone-treated leaf was fumigated with 10% CO (•).
Fumigation of rotenone-treated leaf discs with CO restored a significant dark increase in the apparent Fo (Fig. 4). Thus, although rotenone significantly diminished the rate of dark PQ reduction in light-acclimated sunflower leaves, dark reduction was not entirely inhibited because PQH2 accumulated when PQH2 oxidation was inhibited by CO. It should also be noted that in the presence of rotenone it is evident that CO fumigation induces a rapid decrease in the apparent Fo level. This observation, in combination with the fact that CO-treated leaves generally exhibit a lower maximum apparent Fo during prolonged dark incubation (Fig. 1), suggests that CO may modestly quench fluorescence by a mechanism independent of its inhibition of chlororespiration.
Dark PQ Reduction Activity Affects the Activation/Reduction Status of the Chloroplast ATP Synthase
The chloroplast ATP synthase is an intricately regulated, reversible F1Fo-type H+-ATPase embedded in the thylakoid membrane. Because the catalytically active state of this enzyme requires the maintenance of a sizable ΔμH+, it is generally assumed that the enzyme complex rapidly deactivates following a light-to-dark transition (for review, see Ort and Oxborough, 1992). However, the prospect of proton-coupled chlororespiratory electron flux introduces an intriguing possibility that an activated chloroplast ATP synthase/ATPase could be maintained for extended periods of darkness.
The chloroplast ATP synthase activation status has been studied in intact leaves by analyses of flash-induced electrochromic change-decay kinetics (Kramer and Crofts, 1989; Ort and Oxborough, 1992; Oxborough and Ort, 1995). This measurement allows the fate of the electrical component of the ΔμH+ to be monitored because of the effect that the membrane potential has on the absorption spectrum of a special subset of carotenoids and chlorophyll b molecules within the thylakoid membrane (Witt, 1979). Under conditions in which the ATP synthase is activated, a rapid (i.e. tens of milliseconds) depolarization of the flash-induced electric field occurs due to H+ efflux coupled to the synthesis of ATP (Witt, 1979; Kramer and Crofts, 1989; Ort and Oxborough, 1992). When the ATP synthase is inactive, the flash-induced electric field is depolarized by much slower (i.e. hundreds of milliseconds) ion movements across the thylakoid membrane (Kramer and Crofts, 1989).
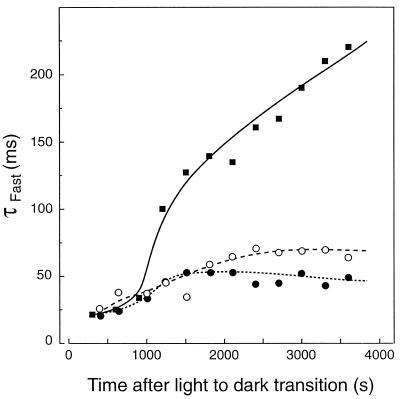
In Figure 5, the changes in the ΔA518 fast-relaxation time constant (τfast) from the light-acclimated state to the dark-adapted state are compared for sunflower leaves with different preillumination histories. In all cases, leaves were initially preilluminated for 5 min under 1000 μmol quanta m−2 s−1 green light. Clearly, the ionic conductance of the thylakoids is much greater in sunflower leaves that exhibited nonphotochemical reduction of PQ compared with those that did not (Fig. 5). Although the relaxation of the ΔA518 in light-acclimated leaves slowed somewhat during the 1 h of dark adaptation (i.e. from approximately 25 to approximately 60 ms), the slowing of τfast was much greater in the leaves of dark-adapted plants (τfast > 200 ms after 1 h of dark adaptation). Light-acclimated leaves fumigated with CO exhibited a similar response to the untreated, light-acclimated leaves during the first 25 min of dark adaptation (Fig. 5). During prolonged dark adaptation, CO fumigation appeared to stabilize τfast.
Figure 5.
Postillumination changes in the fast-relaxation time constant (τfast) for the single-turnover flash-induced electrochromic change measured at 518 nm in sunflower leaves. Changes in τfast were measured in leaves light acclimated for 4 h under 650 μmol quanta m−2 s−1 PPFD and then preilluminated at 1000 μmol quanta m−2 s−1 for 5 min with (•) and without (○) CO added. Results are also shown for a leaf that was preilluminated after a 10-h dark-adaptation period (▪).
