Abstract
Objective
We examined several risk factors as possible independent predictors of aortic stiffness progression among a population based sample of US men.
Methods and Results
A total of 240 men aged 40–49 from the Allegheny County site of the ERA JUMP Study, who were free of CVD at baseline were evaluated. Aortic stiffness was measured as carotid-femoral pulse wave velocity (cfPWV) at baseline and after 4.6±0.21(mean±SD) years of follow-up. Progression of cfPWV was evaluated as relative annual change in cfPWV (% change/year). Using linear regression, both baseline potential risk factors and their annual changes were evaluated as possible risk factors for cfPWV progression. Baseline age, follow-up time, race, heart rate, and medications use were forced in all models. During follow-up, relative to baseline level, cfPWV increased 0.3%±5.3% per year. In final models the independent predictors of degree of cfPWV progression were lower levels of adiponectin (β(SE): −1.8(0.8), P=0.03), higher levels of systolic blood pressure (SBP) (β(SE): 0.07(0.03), P=0.02), greater annual change in SBP (β(SE): 0.3(0.2), P=0.04), and alcohol consumption ≥ 2 times/week (β(SE): 1.6(0.7), P=0.02).
Conclusions
Lower levels of adiponectin, higher levels and annual changes of SBP, and alcohol consumption ≥2 times/week are associated with greater progression in aortic stiffness among relatively healthy middle-aged US men.
Keywords: arteriosclerosis, hypertension, risk factors, pulse wave velocity, stiffness
Stiffening in the central arteries, such as the aorta, has been identified as an independent predictor of coronary heart disease and stroke.1,2 It is a process of structural changes in the arteries accompanied by collagen increase, elastin degeneration, and vascular smooth muscle proliferation.3 Greater arterial stiffness can lead to increased cardiac afterload, impaired coronary blood flow, and increased arterial wall stress,4,5 which collectively contribute to making arteries more susceptible to endothelial injury and vascular damage. 6
Pulse wave velocity (PWV) is the gold standard method for measuring arterial stiffness,7 and carotid-femoral PWV (cfPWV), in particular, is recognized as the best established noninvasive measure for assessing central arterial stiffness (aortic stiffness).4,8 Several cross-sectional studies have assessed factors associated with higher arterial stiffness. High blood pressure, diabetes,9,10,11 heart rate,12 presence of dyslipidemia, 13,14 and smoking, 15 were reported to be independently associated with greater arterial stiffness. The degree to which these factors and other potential risk factors are involved in the progression of arterial stiffness is not completely clear. Studies that assessed possible determinants of progression of arterial stiffness were mainly conducted in diseased populations (e.g. individuals with chronic kidney disease or hypertension), 16–21 included a small number of participants,18–22 or followed subjects for a short period of time.18,19,21–24
Results from prospective studies were inconclusive. Factors that were identified as possible determinants of arterial stiffness progression were not consistent. This could be related to methodological variations such as; use of different measures for arterial stiffness (central vs. peripheral), characteristics of study populations (apparently healthy vs. symptomatic subjects), and statistical methods.
Using a population-based sample of apparently healthy White and African American men from the Electron-Beam computed Tomography and Risk Factor Assessment in Japanese and US Men in the Post-World War II Birth Cohort (ERA JUMP) study, we evaluated the determinants of aortic stiffness progression over a maximum of 6 years of follow-up. Factors that were assessed for their associations with aortic stiffness progression included life-style measures, traditional cardiovascular risk factors, inflammatory, hemostatic, and adipocytokines markers.
Materials and Methods
Subjects
The ERA JUMP study is a population-based study of men aged 40 to 49 years who were free of cardiovascular disease (CVD), type 1 diabetes, or other severe diseases at baseline visit (2000-2006).25,26 Subjects were recruited from four sites as follows: Allegheny County, Pennsylvania, US; Kusatsu, Shiga, Japan; Honolulu, Hawaii, US; and Ansan, South Korea. Only the Allegheny county site has data on cfPWV at two time points (baseline and a follow-up visit) and therefore was included in the current study.
At baseline, 417 participants (310 White and 107 African American) were available from the Allegheny County site for an assessment of cfPWV. We were unable to obtain a sufficient wave form in either carotid or femoral artery to calculate cfPWV on 50 (12%) participants. Of 367 participants with baseline cfPWV, 269 (73.3%) were available for follow-up assessment for cfPWV. Of those, 29 (11%) participants were excluded due to technical issues with their cfPWV follow-up measures. Thus, our final sample was 240 participants (198 White and 42 African American) with two time points of (baseline and follow-up) cfPWV measures. Participants who were excluded from the current analyses due to technical issues or loss to follow-up (n=177) were more likely to be African American, non-frequent alcohol drinker (nondrinker or drink <2 times/week), have higher levels of BMI (mean BMI=29.9 kg/m2 vs. 27.7 kg/m2), have higher levels of glucose and fibrinogen, and have lower levels of triglycerides. There were no significant differences in baseline measures of cfPWV, age, systolic blood pressure, heart rate, total cholesterol, HDL, LDL, insulin, adiponectin, PAI-1, and smoking status. Baseline use of antihypertensive, antidiabetic, and lipid-lowering medications were similar between those who were included in the current analyses and those who were not.
Informed consent was obtained from all participants. The study was approved by the Institutional Review Board of University of Pittsburgh, Pittsburgh, Pennsylvania, US.
Study Variables
All participants underwent a physical examination, laboratory assessment, and lifestyle questionnaire as described previously.25,26 Body weight and height were measured while the participant was wearing light clothing without shoes. BMI was calculated as weight in kg/(height in meter)2. Blood pressure and heart rate were measured in the right arm of the seated participant after he emptied his bladder and sat quietly for 5 minutes, using an automated sphygmomanometer (BP-8800; Colin Medical Technology, Komaki, Japan) and an appropriate-sized cuff. The average of two measurements was used.
