Abstract
Advanced dementia is characterized by the onset of infections and antimicrobial use is extensive. The extent to which this antimicrobial use is appropriate and contributes to the emergence of antimicrobial resistant bacteria is not known. The object of this report is to present the methodology established in the Study of Pathogen Resistance and Exposure to Antimicrobials in Dementia (SPREAD), and describe how challenges specific to this research were met. SPREAD is an ongoing, federally-funded, 5-year prospective cohort study initiated in September 2009. Subjects include nursing home residents with advanced dementia and their proxies recruited from 31 Boston-area facilities. The recruitment and data collection protocols are described. Characteristics of participant facilities are presented and compared to those nationwide. To date, 295 resident/proxy dyads have been recruited. Baseline and selected follow-up data demonstrate successful recruitment of subjects and repeated collection of complex data documenting infections, decision-making for these infections, and antimicrobial bacteria resistance among the residents. SPREAD integrates methods in dementia, palliative care and infectious diseases research. Its successful implementation further establishes the feasibility of conducting rigorous, multi-site NH research in advanced dementia, and the described methodology serves as a detailed reference for subsequent publications emanating from the study.
Keywords: dementia, palliative care, infections, nursing home, methodology
Advanced dementia is the sixth leading cause of death in the United States.1 Emerging data suggests that their end-of-life experience is not optimal.2–7 Improving care in advanced dementia has been identified as a research priority.8
Advanced dementia is characterized by the onset of infections and antimicrobial use is extensive.9 Approximately 40% of nursing home (NH) residents with advanced dementia receive an antimicrobial in the last two weeks of life. However, it remains unclear as to whether antimicrobial treatment confers any life-prolonging or symptomatic benefit in these terminally ill patients, for whom the goal of care is often palliation.7, 10 Prior research suggests substantial antimicrobial misuse in NHs,11–15 however this issue has never been specifically examined among residents with advanced dementia.
Antimicrobial use is a main factor leading to the emergence of antimicrobial-resistant bacteria (ARB); a critical public health problem. Growing concern has focused on the increase of ARB in NHs,16, 17 where it is estimated that up to 60% of residents are colonized with at least one ARB.18, 19 NH residents contribute to the influx of ARB into hospitals.20–22 Our prior work has shown that ARB colonization rates among NH residents with advanced dementia are 3 times higher than those of other residents.23 Taken together, antimicrobial misuse in advanced dementia raises concerns not only from the perspective of individual benefits and burdens near the end-of-life, but also from a public health standpoint with respect to the emergence of ARB.
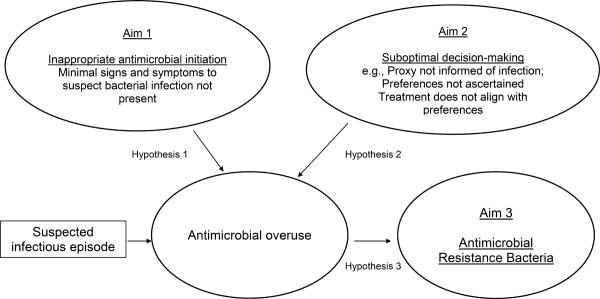
With this foundation, in 2009, the National Institutes of Health (NIH) funded a 5 year study entitled: the Study of Pathogen Resistance and Exposure to Antimicrobials in Dementia (SPREAD). The over-riding goals of SPREAD were to conduct a prospective cohort study to examine antimicrobial exposure in NH residents with advanced dementia and further our understanding of how that exposure contributes to antimicrobial resistance. The specific aims were: 1. To describe the occurrence and management of suspected infectious episodes, and identify potentially inappropriate antimicrobial initiation based on consensus guidelines (e.g., lack of adequate signs and symptoms of an infection); 2. To assess decision-making by health care proxies for the management of infections; and 3. To describe and identify factors associated with ARB colonization (prevalence, acquisition, loss, and persistence). The hypotheses underlying these aims (Figure 1), were that we would detect a high rate of inappropriate antimicrobial use (Aim 1) and suboptimal proxy decision-making (Aim 2) (e.g., proxy not informed or counseled about infections, treatment does not align with goals of care), both of which would contribute to the misuse of antimicrobials and subsequently the emergence of ARB (Aim 3).