Dark PQ Reduction Activity Slows Relaxation of Nonphotochemical Quenching
Nonphotochemical quenching of chlorophyll fluorescence is widely accepted as an indicator of a highly regulated and complex pathway for the direct dissipation of excitation energy as heat within the PSII antenna. Like the activation of the chloroplast ATP synthase discussed above, the formation and maintenance of a ΔμH+ is a prerequisite for nonphotochemical quenching of chlorophyll fluorescence; therefore, it was of interest to determine the influence of chlororespiratory electron flux on the lifetime of nonphotochemical down-regulation of PSII following a light-to-dark transition.
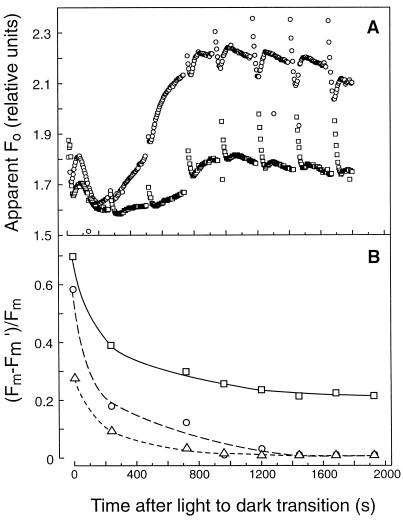
To determine whether dark PQ reduction drove nonphotochemical down-regulation of PSII, changes in the Fm and apparent Fo yields were measured to calculate changes in postillumination nonphotochemical fluorescence quenching. Figure 6 depicts the changes in Fm and apparent Fo of sunflower leaves exposed to two different illumination treatments (4 or 11 h of preillumination) following a light-to-dark transition. Both samples exhibited dark reduction of PQ to PQH2, as was evident from the increase in apparent Fo during dark adaptation (Fig. 6A). Saturation pulses were applied every 4 min during the dark-adaptation period.
Figure 6.
Changes in apparent Fo (A) and Fm quenching (Fm − Fm′/Fm; B) measured with saturation pulses in sunflower leaves exposed to 650 μmol quanta m−2 s−1 PPFD for 4 h (○) and 11 h (□). Saturation pulses (3500 μmol quanta m−2 s−1) were 400 ms long and given at 240-s intervals. Leaves were preilluminated for 5 min at 1000 μmol quanta m−2 s−1 as before. A leaf sample that was dark adapted for 10 h and then preilluminated is included for comparison (▵).
The dark-adapted Fm was also determined after the light-acclimated leaves were incubated in the dark for 10 h. When the extent of nonphotochemical Fm quenching (Fm − Fm′/Fm) was calculated, it was apparent that a small amount of Fm quenching was maintained for a longer time in leaves exhibiting dark PQ reduction compared with a leaf that did not reduce PQ (Fig. 6B). The relaxation kinetics for Fm quenching were slower in leaves acclimated to 11 h compared with 4 h of growth-chamber illumination (Fig. 6B). Initially, it may seem contradictory that the postillumination increase in apparent Fo, which is indicative of the net nonphotochemical PQ reduction rate, was less in the 11-h than the 4-h light-treated leaf, whereas the extent of nonphotochemical quenching, which is indicative of the size of the ΔμH+ maintained during the dark adaptation, was greater in the 11-h than in the 4-h light-treated leaf. However, as discussed below, this may reflect the effect of the greater nonphotochemical quenching on apparent Fo yield in the 11-h light-acclimated leaf.