Venipuncture was performed early in the clinic visit after a 12-hour fast. Serum samples were stored at −80°C and shipped on dry ice to the Heinz Nutrition Lab, University of Pittsburgh (lipids, glucose, insulin, and adiponectin) and University of Vermont (C-reactive protein (CRP), fibrinogen, and plasminogen activator inhibitor-1 (PAI-1)) for analysis. Serum lipids, including total cholesterol, low-density lipoprotein cholesterol (LDL-c), high-density lipoprotein cholesterol (HDL-c), and triglycerides, were determined with standardized methods according to the Centers for Disease Control and Prevention. Serum glucose was determined using a hexokinase glucose-6-phosphate-dehydrogenase enzymatic assay, serum insulin and adiponectin using a radioimmunoassay (Linco Research Inc., St. Charles, Missouri), CRP using a colorimetric competitive enzyme-linked immunosorbent assay, fibrinogen using an automated clot-rate assay (Diagnostica Stago, Parsippany, New Jersey), and PAI-1 levels were determined as the free form (both latent and active) with an assay originally developed by Dr. Desire Collen and colleagues.27
A self-administered questionnaire was used to obtain information on demographics, smoking habits, alcohol consumption, and other factors. Smoking was assessed as current, former or never. Alcohol consumption was assessed as whether the participant drank beer, wine, liquor, sake (Japanese rice wine), or other alcoholic beverages, with quantity and frequency. Alcohol drinkers were defined as those who drank alcohol ≥2 times per week. Uses of medications (antihypertensive, antidiabetic, and lipid-lowering) were reported as yes/no.
All study measures were collected at both baseline and follow-up visits except for adiponectin, fibrinogen, and PAI-1, which were only available from the baseline visit.
cfPWV
Carotid-femoral PWV was measured at both baseline and follow-up visits using a noninvasive automated waveform analyzer (VP2000, Omron Co., Komaki, Japan).28 Following 10 minutes of rest in a supine position, the participant had occlusion and blood pressure monitoring cuffs placed around both arms and ankles. ECG electrodes were placed on both wrists, and a phonocardiogram, a microphone for detecting heart sounds, was placed on the left edge of sternum. Sonographers palpated the left femoral artery and the left carotid artery and placed handheld multiarray tonometers over these two pulse areas to obtain femoral and carotid pulse waveforms simultaneously. A foot-pedal was used to start the recording. PWV (cm/sec) was calculated as the path length between arterial sites of interest divided by the time delay between the foot of the respective waveforms. For cfPWV path length, three distances (in cm) were measured over the surface of the body with a tape measure: 1) from the suprasternal notch to the sampling site on the left common carotid artery; 2) from the suprasternal notch to the inferior edge of the umbilicus; and 3) from the inferior edge of the umbilicus to the sampling site on the left common femoral artery. The final carotid to femoral path length (Lcf) was calculated by subtracting measurement 1 from the sum of 2 and 3. Data were collected two times for each participant and the values were averaged. Intra-class correlation for re-examination was 0.76.
Statistical analyses
It has been argued that including the baseline measure as a covariate in analyses of change usually lead to an overestimation of results and biased regression coefficients for predictors. 29 To account for baseline level of cfPWV without introducing the bias described above, aortic stiffness progression was evaluated as the relative annual change in cfPWV. This was calculated as (change of cfPWV since baseline) X100 / (baseline measure of cfPWV X follow-up time).
For covariates that were measured at both time points, annual changes were calculated and evaluated. Relative annual change in cfPWV was normally distributed. The distribution for triglycerides, PAI-1, fibrinogen, and adiponectin were highly skewed, and therefore, log transformation was applied to achieve normality. Linear regression was used to evaluate the effect of baseline measures as well as annual change in study measures on relative annual change in cfPWV. First, univariate analyses were performed to determine potential factors to be included in multivariabel analyses. All risk factors that were found to be associated with study outcome at p-value ≤0.25 were considered for multivariable analyses. Factors were entered into multivariable model based on univariate analyses p-values. Factors with lower p-values were entered first in to the models followed by factors with higher p-values. Factors were dropped from final models at p-value ≥ 0.1. Age at baseline, follow-up time, race, heart rate, use of antihypertensive and lipid-lowering medication were forced in all models. Quartiles for baseline adiponectin were evaluated and tested in relation to relative annual change in cfPWV to identify any possible threshold effect. Analyses were performed with SAS v9.2 (SAS Institute, Cary, NC). Models were 2-sided.
Results
Study participants were followed for a mean follow-up time of 4.6±0.2 years (range:3.9–6.1 years). The percent annual change in cfPWV relative to the baseline level ranged from −16.6% per year to 23.1% per year, with negative values representing a reduction and positive values representing an increase in cfPWV over the follow-up time relative to the baseline level.
Table 1 presents summary statistics for both baseline and annual change in study variables. Participants were mainly White (82.5%) with a mean age at baseline of 45.0 years old. The majority of the study population never smoked (70.4%) while approximately half of the study population were consuming alcohol ≥2 times per week (46.7%).
Table 1.
Baseline Characteristics and Annual Changes Characteristics of the Study Participants
| Variable | Mean(SD), Median (Q1, Q3) Or No. (%) |
|---|---|
| Baseline | |
| Age, Years | 45.0(2.9) |
| White, n (%) | 198 (82.5) |
| BMI, kg/m2 | 27.7(4.1) |
| SBP, mm Hg | 122.9(11.5) |
| Heart rate/minute | 64.6(8.8) |
| Medication for hypertension, n (%) | 24 (10.0) |
| LDL-c, mg/dL | 134.5(32.8) |
| HDL-c, mg/dL | 48.5(12.8) |
| Triglycerides, mg/dL | 131.0(94.0,193.0) |
| Total cholesterol, mg/dL | 212.8(37.3) |
| Medication for lipids, n (%) | 27 (11.3) |
| Glucose, mg/dL | 98.0(92.0,105.0) |
| Insulin, uU/mL | 12.8(10.2,17.1) |
| Medication for diabetes, n (%) | 3 (1.3) |
| Adiponectin, µg/mL | 9.8(7.0,13.0) |
| PAI-1, ng/mL | 25.9(15.3,39.2) |
| CRP, mg/L | 1.0(0.5, 2.2) |
| Fibrinogen, mg/L | 290.0(252.0,326.0) |
|
Smoking status Current, n (%) Former, n (%) Never, n (%) |
26 (10.8) 45 (18.8) 169 (70.4) |
|
Alcohol consumption ≥2 times/week, n (%) |
112 (46.7) |
| Baseline PWV, cm/s | 835.8(744.0, 934.3) |
| Annual Change | |
| BMI change, kg/m2 per Year | 0.2(0.4) |
| SBP change, mm Hg per Year | 0.7(2.3) |
| LDL-c change, mg/dL per Year | −0.4(7.3) |
| HDL-c change, mg/dL per Year | 0.3(2.0) |
| Triglycerides change, mg/dL per year* | −0.001(0.1) |
| Glucose change, mg/dL per year* | 0.02(0.03) |
| Insulin change, uU/mL per year* | −0.01(0.3) |
| CRP change, mg/L per year* | 0.02(0.2) |
| Relative cfPWV change, % change per year | 0.3(5.3) |
BMI: body mass index; SBP: systolic blood pressure; LDL-c: low density lipoprotein cholesterol; HDL-c: high density lipoprotein cholesterol; PAI-1: plasminogen activator inhibitor; CRP:C reactive protein; cfPWV: carotid femoral pulse wave velocity.