Figure 1.
SPREAD Aims and Hypotheses
SPREAD's unique design leveraged considerable research infrastructure and expertise in the areas of advanced dementia, palliative care, and infectious diseases in the NH setting.7, 9, 23–26 The objective of this report is to describe the detailed methodology of SPREAD and the challenges and successes implementing that methodology to date. This report will serve as a resource for others embarking on this field of investigation, and for future publications that will emerge from the findings of SPREAD.
METHODS
The conduct of this study was approved by the Institutional Review Board (IRB) at Hebrew SeniorLife.
Study Facilities
Participant facilities were a convenience sample of NHs that had greater than 45 beds and were located within a 60-mile radius of metropolitan Boston. For descriptive purposes, data were collected characterizing the NHs organizational structure, staffing, and quality of care markers relevant to advanced dementia and infection management. Data obtained from the LTCFocUS.org website27 included: number of beds; for-profit status; whether the facility was part of a chain; proportion of white residents; registered nurse, licensed nurse and certified nurse assistant hours per resident per day; whether the facility had a special care dementia unit; whether the facility employed a physician assistant or nurse practitioner; and proportion of residents with do-not-resuscitate orders. Quality of care measures obtained from the Medicare NH Compare website,28 included: the number of health deficiencies on the most recent state inspection; and the proportion of long-stay residents with pressure ulcers, foley catheters, urinary tract infections, and pneumococcal and influenza vaccinations. Several variables were unavailable from two facilities which were licensed as hospitals rather than NHs. For comparative purposes, characteristics were also obtained for NHs nationwide.
Study Population
The study population is composed of two groups (dyads); NH residents with advanced dementia and their health care proxies. Resident eligibility criteria included the following: 1. ≥ 65 years, 2. length of NH stay > 30 days, 3. dementia (any type), 4. Global Deterioration Scale (GDS) equal to 7,29 and 5. formally or informally appointed proxy was available and could communicate in English. Residents in short-term rehabilitation units were excluded. GDS stage 7 is distinguished by the following features: very severe cognitive dysfunction (cannot recognize close family members), verbal ability limited to < 5 words, incontinence of urine and stool, and loss of basic psychomotor skills (e.g., ability to walk).
At the time of initial NH recruitment and every 3 months thereafter, research assistants asked nurses on each NH unit to identify residents with dementia who were at GDS stage 7. A diagnosis of dementia was confirmed by chart review. Proxies of eligible residents were mailed written information and telephoned two weeks later to solicit their participation and obtain informed consent for themselves and the residents.