DISCUSSION
This study provides evidence that a chlororespiratory electron-transport pathway functions in the chloroplasts of preilluminated sunflower leaves. Our experiments show that the PQ pool can be reduced in the dark by a rotenone-sensitive process, consistent with the involvement of NAD(P)H dehydrogenase-like activity. Our experiments further show that the dark-aerobic oxidation of PQH2 relies on a CO-sensitive oxidase, suggesting the existence of a Cyt oxidase-like activity in sunflower chloroplasts. Although one anticipates intervening electron-transfer events between PQ reduction and electron donation to molecular oxygen, there is no evidence from higher plants to suggest what additional redox components might be involved in chlororespiration. Studies with C. reinhardtii mutants indicate that the chlororespiratory pathway functions in this single-celled alga without the involvement of Cyt f, the Reiske-center protein, plastocyanin, or the chloroplast ATP synthase (Bennoun, 1993). On the other hand, in prokaryotic cyanobacteria it is well established that the Cyt b6/f complex works as a common transducer of electrons from PQH2 to PSI in photosynthesis and to a terminal oxidase during respiration (Scherer, 1990; Schmetterer, 1994).
In cyanobacteria a CO-sensitive, aa3-type terminal Cyt c oxidase has been well characterized (Schmetterer, 1994), possibly suggesting that an analog of this complex may be present in the chloroplasts of algae and plants. There is also evidence for a thylakoid-bound Cyt c oxidase in the chlorophyll c-containing alga Pleurochloris meiringensis (Buchel and Garab, 1995). However, in this organism inhibition of the terminal oxidase required 25 times the concentration of KCN needed to inhibit the aa3-type oxidase of mitochondria (Buchel and Garab, 1995). There are also reports that Synechocystis sp. strain PCC 6803 contains a KCN/CO-sensitive oxidase other than the aa3-type Cyt oxidase (Schmetterer et al., 1994).
Direct study of the chloroplast oxidase is hindered by an inability to reconstitute a CO-sensitive oxidase activity in isolated thylakoids of algae or plants; this includes our own unsuccessful efforts. A likely explanation is that the oxidase is removed or otherwise inactivated during thylakoid isolation. To complicate matters further, some cyanobacteria and eukaryotic algae appear to have alternative (i.e. not KCN/CO-sensitive) respiratory oxidases. For example, in C. vulgaris chlororespiration is sensitive to SHAM (Bennoun, 1982), an inhibitor of the alternative oxidase of plant mitochondria. A SHAM-sensitive respiratory oxidase has also been reported in the chloroplasts of Dunaliella tertiolecta (Casper-Lindley and Björkman, 1997), and a SHAM-sensitive glycolate-quinone oxidoreductase activity has been observed in the chloroplasts of D. tertiolecta, C. reinhardtii, and spinach (Goyal and Tolbert, 1996).
The rotenone sensitivity of the increase in the apparent Fo of light-acclimated sunflower leaves indicates that a NAD(P)H-dehydrogenase activity is the primary entry point for electrons from NADPH into the PQ pool. Our findings are consistent with earlier studies of spinach thylakoids showing that exogenous NADPH reduced PQ in the dark (Mills et al., 1979). Metabolically, NADPH is likely to be the most important physiological donor to PQ because several photosynthetic metabolites in the chloroplast stroma can be coupled to NADPH formation (e.g. triose-phosphates, malate, etc.). Starch breakdown would produce a significant flux of NADPH that could enter the PQ pool through NADPH oxidation by chlororespiration, as has been shown in algae (Bennoun, 1982; Gfeller and Gibbs, 1985). In the presence of CO, however, net dark reduction of PQ was still possible after rotenone treatment (Fig. 4). Although this may indicate a rotenone-insensitive pathway for dark PQ reduction, it is equally likely that incomplete infiltration of rotenone into the leaves is the explanation.
Other possible reductants for PQ in the dark include ascorbate, which can reduce PQ and is present in concentrations in chloroplasts exceeding 20 mm (Aristarkhov et al., 1987), but there is little indication of how an electron cycle involving ascorbate would operate in the dark. It has been suggested that electrons can enter the PQ pool in the dark in C. reinhardtii from succinate donors (Willeford et al., 1989), but succinate-dependent redox transfer to PQ is unknown in plants. Ferrodoxin-quinone reductase activity is another possible route for stromal donors to shuttle electrons to PQ, but this activity has been observed to operate only in the light (Bendall and Manasse, 1995). The situation is similar for glycolate-PQ-oxidoreductase activity in algae and spinach chloroplasts, in which glycolate reduces PQ in the light (Goyal and Tolbert, 1996), but it seems very unlikely that glycolate pool sizes could be large enough to account for sustained PQ reduction in the dark.