log transformed
The univariate associations between study variables (baseline and annual changes since baseline) and the relative annual change in cfPWV are shown in Table 2. Of all assessed factors, only the baseline measure of adiponectin was significantly associated with progression in aortic stiffness. Each 1 log unit decrease in adiponectin was associated with 1.8% increase in cfPWV per year relative to the baseline level (P=0.01). Using a significant level (P-value) of 0.25, systolic blood pressure (SBP), log adiponectin, log PAI-1, alcohol consumption, annual change in BMI, annual change in SBP, and annual change in log CRP, were all considered as potential risk factors and were evaluated in multivariable analyses to identify independent predictors of aortic stiffness progression. According to the standardized coefficients in Table 2, an increase of 1SD unit of log adiponectin or of annual change in SBP produces greater amount of aortic progression (0.16% reduction for adiponectin and 0.11% increase for annual change in SBP) compared to other assessed factors.
Table 2.
Univariate Effects of Baseline and Annual Change in Study Variables on Relative Annual Change in cfPWV (% change per year)
| Variable, increment | β (SE) | Standardized coefficient |
P value |
|---|---|---|---|
| Baseline | |||
| Age, Years | 0.1(0.1) | 0.03 | 0.6 |
| White | −0.88(0.9) | −0.06 | 0.3 |
| BMI, kg/m2 | −0.001(0.1) | −0.001 | 0.9 |
| SBP, mm Hg | 0.05(0.03) | 0.10 | 0.1 |
| Heart rate per minute | 0.03(0.04) | 0.05 | 0.4 |
| Medication for hypertension | 1.0(1.1) | 0.06 | 0.4 |
| LDL-c, mg/dL | 0.004(0.01) | 0.03 | 0.7 |
| HDL-c, mg/dL | 0.01(0.03) | 0.03 | 0.6 |
| Triglycerides, mg/dL* | 0.7(0.7) | 0.06 | 0.3 |
| Total cholesterol, mg/dL | 0.01(0.01) | 0.06 | 0.3 |
| Medication for lipids | 0.2(1.1) | 0.01 | 0.8 |
| Glucose, mg/dL* | 3.1(2.9) | 0.07 | 0.3 |
| Insulin, uU/mL* | 0.9(0.8) | 0.08 | 0.3 |
| Medication for diabetes | −1.2(3.1) | −0.03 | 0.7 |
| Adiponectin, µg/mL * | −1.8(0.7) | −0.16 | 0.01 |
| PAI-1, ng/mL* | 0.6(0.5) | 0.09 | 0.18 |
| CRP, mg/L* | 0.1(0.4) | 0.02 | 0.8 |
| Fibrinogen, mg/L* | −0.2(1.5) | −0.01 | 0.9 |
|
Smoking status Current Former Never |
0.1(1.1) 0.7(0.9) --- |
0.01 0.05 --- |
0.9 0.4 --- |
|
Alcohol consumption ≥2 times/week |
1.1(0.7) |
0.10 |
0.1 |
| Annual Change | |||
| BMI change, kg/m2 per Year | 1.0(0.8) | 0.08 | 0.2 |
| SBP change, mm Hg per Year | 0.2(0.1) | 0.11 | 0.1 |
| LDL-c change, mg/dL per Year | −0.04(0.05) | −0.06 | 0.3 |
| HDL-c change, mg/dL per Year | −0.08(0.2) | −0.03 | 0.6 |
| Triglycerides change, mg/dL per year* | −0.7(3.4) | −0.01 | 0.8 |
| Glucose change, mg/dL per year* | −1.4(13.0) | −0.007 | 0.9 |
| Insulin change, uU/mL per year* | −0.3(4.2) | −0.005 | 0.9 |
| CRP change, mg/L per year* | −2.8(1.8) | −0.10 | 0.1 |
BMI: body mass index; SBP: systolic blood pressure; LDL-c: low density lipoprotein cholesterol; HDL-c: high density lipoprotein cholesterol; PAI-1: plasminogen activator inhibitor; CRP:C reactive protein; cfPWV: carotid femoral pulse wave velocity.
log-transformed
Multivariable analyses adjusted for age at baseline, race, follow-up time, heart rate, and use of antihypertensive and lipid-lowering medications, revealed that baseline levels of adiponectin, SBP, and alcohol consumption, as well as annual change in SBP to be the main determinants of aortic stiffness progression among apparently healthy middle-aged men, Table 3. The adjusted standardized coefficients indicated that the amount of change in aortic stiffness progression per 1 SD increase in any of these factors is approximately the same. Additional analyses were performed adjusting this final model for BMI, annual change in BMI, LDL, annual change in LDL, and smoking status. Results were similar to model without adjustment (data not shown). Only parsimonious model was presented.
Table 3.