Resident Assessments
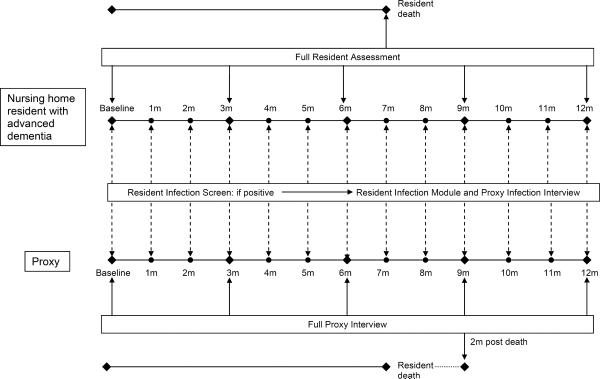
Figure 2 presents a schematic illustration of the data collection protocol and Table 1 describes the data elements. Resident data were collected for up to 12 months and obtained from the NH charts, nursing interviews, cognitive examination, and nasal and rectal swabs. There were three types of resident assessments: full assessments, infection screens, and infection modules. Full assessments were conducted at baseline, quarterly, and within 14 days of death. Demographic information and comorbid conditions were abstracted from the charts at baseline. Demographic data included: age, gender, race, ethnicity, education, marital status, length of NH stay, and whether they were in a special care dementia unit. The following data were collected at each full assessment to reflect the residents' status during the interval between assessments or in the 30 days prior to study enrollment in the case of the baseline assessment: daily oral medications, advance directives, use of devices (feeding tubes, foley catheters, and parenteral therapy), health care utilization, pressure ulcers (stage I–IV), new major acute illnesses (e.g., gastrointestinal bleeds, fractures, strokes), weight, and laboratory data. Advance directives included the following orders: (do-not-resuscitate (DNR), do-not-hospitalize (DNH), withhold parenteral hydration, withhold parenteral and/or oral antimicrobials, and no tube-feeding. Health care utilization included hospitalizations, emergency room visits, intensive care unit admissions, and hospice enrollment. If available, the most recent hematocrit, serum albumin, and creatinine were ascertained. At baseline and quarterly assessments, nurses were interviewed to quantify the residents' functional status using the Bedford Alzheimer's Nursing Severity-Subscale (BANS-S) (range 7–28; higher scores indicate greater disability).30 A brief resident cognitive examination was conducted at baseline only using the Test for Severe Impairment (TSI) (range 0–24, lower scores indicate greater impairment).31 If the resident died, the date and location of death were obtained from the record.
Figure 2.
SPREAD study design
Table 1.
SPREAD Data Collection Elements
| Data Element | From | When |
|---|---|---|
|
| ||
| Resident Full Assessment | ||
|
| ||
| Demographics | Chart | Baseline only |
| Comorbid conditions | Chart | |
| Cognitive Status | Resident | |
|
| ||
| Daily oral medications | Chart | Baseline, 3m, 6m, 9m, 12m, Death |
| Advance directives | Chart | |
| Devices | Chart | |
| Health care utilization | Chart | |
| Pressure ulcers | Chart, Nurse | |
| Acute Illnesses | Chart | |
| Weight | Chart | |
| Laboratory data | Chart | |
| Functional Status | Nurse | |
|
| ||
| Rectal and nasal swabs | Resident | Baseline, 3m, 6m, 9m, 12m only |
|
| ||
| Resident infection screen | ||
|
| ||
| Suspected infection | Chart | Baseline, qmonth, death |
| Antimicrobial use | ||
|
| ||
| Resident Infection module | ||
|
| ||
| Suspected source | Chart | At baseline, qmonth, and death if infection screen detects an infectious episode. |
| Antimicrobial use | ||
| Hospital transfer | ||
| Signs and symptoms | ||
| Investigations | ||
| Proxy/provider discussion | ||
| Physician/physician extender examination | ||
|
| ||
| Proxy Full Interviews | ||
|
| ||
| Demographics | Telephone interview | Baseline, 3m, 6m, 9m, 12m, 2 month post-death |
| Advance care planning | ||
| Communication with providers | ||
|
| ||
| Proxy Infection Interviews | ||
|
| ||
| Aware of infection | Telephone interview | At baseline, qmonth, and 2-month post death if infection screen detects an infectious episode. |
| Knowledge of infection management | ||
| Participation in decision-making | ||
| Decision Satisfaction Index | ||
Rectal and nasal specimens were collected from the resident at the baseline and quarterly assessments by trained research nurses to assess colonization with methicillin resistant staphylococcus aureus (MRSA), vancomycin-resistant enterococci (VRE), and multidrug-resistant gram-negative bacteria (MDRGN). MDRGN resistance was defined as resistance to ≥ 3 of the following: ampicillin/sulbactam or piperacillin/tazobactam, ceftriaxone or ceftazidime, ciprofloxacin, gentamicin, meropenem.32 Colonization was defined as the recovery of at least one ARB at one or more sites. Nasal specimens were obtained by rotating a cotton swab (Culturette II™, Becton-Dickinson, Cockeysville, MD) in each anterior nares 5 times. Rectal specimens were obtained by swabbing the perianal area. To minimize resident and staff burden, all