Estimating from the recovery kinetics for apparent Fo following a far-red pulse (Fig. 2), the net rate of dark PQH2 formation in light-acclimated sunflower leaves is about 0.28 meq mol−1 chlorophyll s−1. This calculation assumes that the pool size of reducible PQ is 16.5 meq mol−1 chlorophyll s−1 (Graan and Ort, 1984). When CO was added (Fig. 2), recovery of the apparent Fo was nearly twice as fast, corresponding to a net rate of dark PQ reduction of 0.55 meq mol−1 chlorophyll s−1. To estimate the true gross electron flux to PQ in the dark, the nonenzymatic aerobic oxidation of PQH2 by molecular oxygen must be considered. Using the data of Graan and Ort (1984) for spinach thylakoids, in which aerobic PQH2 oxidation occurs as a first-order reaction with a 60-s half-time, we assigned a flux of 0.14 meq mol−1 chlorophyll s−1 to the nonenzymatic aerobic oxidation of PQH2. This yields a gross electron flux to PQ in darkness of about 0.7 meq mol−1 chlorophyll s−1 (i.e. approximately 0.3% of light-saturated photosynthetic electron flux in sunflower leaves).
Regardless of the identities of functioning donor molecules, it is clear that a redox pool of considerable size supplies electrons to PQ during darkness. Dark PQ reduction persisted for 45 to 70 min in light-acclimated sunflower leaves under the conditions studied here. Taking the estimate for the gross electron transport rate reducing PQ, along with the duration observed for nonphotochemical PQ reduction in sunflower leaves, an estimate of the potential chlororespiratory electron donor pool size for PQ reduction is at least 75-fold larger than the electron storage capacity provided by the PQ pool. The size of the chlororespiratory electron donor pool may indicate that the transfer of reducing equivalents and adenylates from the cytosol and/or mitochondria may be crucial for sustaining activity (Garab et al., 1989; Bennoun, 1994). These observations are consistent with a number of studies that have shown a substantial reserve of reductant in chloroplasts for P700+ formed by far-red illumination following a light-to-dark transition (Asada et al., 1992; Havaux, 1996).
The function of chlororespiration in both plant leaves and algae remains an enigma (Scherer, 1990; Peltier and Schmidt, 1991), but its potential role in starch mobilization at night is an intriguing and plausible possibility. The full biochemical details for the degradation and mobilization of starch from higher plant chloroplasts are unknown, but the process may involve parallel pathways and significant species-specific differences (Preiss, 1988; Stitt, 1990). It is clear that sustained starch degradation and mobilization from the chloroplast in the dark requires the regenerative cycling of adenine nucleotides and phosphate and possibly the maintenance of transmembrane pH differences that regulate the activity of key enzymes in the pathway(s) (Stitt, 1996).
These considerations place special significance on the effect of chlororespiratory activity on the regulation of the chloroplast ATP synthase. Activation of the chloroplast ATP synthase and the maintenance of its catalytic activity requires the formation and maintenance of a sizable ΔμH+, after which the energetic and catalytic properties of the enzyme are further modified by thioredoxin-mediated reduction of the regulatory disulfide of the γ-subunit (for review, see Ort and Oxborough, 1992). Whether chlororespiratory activity generates a ΔμH+ of sufficient magnitude to drive net ATP formation or even to maintain an activated ATP synthase/ATPase at flux equilibrium is uncertain. The decay rate of the electrochromic change indicated that light acclimation caused the thylakoid ionic conductance to remain high following prolonged dark adaptation (Fig. 5). However, it was unclear whether chlororespiration actually prolonged the duration of the activated state of the ATP synthase in the dark or whether only the reduced state of the regulatory disulfide of the γ-subunit was maintained by a redox buffering pool formed during light acclimation (Kramer and Crofts, 1989; Gabrys et al., 1994).
These alternatives are difficult to resolve because a single saturating flash can initiate ATP synthase activation and the net synthesis of ATP when the γ-subunit is reduced, even in the absence of a preexisting ΔμH+ (Ort and Oxborough, 1992). Thus, the insensitivity of the rapid ΔA518 decay kinetics in light-acclimated leaves to CO fumigation (Fig. 5) would be expected whether or not chlororespiration supported the maintenance of a large ΔpH in the dark. In fact, the maintenance of more rapid ΔA518 decay kinetics in CO-treated leaves during prolonged dark adaptation (Fig. 5, >25 min) may be the result of the maintenance of the chloroplast reductant pool due to CO inhibition of chlororespiration.