Final Predicted Model for Determinants of Relative Annual Change in cfPWV (% change per Year)
| Variable, increment | β (SE) | Standardized coefficient |
P value |
|---|---|---|---|
| Adiponectin* | −1.8(0.8) | −0.15 | 0.03 |
|
Alcohol consumption ≥2 times/week |
1.6(0. 7) |
0.15 |
0.02 |
| SBP | 0.07(0.03) | 0.15 | 0.03 |
| Change in SBP per Year | 0.3(0.2) | 0.14 | 0.04 |
Adjusted for age at baseline, race, follow-up time, heart rate, use of antihypertensive, and lipid lowering medications. SBP: systolic blood pressure; cfPWV: carotid femoral pulse wave velocity.
log transformed
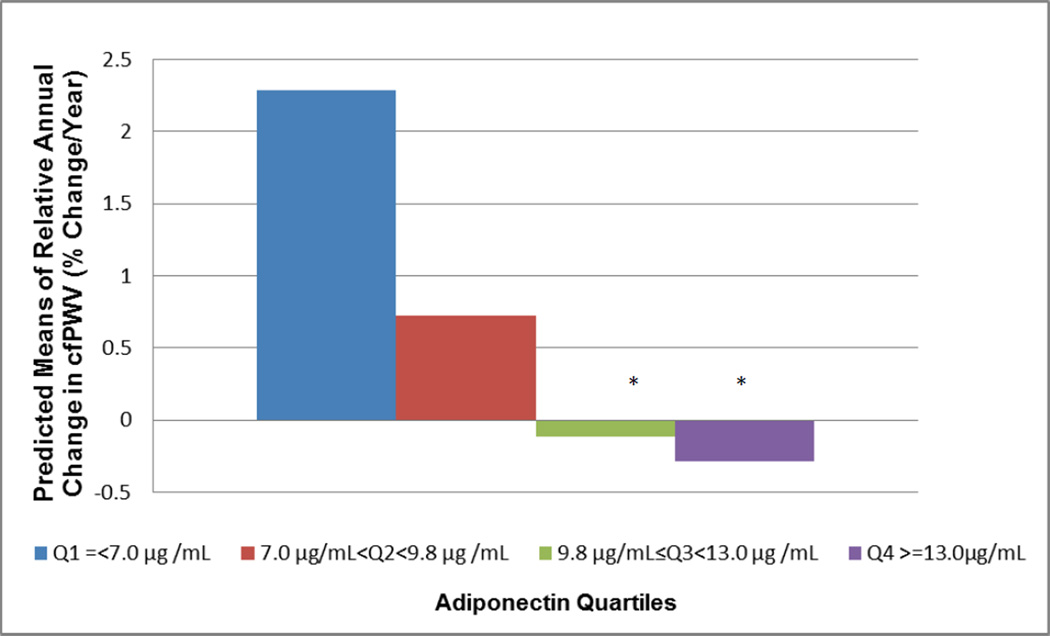
There was a trend for an association between log baseline level of adiponectin and relative annual change in cfPWV (P=0.01). Evaluating relative annual change in cfPWV by quartiles of adiponectin showed a threshold effect (Figure 1). Adiponectin levels within the third (9.8 µg/Ml ≤ adiponectin <13.0 µg /mL) and the fourth (adiponectin ≥ 13.0 µg/mL) quartiles were associated with greater reduction in relative annual aortic stiffnes progression (Q3: 2.4%±1.02% reduction/year, p=0.02; Q4: 2.57%±1.01% reduction/year, p=0.01, respectively) compared to levels within the first quartile (adiponectin ≤ 7.0 µg/mL).
Figure 1. Relative Annual Change in cfPWV by Adiponectin Quartiles.
Adjusted for age at baseline, race, follow-up time, heart rate, use of antihypertensive, lipid lowering medications, alcohol consumption, SBP, annual change in SBP. cfPWV: carotid femoral pulse wave velocity.
* Relative annual changes in cfPWV at Q3 and Q4 significantly differ from that at Q1, P<0.05
† Trend P value =0.01
Discussion
Using a population-based sample of middle-aged apparently healthy men from the ERA JUMP study Allegheny County site, we evaluated several potential risk factors as possible determinants of progression of aortic stiffness, using the gold standard measure, cfPWV, 4,8 over an extended period of time. The results from this study are novel and of great interest given that most of the previous longitudinal studies were conducted among symptomatic participants. 16–21 Independent of aging, follow-up time, race, heart rate, and use of medications for blood pressure and lipids, lower baseline levels of adiponectin, higher baseline levels of SBP, greater annual change in SBP, and baseline level of alcohol consumption ≥2 times/week were all found to be independently associated with greater annual increase (progression) in cfPWV relative to the baseline level. The magnitude of effect for each of these predictors on central arterial stiffness progression was similar, suggesting that each of these factors should be a target for early intervention. Further, the threshold identified in the current study for adiponectin level of ≥9.8 µg/mL underscore the level at which adiponectin appears to be protective against more progression in central arterial stiffness. Baseline levels of adiponectin ≥9.8 µg/mL were found to be associated with a reduction in arterial stiffness overtime among this sample of apparently healthy middle-aged men.
Very few studies have assessed adiponectin as a potential predictor of progression of arterial stiffness. 19,22 Youn et al, reported a similar effect of plasma levels of adiponectin on heart-femoral PWV progression in adjusted analyses among 141 patients with treated essential hypertension followed for 24 months.19 Moreover, Störk et al, evaluated associations between plasma levels of adiponectin and changes in carotid distensibility (a measure of arterial stiffness assessed by high-resolution ultrasound) and intima-media thickness among 142 non-diabetic postmenopausal women after 1 year of follow-up. Lower levels of adiponectin were associated with adverse changes in distensibility and intima media-thickness of the carotid artery independent of other potential risk factors.22 Our finding of a significant effect of baseline level of adiponectin on aortic stiffness progression was in agreement with these two studies19,22 and extend their findings to apparently healthy middle-aged men who were followed for a longer duration of time (maximum of 6 years).
Adiponectin is an adipokine that is predominantly secreted by adipocytes and more abundantly presented in plasma than other adipokines.30,31 Low levels of adiponectin have been reported as an independent risk factor for hypertension development, 32 myocardial infarction in men, 33 ischemic cerebrovascular disease,34 and type 2 diabetes.35 Several mechanisms have been suggested to link adipoenctin with CVD. Adiponectin shows beneficial effects on energy metabolism, insulin action, lipid metabolism, and inflammation.31 It has been found to be synthesized and secreted by human cardiomyocytes.36 Furthermore, studies have shown that adiponectin receptors also exist in endothelial cells 37,38 and cardiomyocytes.36 These findings, suggest a direct effect of adiponectin on cardiovascular tissues. Moreover, adiponectin was proposed as having a direct vasoprotective effect via stimulating endothelial nitric oxide synthase.32,39 Lower levels of adiponectin are associated with greater smooth muscle proliferation after arterial injury. In an in vivo study, severe neointimal thickening and increased proliferation of vascular smooth muscle cells were reported in adiponectin deficient mice. Furthermore, adenovirus-mediated supplementation of adiponectin was found to decrease neointimal proliferation.40 The findings from the current study, as well as from other studies,19,22 highlight adiponectin as an important potential therapeutic target for early intervention of aortic stiffness progression.