swabbing procedures were conducted early in the morning while residents were still in bed. Specimens were couriered to a microbiology laboratory where they were frozen at −70°C prior to processing. To process specimens, swabs were plated onto selective media that allowed growth of only those ARBs under investigation. Species identification, using Microscan (Baxter, Parsipanny, NJ) and susceptibility testing, was performed as per the Clinical and Laboratory Standards Institute methodology.33, 34 To determine if there was resident-to-resident spread of ARB, the clonal relatedness of isolates was examined using pulsed field gel electrophoresis which generated DNA fingerprint profiles of individual ARB strains,35 that were interpreted using the criteria of Tenover et al.36
At the baseline, monthly, and death assessments, the residents' charts were reviewed to determine if a suspected infectious episode occurred over the prior 30 days (or since the last screen prior to death) defined by any of the following: 1. documentation by a physician, physician assistant, or nurse that an infection was suspected based on clinical signs or symptoms (e.g., cough, mental status change); 2. antimicrobial use; or 3. a recorded temperature > 37.9 °C (by any method). If a suspected infectious episode occurred, one of the following infection modules was completed depending on the suspected source: urinary tract module, respiratory tract module, skin module, or febrile episode of unknown source module. For each module, the following details about each episode were ascertained from the record: i. antimicrobial use, ii. hospital transfer, iii. signs and symptoms including: vital signs, mental status changes, localized signs (e.g., skin rubor, lung auscultation, hematuria), and localized symptoms (e.g., cough, pain), iv.. laboratory or radiological evidence of infection if obtained (e.g., urinalysis, cultures, x-rays, white blood cell counts,), v. whether or not discussion between providers and proxies was documented , and vi. documentation and timing of a physician, nurse practitioner or physician assistant visit to evaluate the resident.
Details of all antimicrobial exposure were determined during the monthly infection screens. A separate antimicrobial course was considered to have started after a 3-day interval free of any antimicrobial. For each course, the following data were collected: suspected diagnosis, agent, dose, route, and duration. Additional details were collected regarding the initial order to start antimicrobials including: prescribing practitioner (physician, nurse practitioner, or physician assistant), mode (telephone vs. in-person order), and time of day.
Proxy Data
All proxy data were acquired by telephone interviews. There were two types of proxy interviews: full interviews and infection interviews. Full assessments were conducted at baseline, quarterly for up to 12 months, and 2-months after a resident's death. At the baseline interview, the following proxy data were obtained: age, gender, race, ethnicity, primary language, education, household income, marital status, work status, relation to the resident, number of years as proxy, and number of hours spent visiting the resident each week. At the baseline, quarterly, and 2-month post-death interviews, proxies were asked questions related to advance care planning and communication with NH providers. At the post-death proxy interview, proxies were instructed to report their experiences relative to the last month of the resident's life. Advance care planning questions included: the extent to which proxies felt comfort was the primary goal of care, the extent to which they felt life prolongation was the primary goal of care, whether they perceived dementia is a terminal condition, and how close they felt the resident was to the end of life. Specifically related to infections, proxies were asked whether they thought infections were expected in advanced dementia, and whether they have made any prior decisions and/or formal directives related to the treatment of infections. With respect to communication, proxies were asked whether a NH primary care provider ever did the following: provided counseling about resident's prognosis, elicited the proxies' opinion regarding goals of care, explained the expected health problems in advanced dementia (including infections), described treatment options for infections, explained the risks and benefits of antimicrobials, or solicited their preferences regarding the use of antimicrobials. Proxies were also asked to rate their own health as excellent, very good, good, fair, or poor.