The strongest evidence that chlororespiration can support the formation of a sizable ΔpH in the dark is the demonstration in unicellular algae of the induction of ΔpH-dependent nonphotochemical quenching by chlororespiration (Ting and Owens, 1993; Endo and Asada, 1996). In P. tricornutum, Ting and Owens (1993) found increases approaching 10% in both Fm and Fo upon the addition of the uncoupler m-chlorocyanocarbonyl phenylhydrazone in the dark (Ting and Owens, 1993). In sunflower leaves, we observed that the apparent Fo was reduced by nearly one-half in leaves treated with 11 h of growth-chamber light compared with those that had received only 4 h of light acclimation (Fig. 6). However, only a portion of this quenching was CO sensitive, indicating that both chlororespiration-dependent ΔpH-induced quenching and sustained PSII down-regulation contributed to the lowering of the apparent Fo between 4 and 11 h of light acclimation.
Although the magnitude of this chlororespiration-dependent ΔpH in sunflower chloroplasts is unknown, most current evidence suggests that the ΔpH threshold for energy-dependent fluorescence quenching is normally higher than the energetic threshold for ATP synthase/ATPase activation (Schönknecht et al., 1995; Horton et al., 1996). This relationship implies that chlororespiratory activity in sunflower leaves should be sufficient to maintain an activated chloroplast ATP synthase/ATPase during prolonged dark periods. If so, seemingly cryptic diurnal patterns reported for the dark inactivation of the chloroplast ATP synthase in intact plants (Kramer and Crofts, 1990) may be accounted for by chlororespiratory activity acquired during the course of the day.
We observed an apparent light-dependent activation requirement for chlororespiration that was not reported in previous studies with algae (Peltier and Schmidt, 1991). Net nonphotochemical reduction of PQ was not seen in fully dark-adapted sunflower leaves or in leaves illuminated for 30 min under 1000 μmol quanta m−2 s−1 light, even following CO treatment. Approximately 4 h under growth-chamber light was required to induce chlororespiration in sunflower; we do not know the requirements for activation of chlororespiratory electron transport. Activation of chlororespiration in leaves could require gene expression and de novo protein synthesis and/or accumulation of suitably large electron donor pools. Considering the possible role of chlororespiration in the maintenance of dark starch metabolism, the dependence of nonphotochemical PQ reduction on prolonged light exposure time may be linked to the accumulation of starch in the chloroplast stroma.
Evidence has been presented that the nonphotochemical reduction of PQ in the dark observed previously in sunflower originates from chlororespiration. It is still unclear what the function(s) of chlororespiration may be in the dark. Although chlororespiration appears to generate an electrochemical potential in sunflower leaves, its magnitude is unknown. Future studies need to address the possible relationships between chlororespiration and starch metabolism in leaves. Studies addressing the molecular biology and protein chemistry aspects of NAD(P)H dehydrogenase and particularly the unknown CO-sensitive oxidase are critical to an understanding of the mechanism and function of chlororespiration. Clarification of the factors responsible for light activation of chlororespiration deserves careful study. Additionally, the possible influences of environmental factors (e.g. heat stress) that may modulate the rate of nonphotochemical PQ reduction (Havaux, 1996) remain to be clarified in plant leaves.
ACKNOWLEDGMENT
Useful advice by Dr. John Whitmarsh was appreciated at all stages of this project.
Abbreviations:
- apparent Fo
observed fluorescence yield following dark adaptation
- ΔμH+
transmembrane electrochemical potential
- Fm
maximal fluorescence yield
- Fo
minimum fluorescence yield when QA is fully oxidized and nonphotochemical quenching fully relaxed
- MV
methyl viologen
- PQ
plastoquinone
- PQH2
plastoquinol
- QA
primary quinone acceptor of PSII
- QB
secondary quinone acceptor of PSII
- SHAM
salicylhydroxamic acid
Footnotes
This work was supported in part by an Integrative Photosynthesis Research training grant from the Department of Energy (no. DEFGO2-92ER20095), funded under the Program for Collaborative Research in Plant Biology.