We reported a significant positive effect of baseline alcohol consumption ≥2 times/week on aortic stiffness progression among middle aged men. Sierksma et al, found a J-shaped association between alcohol consumption and cfPWV among men aged 40–80 years in a cross-sectional analysis.41 On the other hand, Nakanishi et al, assessed the role of alcohol consumption on the development of increased cfPWV of at least 8.0m/sec during 10,598 person-years of follow-up among 1,358 Japanese men aged 35-59 years. The authors reported a dose-dependent association between alcohol consumption and cfPWV increase; mainly in leaner non-smoker men.42 Our findings of greater aortic stiffness progression among those who are consuming alcohol ≥2times/week are in agreement with Nakanishi et al.42 Large intakes of alcohol are associated with greater LDL oxidation, 43 which in turn may result in greater titers of oxidized LDL antibodies that were found to be associated with accelerated progression of atherosclerosis.44
Given the nature of our alcohol consumption data (self-reported with limited details) we were not able to assess if there is any possible dose-dependent association as was reported by Nakanishi et al.42 Further, there is a possibility that the current analyses may overestimate the relationship between alcohol consumption and aortic stiffness progression given the fact that those who were excluded because of missing for baseline and/or follow-up cfPWV were more likely to be non-drinker or less frequent drinkers (drink alcohol < 2 times/week). Future longitudinal studies should evaluate the association between alcohol consumption and aortic stiffness progression using a more reliable method to collect alcohol consumption data with extensive details about type, frequency and habit.
The current study did not find any significant associations between baseline or annual changes in lipids, BMI, glucose, insulin, and CRP with the progression of central arterial stiffness in final models. This was in line with what has been reported to date.10 Most previous studies that assessed associations between potential risk factors and arterial stiffness, whether cross-sectional or longitudinal, confirmed that blood pressure is associated with greater arterial stiffness. There is some evidence for diabetes to accelerate arterial stiffness while the role of lipids, smoking, and other factors remain unclear.10
The relationship between BMI and arterial stiffness is still under investigation. In a cross-sectional analysis by Zebekakis et al, the authors have reported an increase in arterial stiffness with higher BMI in middle-aged and older women, but not in men of any age.45 A prospective study by Wildman et al, has shown that weight gain was independently associated with an increase in arterial stiffness in young subjects (mean age=30.4 years).23 Birru et al, found larger waist circumference but not BMI to be a significant predictor of PWV progression among middle-aged women.24 Our finding of no significant effect of baseline BMI among middle-aged men on arterial stiffness progression is in agreement with the cross-sectional analysis by Zebekakis et al. 45 The discrepancies between our null findings for baseline and annual change in BMI and those reported by Wildman et al, may be due to differences in statistical methodology (adjusting for baseline level vs. calculating change relative to baseline level) and the younger age group assessed by Wildman et al.23 Since most of the previous prospective studies of the determinants of arterial stiffness progression were conducted among diseased populations, more longitudinal studies are required to evaluate the impact of obesity on arterial stiffness in a larger group of apparently healthy subjects.
We did not find any effect of either baseline measures or annual changes in lipid makers on arterial stiffness progression among middle aged men. The effect of serum lipids on arterial stiffness remains unclear.3 In both studies among participants with familial hypercholesterolemia 46,47 and those among general population,13,14 the associations between serum lipids and arterial stiffness (assessed as PWV, compliance, or distensibility) are not consistent; with studies reporting positive,47,14 negative ,46 or null associations.11,48
Certain aspects of the current study need to be considered when interpreting our findings. The current study only included middle-aged men (baseline age: 40–49 years), and therefore, our findings may not be generalizable to women or other age groups. We did not have both baseline and follow-up cfPWV on 177 of the study participants, and this may introduce selection bias, however, statistical testing showed that those who were excluded due to missing the main study outcome were not significantly different from those who were included in baseline measures of cfPWV, age, SBP, heart rate, total cholesterol, HDL-c, LDL-c, insulin, adiponectin, PAI-1, smoking status and medication use. We did not measure adiponectin at follow-up time, and therefore, we were not able to evaluate if a change in adiponectin over time would have a similar effect to baseline adiponectin on the progression of aortic stiffness. Although we did not measure high-molecular weight form of adiponectin, which has been reported as the most active form of adiponectin,30 we as well as others 19,22 were able to report a significant effect of total measure of adiponectin. The major strengths of the current study include: 1) Evaluating determinants of arterial stiffness using cfPWV in a population-based sample of apparently healthy middle-aged men followed for a maximum of 6 years; 2) Assessing the potential role of several risk factors, both as baseline measures and as annual changes since baseline, on aortic stiffness progression.
In conclusion, lower levels of adiponectin, higher levels of SBP, greater annual change in SBP, and alcohol consumption ≥2 times/week were found to be independently associated with greater progression in aortic stiffness among relatively healthy middle-aged US men. We confirmed the potential impact of greater SBP and changes in SBP on progression of aortic stiffness. We reported for the first time, among apparently healthy subjects, that lower levels of baseline adiponectin could be a novel marker for greater risk of aortic stiffness progression overtime. This underscores the importance of further investigation into the role of adiponectin as a potential prophylactic therapy for vascular changes. There is a strong negative association between plasma levels of adiponectin and fat mass. Adiponectin decreases with obesity and increases during weight reduction.49,50 Therefore, weight loss intervention could be one potential method to increase adiponectin to levels that may reduce aortic stiffness progression. The finding of alcohol consumption as a determinant of aortic stiffness progression warrants more investigation to assess if there is a specific dose-dependent effect on central arterial stiffness.