If a suspected infection was detected on the monthly resident infection screens, an attempt was made to contact the proxy by telephone within 8 weeks of the infection. At these infection interviews, proxies were asked whether they were aware the resident recently had a suspected infection. If unaware, no further questions were asked. If the proxy was aware, they were asked about their knowledge of the following: i. suspected source of infection, ii. treatment received, iii. whether the resident was hospitalized, and iv. whether a NH provider informed them about the episode, and if so, who (e.g., physician, nurse), at what time of day, and by what mode (telephone or in-person), and v. whether they proxy participated in a treatment decision for the infection. If the proxy did not participate in decision-making, they were asked if they had wanted to do so. If they did participate in the treatment decision, the following additional questions were asked: did a NH provider explain the treatment options, and if yes, what options were presented, and were the risks and benefits of each option explained. Finally, the Decision Satisfaction Inventory (DSI) was administered to all proxies who participated in a treatment decision for the episode (range, 20–100, higher scores indicate greater satisfaction).37
Defining appropriate antimicrobial initiation
Using data collected on the infection modules, we wanted to categorize whether antimicrobials were initiated appropriately for each episode (e.g., minimal signs and symptoms were present to suggest a bacterial infection). The determination of whether or not minimal clinical criteria for antimicrobial initiation were met were based on consensus guidelines developed for the NH setting by the Society for Healthcare Epidemiology of America (SHEA) for suspected urinary tract, respiratory tract, and skin infections, as well as febrile episodes.38 Operationalization of the guidelines using SPREAD data, with slight adaptations for residents with advanced dementia, is presented in Table 2.
Table 2.
Minimal criteria for initiation of antimicrobials use for a suspected infections in nursing home residents with advanced dementia
| Suspected urinary tract infection | Suspected lower respiratory tract infection | Suspected skin infection | Febrile episode |
|---|---|---|---|
|
| |||
|
a. No indwellina folev catheter Acute dysuria alone OR Temperature>37.9°C AND ≥ 1 of following2:
≥ 1 of following:
|
a. Temperature >38.9°C ≥ 1 of following:
New productive cough AND ≥ 1 of the following:
New/increased cough with purulent sputum |
New or increased purulent drainage OR ≥ 1 of following2:
|
Temperature>37.9°C AND ≥ 1 of following: |
Diagnostic criteria for delirium can be difficult to evaluate in advanced dementia, therefore any change from baseline mental status considered.
Urgency, frequency, skin tenderness, costovertebral tenderness and suprapubic pain may be difficult to evaluate in advanced dementia but accepted criteria if present
Unstable vital signs= systolic blood pressure < 90 mmHg systolic OR heart rate ≥ 100 beats/min OR respiratory rate ≥ 25 breaths/minute
Results
The intent of the results presented below is to describe our experience implementing the SPREAD study protocol to date. A brief description of the baseline cohort is provided to illustrate the success of the recruitment scheme. Data relating to the number of full resident and proxy assessments, infections screens, proxy infection interviews, and nasal and rectal swabs are provided to show success of the data collection protocol. As data collection is on-going, findings related to the primary outcomes and specific aims of the study are forthcoming.
Study Facilities
To date, senior administrators representing approximately 100 NHs were approached to be involved in this study, of which 31 agreed to participate. Participant facilities varied with respect to demographic ownership, staffing, and quality of care features and were comparable to NHs nationwide in these characteristics with only a few exceptions (Table 3). SPREAD facilities had a relatively higher mean number of beds (149.2 ± standard deviation (SD) 82.6 vs. 106.1 ± (SD) 63.9), were more likely to have special care dementia units (45.2% vs. 18.2%), and displayed a fewer number of mean deficiencies on the most recent state inspection (4.6 ± (SD) 6.9 vs. 8) compared to NHs nationwide.
Table 3.