LITERATURE CITED
- Aristarkhov AI, Nikandrov VV, Krasnovskii AA. Ascorbate permeability of chloroplast thylakoid membrane; reduction of plastoquinone and cytochrome f. Biokhimiya. 1987;52:1729–1808. [Google Scholar]
- Asada K, Heber U, Schreiber U. Pool size of electrons that can be donated to P700+, as determined in intact leaves: donation of P700+ from stromal components via the intersystem chain. Plant Cell Physiol. 1992;33:927–932. [Google Scholar]
- Bendall DS, Manasse RS. Cyclic photophosphorylation and electron transport. Biochim Biophys Acta. 1995;1229:23–38. [Google Scholar]
- Bennoun P. Evidence for a respiratory chain in the chloroplast. Proc Natl Acad Sci USA. 1982;79:4352–4356. doi: 10.1073/pnas.79.14.4352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennoun P. Effects of mutations and of ionophores on chlororespiration in Chlamydomonas reinhardtii. FEBS Lett. 1993;156:363–365. [Google Scholar]
- Bennoun P. Chlororespiration revisited: mitochondrial-plastid interactions in Chlamydomonas. Biochim Biophys Acta. 1994;1186:59–66. [Google Scholar]
- Berger S, Ellersiek U, Westhoff P, Steinmuller K. Studies on the expression of NDH-H, a subunit of the NAD(P)H-plastoquinone-oxidoreductase of higher plant chloroplasts. Planta. 1993;190:25–31. [Google Scholar]
- Buchel C, Garab G. Evidence for the operation of a cyanide-sensitive oxidase in chlororespiration in the thylakoids of the chlorophyll c-containing alga Pleurochloris meiringensis (Xanthophyceae) Planta. 1995;197:69–75. [Google Scholar]
- Casper-Lindley C, Björkman O. Nigericin insensitive post-illumination reduction in fluorescence yield in Dunaliella tertiolecta (Chlorophyte) Photosynth Res. 1997;50:209–222. doi: 10.1007/BF00033120. [DOI] [PubMed] [Google Scholar]
- Chylla RA, Garab G, Whitmarsh J. Evidence for slow turnover in a fraction of photosystem II complexes in thylakoid membranes. Biochim Biophys Acta. 1987;894:562–571. [Google Scholar]
- Endo T, Asada K. Dark induction of the non-photochemical quenching of chlorophyll fluorescence by acetate in Chlamydomonas reinhardtii. Plant Cell Physiol. 1996;37:551–555. [Google Scholar]
- Gabrys H, Kramer DM, Crofts AR, Ort DR. Mutants of the chloroplast coupling factor reduction in Arabidopsis. Plant Physiol. 1994;104:769–776. doi: 10.1104/pp.104.2.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garab G, Lajko F, Mustardy L, Marton L. Respiratory control over photosynthetic electron transport in chloroplasts of higher plant cells. Evidence for chlororespiration. Planta. 1989;179:349–358. doi: 10.1007/BF00391080. [DOI] [PubMed] [Google Scholar]
- Genty B, Briantais J-M, Baker NR. The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim Biophys Acta. 1989;990:87–92. [Google Scholar]
- Gfeller RP, Gibbs M. Fermentative metabolism of Chlamydomonas reinhardtii. III. Role of plastoquinone. Plant Physiol. 1985;77:509–511. doi: 10.1104/pp.77.2.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goyal A, Tolbert NE. Association of glycolate oxidation with photosynthetic electron transport in plant and algal chloroplasts. Proc Natl Acad Sci USA. 1996;93:3319–3324. doi: 10.1073/pnas.93.8.3319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graan T, Ort DR. Quantitation of the rapid electron donors to P700, the functional plastoquinone pool, and the ratio of the photosystems in spinach chloroplasts. J Biol Chem. 1984;259:14003–14010. [PubMed] [Google Scholar]
- Groom Q, Kramer DM, Crofts AR, Ort DR. The non-photochemical reduction of plastoquinone in leaves. Photosynth Res. 1993;36:205–215. doi: 10.1007/BF00033039. [DOI] [PubMed] [Google Scholar]