Acknowledgments
Funding Sources
This research was supported by grants R01 HL68200 and HL071561 from the National Institutes of Health as well as B 16790335 and A 13307016, 17209023, and 21249043 from the Japanese Ministry of Education, Culture, Sports, Science and Technology.
Footnotes
El Khoudary Determinants of Aortic Stiffness Progression
Disclosures: The authors declare no conflict of interest.
References
- 1.Mattace-Raso FUS, van der Cammen TJM, Hofman A, van Popele NM, Bos ML, Schalekamp MADH, Asmar R, Reneman RS, Hoeks APG, Breteler MMB, Witteman JCM. Arterial stiffness and risk of coronary heart disease and stroke: the Rotterdam Study. Circulation. 2006;113:657–663. doi: 10.1161/CIRCULATIONAHA.105.555235. [DOI] [PubMed] [Google Scholar]
- 2.Willum Hansen T, Staessen JA, Torp-Pedersen C, Rasmussen S, Thijs L, Ibsen H, Jeppesen J. Prognostic value of aortic pulse wave velocity as index of arterial stiffness in the general population. Circulation. 2006;113:664–670. doi: 10.1161/CIRCULATIONAHA.105.579342. [DOI] [PubMed] [Google Scholar]
- 3.Cecelja M, Chowienczyk P. Dissociation of aortic pulse wave velocity with risk factors for cardiovascular disease other than hypertension: a systematic review. Hypertension. 2009;54:1328–1336. doi: 10.1161/HYPERTENSIONAHA.109.137653. [DOI] [PubMed] [Google Scholar]
- 4.Safar ME, Levy BI, Struijker-Boudier H. Current perspectives on arterial stiffness and pulse pressure in hypertension and cardiovascular diseases. Circulation. 2003;107:2864–2869. doi: 10.1161/01.CIR.0000069826.36125.B4. [DOI] [PubMed] [Google Scholar]
- 5.O'Rourke MF, Hashimoto J. Mechanical factors in arterial aging: a clinical perspective. J Am Coll Cardiol. 2007;50:1–13. doi: 10.1016/j.jacc.2006.12.050. [DOI] [PubMed] [Google Scholar]
- 6.Sutton-Tyrrell K, Mackey RH, Holubkov R, Vaitkevicius PV, Spurgeon HA, Lakatta EG. Measurement variation of aortic pulse wave velocity in the elderly. Am J Hypertens. 2001;14:463–468. doi: 10.1016/s0895-7061(00)01289-9. [DOI] [PubMed] [Google Scholar]
- 7.Laurent S, Cockcroft J, Van Bortel L, Boutouyrie P, Giannattasio C, Hayoz D, Pannier B, Vlachopoulos C, Wilkinson I, Struijker-Boudier H. European Network for Non-invasive Investigation of Large Arteries. Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J. 2006;27:2588–2605. doi: 10.1093/eurheartj/ehl254. [DOI] [PubMed] [Google Scholar]
- 8.Asmar R, Benetos A, Topouchian J, Laurent P, Pannier B, Brisac AM, Target R, Levy BI. Assessment of arterial distensibility by automatic pulse wave velocity measurement. Validation and clinical application studies. Hypertension. 1995;26:485–490. doi: 10.1161/01.hyp.26.3.485. [DOI] [PubMed] [Google Scholar]
- 9.Reference Values for Arterial Stiffness' Collaboration. Determinants of pulse wave velocity in healthy people and in the presence of cardiovascular risk factors: 'establishing normal and reference values'. Eur Heart J. 2010;31:2338–2350. doi: 10.1093/eurheartj/ehq165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Benetos A, Waeber B, Izzo J, Mitchell G, Resnick L, Asmar R, Safar M. Influence of age, risk factors, and cardiovascular and renal disease on arterial stiffness: clinical applications. Am J Hypertens. 2002;15:1101–1108. doi: 10.1016/s0895-7061(02)03029-7. [DOI] [PubMed] [Google Scholar]
- 11.Taquet A, Bonithon-Kopp C, Simon A, Levenson J, Scarabin Y, Malmejac A, Ducimetiere P, Guize L. Relations of cardiovascular risk factors to aortic pulse wave velocity in asymptomatic middle-aged women. Eur J Epidemiol. 1993;9:298–306. doi: 10.1007/BF00146267. [DOI] [PubMed] [Google Scholar]
- 12.Sa Cunha R, Pannier B, Benetos A, Siché JP, London GM, Mallion JM, Safar ME. Association between high heart rate and high arterial rigidity in normotensive and hypertensive subjects. J Hypertens. 1997;15:1423–1430. doi: 10.1097/00004872-199715120-00009. [DOI] [PubMed] [Google Scholar]
- 13.Lehmann ED, Hopkins KD, Parker JR, Gosling RS. Hyperlipidaemia, hypertension, and coronary heart disease. Lancet. 1995;345:863. [PubMed] [Google Scholar]
- 14.Wang F, Ye P, Luo L, Xiao W, Qi L, Bian S, Wu H, Sheng L, Xiao T, Xu R. Association of serum lipids with arterial stiffness in a population-based study in Beijing. Eur J Clin Invest. 2011;41:929–936. doi: 10.1111/j.1365-2362.2011.02481.x. [DOI] [PubMed] [Google Scholar]
- 15.Stefanadis C, Tsiamis E, Vlachopoulos C, Stratos C, Toutouzas K, Pitsavos C, Marakas S, Boudoulas H, Toutouzas P. Unfavorable effect of smoking on the elastic properties of the human aorta. Circulation. 1997;95:31–38. doi: 10.1161/01.cir.95.1.31. [DOI] [PubMed] [Google Scholar]
- 16.Benetos A, Adamopoulos C, Bureau JM, Temmar M, Labat C, Bean K, Thomas F, Pannier B, Asmar R, Zureik M, Safar M, Guize L. Determinants of accelerated progression of arterial stiffness in normotensive subjects and in treated hypertensive subjects over a 6-year period. Circulation. 2002;105:1202–1207. doi: 10.1161/hc1002.105135. [DOI] [PubMed] [Google Scholar]