Characteristics of SPREAD Facilities vs. US Nursing Homes
| Characteristics | Study Facilities (N=31) | US facilities (N=15,769) |
|---|---|---|
| Organization | ||
| No. Beds | ||
| mean ± SD | 149.2 ± 82.6 | 106.1 ± 63.9 |
| range | 20 – 405 | Not available |
| Special care dementia unit (%) | 45.2 | 18.2 |
| White residents (%) | 89.4 ± 13.5 | 86.0 ± 13.5 |
| Medicaid bed (%) | 59.0 ± 19.5 | 61.9 ± 6.5 |
| For profit (%) | 51.6 | 62.3 |
| Part of a Chain (%) | 45.2 | 55.0 |
| Staffing1 | ||
| Licensed nursing staff hours per resident per day | ||
| mean ± SD | 0.7 ± 0.2 | 0.8 ± .22 |
| range | 0.3 – 1.2 | 0.08–0.44 |
| Nursing assistant staff hours per resident per day | ||
| mean ± SD | 2.3 ± 0.2 | 2.2 ± .3 |
| range | 1.4 – 3.2 | 1.7–3.1 |
| Registered nurse staff hours per resident per day | ||
| mean ± SD | 0.6 ± 0.2 | 0.6 ± 0.2 |
| range | 0.2–0.4 | 0.2–1.3 |
| Nurse practitioner or physician assistant on staff (%) | 54.8 | 20.6 |
| Quality of Care1,2 | ||
| Residents with do-not-resuscitate orders (%) | ||
| mean ± SD | 56.9 ± 23.0 | 57.4 ± 14.5 |
| range | 23.6–95.0 | 20.0–77.9 |
| High risk residents with pressure sores (%) | ||
| mean ± SD | 8.8 ± 4.4 | 10 |
| range | 2–16 | |
| Residents with restraints (%) | ||
| mean ± SD | 3.1 ± 3.4 | 3 |
| range | 0 – 15 | |
| Residents with indwelling foley catheters (%) | ||
| mean ± SD | 3.7 ± 2.1 | 5 |
| range | 0 – 9 | |
| No. deficiencies on state inspection | ||
| mean ± SD | 4.6 ± 6.9 | 8 |
| range | 0 – 35 | |
| Residents with pneumococcal vaccinations (%) | ||
| mean ± SD | 93.6 ± 6.1 | 90 |
| range | 74 – 100 | |
| Residents with influenza vaccinations (%) | ||
| mean ± SD | 93.5 ± 5.5 | 91 |
| range | 80 – 99 | |
| Residents with urinary tract infections (%) | ||
| mean ± SD | 8.5 ± 3.6 | 9 |
| range | 3–17 |
Standard deviations (SD) and ranges were not available for quality of care variables for all US nursing homes
Quality of care data were unavailable at two SPREAD facility that are licensed as a chronic hospitals.
Study Population
To date, upon screening the 31 participant facilities (total beds=4477), 780 residents met eligibility criteria. Among the eligible residents, 38% (N=295) were recruited into the study. Non-participation of eligible residents was due to physician refusal (N=1) and proxy refusal (n=484) due to lack of interest (N=283), study was too burdensome (N=164), privacy concerns (N=8), and other (N=29). Non-recruited eligible residents did not differ significantly from those recruited with respect to mean age (86.8 ± 7.9 (SD)) years but were less likely to be female (80.8%).
To date, 30% (N=87) of recruited residents have died, 33% (N=99) have survived the full 12-month follow-up, and data collection is on going for 37% (N=109) of the cohort. The final mortality rate is expected to be higher by the study's completion. Only 18 proxies and 4 residents were lost to follow-up; 14 proxies could not be contacted after multiple attempts, and 4 proxies requested that they and the residents both be withdrawn from the study.