- Gruszecki WI, Bader KP, Schmid GH. Light-induced oxygen uptake in tobacco chloroplasts explained in terms of chlororespiratory activity. Biochim Biophys Acta. 1994;1188:335–338. [Google Scholar]
- Guedeney G, Corneille S, Cuine S, Peltier G. Evidence for an association of ndh B, ndh J gene products and ferrodoxin-NADP-reductase as components of a chloroplastic NAD(P) dehydrogenase complex. FEBS Lett. 1996;378:277–280. doi: 10.1016/0014-5793(95)01473-x. [DOI] [PubMed] [Google Scholar]
- Harris GC, Heber U. Effects of anaerobiosis of chlorophyll fluorescence yield in spinach (Spinacea oleracea) leaf discs. Plant Physiol. 1993;101:1169–1173. doi: 10.1104/pp.101.4.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havaux M. Short-term responses of photosystem I to heat stress. Induction of a PSII-independent electron transport through PSI fed by stromal components. Photosynth Res. 1996;47:85–97. doi: 10.1007/BF00017756. [DOI] [PubMed] [Google Scholar]
- Horton P, Ruban AV, Walters RG. Regulation of light harvesting in green plants. Annu Rev Plant Physiol Mol Biol. 1996;47:655–684. doi: 10.1146/annurev.arplant.47.1.655. [DOI] [PubMed] [Google Scholar]
- Joliot P, Joliot A. Dependence of delayed luminescence upon adenosine triphosphatase activity in Chlorella. Plant Physiol. 1980;65:691–696. doi: 10.1104/pp.65.4.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer DM, Crofts AR. Activation of the chloroplast ATPase measured by the electrochromic change in leaves of intact plants. Biochim Biophys Acta. 1989;967:28–41. [Google Scholar]
- Kramer DM, Crofts AR. Diurnal pattern of chloroplast coupling factor oxidation kinetics in leaves of intact sunflower. In: Baltscheffsky M, editor. Current Research in Photosynthesis, Vol III. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1990. pp. 89–92. [Google Scholar]
- Kramer DM, Robinson HR, Crofts AR. A portable multi-flash fluorimeter for measurement of donor and acceptor reactions of photosystem 2 in leaves of intact plants under field conditions. Photosynth Res. 1990;26:181–193. doi: 10.1007/BF00033131. [DOI] [PubMed] [Google Scholar]
- Krause GH, Weis E. Chlorophyll fluorescence and photosynthesis: the basics. Annu Rev Plant Physiol Plant Mol Biol. 1991;42:313–349. [Google Scholar]
- Kubicki A, Funk E, Westhoff P, Steinmuller K. Differential expression of plastome-encoded ndh genes in mesophyll and bundle-sheath chloroplasts of the C4 plant Sorghum bicolor indicates that complex I-homologous NAD(P)H-plastoquinone oxidoreductase is involved in cyclic electron transport. Planta. 1996;199:276–281. [Google Scholar]
- Mills JD, Mitchell PD, Barber J. The cyclic electron transport pathway in chloroplasts. Reduction of plastoquinone by reduced nicotinamide adenine dinucleotide diphosphate in the dark. Photobiochem Photobiophys. 1979;1:3–9. [Google Scholar]
- Nedbal L, Trtilek M. Diffuse-light dual modulation fluorimeter: monitoring of electron transfer reactions in Synechococcus elongatus exposed to intermittent light. In: Mathis P, editor. Photosynthesis: From Light to the Biosphere, Vol V. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1995. pp. 813–816. [Google Scholar]
- Ort DR, Izawa S. Studies on the energy-coupling sites of photophosphorylation. V. Phosphorylation efficiencies (P/e2) associated with aerobic photooxidation of artificial electron donors. Plant Physiol. 1973;53:370–376. doi: 10.1104/pp.53.3.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ort DR, Oxborough K. In situ regulation of chloroplast coupling factor activity. Annu Rev Plant Physiol Plant Mol Biol. 1992;43:269–291. [Google Scholar]
- Oxborough K, Ort DR. In situ evidence that chilling in the light does not cause uncoupling of photophosphorylation or detachment of coupling factor in chilling-sensitive plants. Photosynth Res. 1995;43:93–105. doi: 10.1007/BF00042966. [DOI] [PubMed] [Google Scholar]