- 17.Safar ME, Thomas F, Blacher J, Nzietchueng R, Bureau JM, Pannier B, Benetos A. Metabolic syndrome and age-related progression of aortic stiffness. J Am Coll Cardiol. 2006;47:72–75. doi: 10.1016/j.jacc.2005.08.052. [DOI] [PubMed] [Google Scholar]
- 18.Chen SC, Chang JM, Liu WC, Wang CS, Su HM, Chen HC. The Longitudinal Change of Arterial Stiffness in Patients With Chronic Kidney Disease. Am J Med Sci. 2011;343:109–113. doi: 10.1097/MAJ.0b013e318223e814. Jul 28. 2012. [DOI] [PubMed] [Google Scholar]
- 19.Youn JC, Kim C, Park S, Lee SH, Kang SM, Choi D, Son NH, Shin DJ, Jang Y. Adiponectin and progression of arterial stiffness in hypertensive patients. Int J Cardiol. 2011 Jul 1; doi: 10.1016/j.ijcard.2011.06.061. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 20.Matsumae T, Ueda K, Abe Y, Nishimura S, Murakami G, Saito T. What factors accelerate aortic stiffening in hemodialysis patients? An observational study. Hypertens Res. 2010;33:243–249. doi: 10.1038/hr.2009.219. [DOI] [PubMed] [Google Scholar]
- 21.Jung JY, Hwang YH, Lee SW, Lee H, Kim DK, Kim S, Oh YG, Yang J, Joo KW, Ahn C, Oh KH. Factors associated with aortic stiffness and its change over time in peritoneal dialysis patients. Nephrol Dial Transplant. 2010;25:4041–4048. doi: 10.1093/ndt/gfq293. [DOI] [PubMed] [Google Scholar]
- 22.Störk S, Bots ML, Angerer P, von Schacky C, Grobbee DE, Angermann CE, Seufert J. Low levels of adiponectin predict worsening of arterial morphology and function. Atherosclerosis. 2007;194:e147–e153. doi: 10.1016/j.atherosclerosis.2006.11.044. [DOI] [PubMed] [Google Scholar]
- 23.Wildman RP, Farhat GN, Patel AS, Mackey RH, Brockwell S, Thompson T, Sutton-Tyrrell K. Weight change is associated with change in arterial stiffness among healthy young adults. Hypertension. 2005;45:187–192. doi: 10.1161/01.HYP.0000152200.10578.5d. [DOI] [PubMed] [Google Scholar]
- 24.Birru MS, Matthews KA, Thurston RC, Brooks MM, Ibrahim S, Barinas-Mitchell E, Janssen I, Sutton-Tyrrell K. SWAN Heart Study. African-American ethnicity and cardiovascular risk factors are related to aortic pulse-wave velocity progression. Am J Hypertens. 2011;24:809–815. doi: 10.1038/ajh.2011.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sekikawa A, Curb JD, Ueshima H, El-Saed A, Kadowaki T, Abbott RD, Evans RW, Rodriguez BL, Okamura T, Sutton-Tyrrell K, Nakamura Y, Masaki K, Edmundowicz D, Kashiwagi A, Willcox BJ, Takamiya T, Mitsunami K, Seto TB, Murata K, White RL, Kuller LH ERA JUMP (Electron-Beam Tomography, Risk Factor Assessment Among Japanese and U.S. Men in the Post-World War II Birth Cohort) Study Group. Marine-derived n-3 fatty acids and atherosclerosis in Japanese, Japanese-American, and white men: a cross-sectional study. J Am Coll Cardiol. 2008;52:417–424. doi: 10.1016/j.jacc.2008.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sekikawa A, Ueshima H, Kadowaki T, El-Saed A, Okamura T, Takamiya T, Kashiwagi A, Edmundowicz D, Murata K, Sutton-Tyrrell K, Maegawa H, Evans RW, Kita Y, Kuller LH. Less subclinical atherosclerosis in Japanese men in Japan than in white men in the United States in the post-World War II birth cohort. Am J Epidemiol. 2007;165:617–624. doi: 10.1093/aje/kwk053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Declerck PJ, Alessi MC, Verstreken M, Kruithof EK, Juhan-Vague I, Collen D. Measurement of plasminogen activator inhibitor 1 in biologic fluids with a murine monoclonal antibody-based enzyme-linked immunosorbent assay. Blood. 1988;71:220–225. [PubMed] [Google Scholar]
- 28.Tanaka H, Munakata M, Kawano Y, Ohishi M, Shoji T, Sugawara J, Tomiyama H, Yamashina A, Yasuda H, Sawayama T, Ozawa T. Comparison between carotid-femoral and brachial-ankle pulse wave velocity as measures of arterial stiffness. J Hypertens. 2009;27:2022–2027. doi: 10.1097/HJH.0b013e32832e94e7. [DOI] [PubMed] [Google Scholar]
- 29.Glymour MM, Weuve J, Berkman LF, Kawachi I, Robins JM. When is baseline adjustment useful in analyses of change? An example with education and cognitive change. Am J Epidemiol. 2005;162:267–278. doi: 10.1093/aje/kwi187. [DOI] [PubMed] [Google Scholar]
- 30.Szmitko PE, Teoh H, Stewart DJ, Verma S. Adiponectin and cardiovascular disease: state of the art? Am J Physiol Heart Circ Physiol. 2007;292:H1655–H1663. doi: 10.1152/ajpheart.01072.2006. [DOI] [PubMed] [Google Scholar]
- 31.Harwood HJ., Jr The adipocyte as an endocrine organ in the regulation of metabolic homeostasis. Neuropharmacology. 2011 Dec 17; doi: 10.1016/j.neuropharm.2011.12.010. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 32.Chow WS, Cheung BM, Tso AW, Xu A, Wat NM, Fong CH, Ong LH, Tam S, Tan KC, Janus ED, Lam TH, Lam KS. Hypoadiponectinemia as a predictor for the development of hypertension: a 5-year prospective study. Hypertension. 2007;49:1455–1461. doi: 10.1161/HYPERTENSIONAHA.107.086835. [DOI] [PubMed] [Google Scholar]