At the time of this report, summary statistics were available for 286 recruited residents and proxies dyads. The residents' mean age was 86.4 + (SD) 7.4 years, 85.7% were female, and 93% were white (N=266). The recorded causes of dementia included: Alzheimer's disease (N=217, 75.9%), multi-infarct dementia (N=40, 14.0%), Parkinson's disease (N=15, 5.2%) and other causes (N=14, 4.9%). Recruited residents were severely cognitively impaired as indicated by a mean TSI score of 1.9 ± (SD) 3.9, with 65.4% scoring 0. Functional impairment was also advanced (mean BANS score = 21.6 ± (SD) 2.6). Mean length of stay was 43.2 ± (SD) 34.4 months.
The mean age of proxies was 60.7 ± (SD) 10.2 years, 60.8% (N=174) were female and N=264 (92.3%) were white. The proxies' relationships to the residents were as follows: child, N=187 (65.4%); spouse, N=22 (7.7%); niece or nephew, N=28 (9.8%); sibling, N=9 (3.1%); legal guardian, N=20 (7.0%); friend, N=12 (4.2%), grandchild, N=2 (0.7%); and other, N=6 (2.0%). The distribution of the proxies' self-rated health was: excellent, N=96 (33.6%); very good, N=79 (27.6%); good, N=94 (32.9%); fair N=14 (4.9%); or poor, N=3 (1.0%).
Data Collection
To date, we have completed 985 full resident assessments (285 baselines, 613 quarterlies, and 87 death assessments) and 847 full proxy interviews (293 baselines, 477 quarterlies, and 77 two-month post death). A total of 869 rectal swabs have been obtained from residents (mean, 3.0 swabs per resident, range 1–5 per resident). A total of 847 nasal swabs have been obtained (mean 2.9 nasal swabs/resident, range 1–5 per resident). Only 3.2% (N=29/898) of scheduled rectal swabs were not collected for the following reasons: resident refused, N=25; resident out of bed, N=3; and resident out of facility, N=1. Only 5.7% (N=51/898) of scheduled nasal swabs were not collected for the following reasons: resident refused, N=50, resident out of facility, N=1. All swabs have been processed to determine whether or not ARB were present.
Infection data
To date, 2392 monthly resident infection screens have been conducted. At least one suspected infectious episode was detected on 13.5% of screens (N=324/2392). Ten infection screens detected 2 suspected infectious episodes in the prior month, for a total of 334 episodes. The suspected sources of these episodes were: urinary tract, 40.1% (N=137); respiratory tract, 29.6% (N= 99); skin, 12.6% (N=42); and febrile episode of unknown source, 16.8% (N=56)
Telephone infection interviews were conducted with proxies for 83% (N=269/324) of infectious episodes. We were unable to contact proxies for 17% (N=55/324) of documented infectious episodes, and therefore data regarding the proxy's experience related to decision-making for those episodes were missing. The distribution of the suspected sources of infections for the 55 missing interviews was similar to that for which an interview was conducted: urinary tract, 32.2% (N=21); respiratory tract, 29.1% (N= 16), skin, 12.7% (N=7); and febrile episode of unknown source 23.6 % (N=13).
Discussion
SPREAD is a unique, multi-site, prospective study that applies and integrates research methods in palliative care and infectious diseases. This report demonstrates our success implementing the study design, including the recruitment of NH residents with advanced dementia and their proxies, and the repeated collection of complex data documenting infections, decision-making, and ARB in this cohort residing in 31 NHs. This success portends the likelihood the aims of this NIH-funded project will be achieved and that the study will yield novel findings that will ultimately inform better management of infections in advanced dementia; an area that is in great need of evidence-based research and that is highly significant both from a clinical and public health perspective. Our experience implementing SPREAD will also inform the design and conduct of future NH research related to advanced dementia and infectious diseases.