- Peltier G, Ravenel J, Vermeglio A. Inhibition of a respiratory activity by short saturating flashes in Chlamydomonas: evidence for chlororespiration. Biochim Biophys Acta. 1987;893:83–90. [Google Scholar]
- Peltier G, Schmidt GW. Proc Natl Acad Sci USA. 1991;88:4791–4795. doi: 10.1073/pnas.88.11.4791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preiss J. Biosynthesis of starch and its degradation. In: Preiss J, editor. Biochemistry of Plants, Vol 13. San Diego, CA: Academic Press; 1988. pp. 181–254. [Google Scholar]
- Rutherford AW, Govindjee, Inoue Y. Charge accumulation and photochemistry in leaves studied by thermoluminescence and delayed light. Proc Natl Acad Sci USA. 1984;81:1107–1111. doi: 10.1073/pnas.81.4.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sazanov LA, Burrows P, Nixon PJ. Detection and characterization of a complex I-like NADH-specific dehydrogenase from pea thylakoids. Trans Biochem Soc London. 1996;24:739–743. doi: 10.1042/bst0240739. [DOI] [PubMed] [Google Scholar]
- Scherer S. Do photosynthetic and respiratory electron transport chains share redox proteins? Trends Biochem Sci. 1990;15:458–462. doi: 10.1016/0968-0004(90)90296-n. [DOI] [PubMed] [Google Scholar]
- Schmetterer G. Cyanobacterial respiration. In: Bryant DA, editor. The Molecular Biology of Cyanobacteria. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1994. pp. 409–435. [Google Scholar]
- Schönknecht G, Neimanis S, Gerst U, Heber U. The pH-dependent regulation of photosynthetic electron transport in leaves. In: Mathis P, editor. Photosynthesis: From Light to the Biosphere, Vol II. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1995. pp. 843–846. [Google Scholar]
- Stitt M (1990) The flux of carbon between the chloroplast and the cytoplasm. In DT Dennis, DH Turpin, eds, Advanced Plant Physiology: Interaction and Control of Metabolism. Pitman Publishing, London, pp 319–340
- Stitt M. Metabolic regulation in photosynthesis. In: Baker NR, editor. Photosynthesis and the Environment. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1996. pp. 152–190. [Google Scholar]
- Ting CS, Owens TG. Photochemical and nonphotochemical fluorescence quenching processes in the diatom Phaeodactylum tricornutum. Plant Physiol. 1993;101:1323–1330. doi: 10.1104/pp.101.4.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velthuys BR, Amesz J. Charge accumulation at the reducing side of system 2 in photosynthesis. Biochim Biophys Acta. 1974;333:85–94. doi: 10.1016/0005-2728(74)90165-0. [DOI] [PubMed] [Google Scholar]
- Vernotte C, Etienne AL, Briantais J-M. Quenching of the system II chlorophyll fluorescence by the plastoquinone pool. Biochim Biophys Acta. 1979;545:519–527. doi: 10.1016/0005-2728(79)90160-9. [DOI] [PubMed] [Google Scholar]
- Wakasugi T, Tsudzuki T, Shibata M, Hirai A. Loss of all ndh genes as determined by sequencing the entire chloroplast genome of black pine (Pinus thurgergii) Proc Natl Acad Sci USA. 1994;91:9794–9798. doi: 10.1073/pnas.91.21.9794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willeford KO, Gombos Z, Gibbs M. Evidence for chloroplastic succinate dehydrogenase participating in the chloroplastic respiratory and photosynthetic electron transport chains of Chlamydomonas reinhardtii. Plant Physiol. 1989;90:1084–1087. doi: 10.1104/pp.90.3.1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witt HT. Energy conversion in the functional membrane of photosynthesis. Analysis by light pulse and electric pulse methods. The central role of the electric field. Biochim Biophys Acta. 1979;505:355–427. doi: 10.1016/0304-4173(79)90008-9. [DOI] [PubMed] [Google Scholar]