- 33.Pischon T, Girman CJ, Hotamisligil GS, Rifai N, Hu FB, Rimm EB. Plasma adiponectin levels and risk of myocardial infarction in men. JAMA. 2004;291:1730–1737. doi: 10.1001/jama.291.14.1730. [DOI] [PubMed] [Google Scholar]
- 34.Chen MP, Tsai JC, Chung FM, Yang SS, Hsing LL, Shin SJ, Lee YJ. Hypoadiponectinemia is associated with ischemic cerebrovascular disease. Arterioscler Thromb Vasc Biol. 2005;25:821–826. doi: 10.1161/01.ATV.0000157784.25920.a7. [DOI] [PubMed] [Google Scholar]
- 35.Lindsay RS, Funahashi T, Hanson RL, Matsuzawa Y, Tanaka S, Tataranni PA, Knowler WC, Krakoff J. Adiponectin and development of type 2 diabetes in the Pima Indian population. Lancet. 2002;360:57–58. doi: 10.1016/S0140-6736(02)09335-2. [DOI] [PubMed] [Google Scholar]
- 36.Pineiro R, Iglesias MJ, Gallego R, Raghay K, Eiras S, Rubio J, Dieguez C, Gualillo O, Gonzalez-Juanatey JR, Lago F. Adiponectin is synthesized and secreted by human and murine cardiomyocytes. FEBS Lett. 2005;579:5163–5169. doi: 10.1016/j.febslet.2005.07.098. [DOI] [PubMed] [Google Scholar]
- 37.Motoshima H, Wu X, Mahadev K, Goldstein BJ. Adiponectin suppresses proliferation and superoxide generation and enhances eNOS actvity in endothelial cells treated with oxidized LDL. Biochem Biophys Res Commun. 2004;315:264–271. doi: 10.1016/j.bbrc.2004.01.049. [DOI] [PubMed] [Google Scholar]
- 38.Tan KC, Xu A, Chow WS, Lam MC, Ai VH, Tam SC, Lam KS. Hypoadiponectinemia is associated with impaired endothelium-dependent vasodilation. J Clin Endocrinol Metab. 2004;89:765–769. doi: 10.1210/jc.2003-031012. [DOI] [PubMed] [Google Scholar]
- 39.Chen H, Montagnani M, Funahashi T, Shimomura I, Quon MJ. Adiponectin stimulates production of nitric oxide in vascular endothelial cells. J Biol Chem. 2003;278:45021–45026. doi: 10.1074/jbc.M307878200. [DOI] [PubMed] [Google Scholar]
- 40.Matsuda M, Shimomura I, Sata M, Arita Y, Nishida M, Maeda N, Kumada M, Okamoto Y, Nagaretani H, Nishizawa H, Kishida K, Komuro R, Ouchi N, Kihara S, Nagai R, Funahashi T, Matsuzawa Y. Role of adiponectin in preventing vascular stenosis. The missing link of adipo-vascular axis. J Biol Chem. 2002;277:37487–37491. doi: 10.1074/jbc.M206083200. [DOI] [PubMed] [Google Scholar]
- 41.Sierksma A, Muller M, van der Schouw YT, Grobbee DE, Hendriks HF, Bots ML. Alcohol consumption and arterial stiffness in men. J Hypertens. 2004;22:357–362. doi: 10.1097/00004872-200402000-00020. [DOI] [PubMed] [Google Scholar]
- 42.Nakanishi N, Yoshida H, Kawashimo H, Suzuki K, Nakamura K, Tatara K. Alcohol consumption and risk for increased aortic pulse wave velocity in middle-aged Japanese men. Angiology. 2001;52:533–542. doi: 10.1177/000331970105200805. [DOI] [PubMed] [Google Scholar]
- 43.Altomare E, Grattagliano I, Vendemiale G, Palmieri V, Palasciano G. Acute ethanol administration induces oxidative changes in rat pancreatic tissue. Gut. 1996;38:742–746. doi: 10.1136/gut.38.5.742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Salonen JT, Ylä-Herttuala S, Yamamoto R, Butler S, Korpela H, Salonen R, Nyyssönen K, Palinski W, Witztum JL. Autoantibody against oxidised LDL and progression of carotid atherosclerosis. Lancet. 1992;339:883–887. doi: 10.1016/0140-6736(92)90926-t. [DOI] [PubMed] [Google Scholar]
- 45.Zebekakis PE, Nawrot T, Thijs L, Balkestein EJ, van der Heijden-Spek J, Van Bortel LM, Struijker-Boudier HA, Safar ME, Staessen JA. Obesity is associated with increased arterial stiffness from adolescence until old age. J Hypertens. 2005;23:1839–1846. doi: 10.1097/01.hjh.0000179511.93889.e9. [DOI] [PubMed] [Google Scholar]
- 46.Lehmann ED, Watts GF, Fatemi-Langroudi B, Gosling RG. Aortic compliance in young patients with heterozygous familial hypercholesterolaemia. Clin Sci (Lond) 1992;83:717–721. doi: 10.1042/cs0830717. [DOI] [PubMed] [Google Scholar]
- 47.Lehmann ED, Watts GF, Gosling RG. Aortic distensibility and hypercholesterolaemia. Lancet. 1992;340(8828):1171–1172. doi: 10.1016/0140-6736(92)93210-e. [DOI] [PubMed] [Google Scholar]
- 48.Czernichow S, Bertrais S, Blacher J, Oppert JM, Galan P, Ducimetière P, Hercberg S, Safar M, Zureik M. SU.VI.MAX. Vascular Study. Metabolic syndrome in relation to structure and function of large arteries: a predominant effect of blood pressure. A report from the SU.VI.MAX. Vascular Study. Am J Hypertens. 2005;18:1154–1160. doi: 10.1016/j.amjhyper.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 49.Yang WS, Lee WJ, Funahashi T, Tanaka S, Matsuzawa Y, Chao CL, Chen CL, Tai TY, Chuang LM. Weight reduction increases plasma levels of an adipose-derived anti-inflammatory protein, adiponectin. J Clin Endocrinol Metab. 2001;86:3815–3819. doi: 10.1210/jcem.86.8.7741. [DOI] [PubMed] [Google Scholar]
- 50.Matsubara M, Maruoka S, Katayose S. Inverse relationship between plasma adiponectin and leptin concentrations in normal-weight and obese women. Eur J Endocrinol. 2002;147:173–180. doi: 10.1530/eje.0.1470173. [DOI] [PubMed] [Google Scholar]