Our established relationships with many Boston-area NHs from two earlier studies was advantageous with respect to facility recruitment.7, 25, 26 Nonetheless, similar to those prior studies, only of 30% of senior administrators we approached allowed SPREAD to be conducted in their facilities, reflecting a general reluctance to involve NHs in research activities. A greater number of facilities were needed for SPREAD because, as anticipated, subject recruitment rates (38%) were lower compared to prior studies using similar cohorts (range, 57%– 70%)7, 25, 26, 39 due to proxy concerns about the invasiveness of the nasal and rectal swabs. To meet our target sample size of 400 dyads by the completion of the recruitment period (December 1, 2012), we will need to recruit at least 5 more facilities. Concerns about the generalizability of this study due to its limitation to Boston-area NHs are somewhat mitigated by the observation that characteristics of participant NHs were, for the most part, very similar to those of NHs nationwide. SPREAD was not designed to have adequate power to examine the effects of facility characteristics on infection management or ARB outcomes, however statistical techniques will be employed in the analyses to account for clustering of residents within facilities.40–42
The baseline characteristics of the residents and proxies enrolled in SPREAD are comparable to advanced dementia cohorts included in other studies.7, 25, 26, 39 This observation lessens concerns about important selection bias resulting from the relatively lower recruitment rate. The residents participating in SPREAD are extremely functionally and cognitively impaired, as expected in advanced dementia, reflecting the success of our eligibility criteria and recruitment procedures. Between SPREAD and our prior prospective cohort studies,7, 25, 26 our team has recruited over 1200 NH residents with advanced dementia. We have concluded the most efficient and accurate method to screen general NH populations for these subjects is to directly ask nurses on each unit to identify residents with dementia who display the features of GDS stage 7.29 As recommended by senior researchers in the field, we endorse the broad adoption of GDS stage 7 for defining research subjects with advanced dementia in studies that involve primary data collection.8
To our knowledge, the extensive repeated collection of nasal and rectal swabs in SPREAD represents the largest such research effort in the NH setting to date. Prior studies of ARB in the NH were limited to one or two facilities,19, 23, 32, 43 cross-sectional designs,19, 23, 44 and small cohorts.18, 19, 23 The small proportion of missing swab data in SPREAD demonstrates the feasibility of collecting these specimens on a larger scale. However, it is important to appreciate that our team made a very concerted effort to minimize any patient discomfort of staff disruption by collecting these swabs very early in the morning, often before 7:00AM, while the resident was still in bed. Swabbing procedures were generally well-tolerated, alleviating proxies' and NH administrators' concerns regarding potential resident discomfort. While a resident's ability to express refusal is limited in advanced dementia, only 6% of scheduled nasal swabs were not obtained because the research nurse perceived a lack of willingness by the resident to undergo the procedure.
To date, we have detected 334 suspected infections in our cohort of residents with advanced dementia. The urinary and respiratory tracts are the most common suspected sources of infection, which is consistent with a prior comparable study.9 While infection screening on a monthly basis was extremely labor intensive, this relatively short interval was chosen to try to maximize proxy contact and recollection of these episodes. Despite our best efforts, we were unable to contact proxies for 17% of episodes. Thus, the approach to the proxy decision-making data in Aim 2 will require various sensitivity analyses to assess the potential effect these missing data on the study outcomes.45
The NH is a challenging setting in which to conduct research. By leveraging and expanding on our prior experience, this report further establishes the feasibility of rigorously recruiting and collecting complex primary data from a large cohort of NH residents with advanced dementia and their proxies. SPREAD will yield a rich and unique dataset that will further our understanding of the management of infections and emergence of ARB in this population. As SPREAD and other observational studies refine methodologies in advanced dementia research and elucidate opportunities for to improve advanced dementia care, the stage is being set for tackling the next research priority for this field: the design and testing of interventions targeting those opportunities.8
Acknowledgments
Supported by funding from the grants R01AG032982, K24AG033640 (SLM) and K23 AG034967 (JLG) from the National Institute on Aging
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1. [Accessed July 6, 2011];National Center for Health Statistics;National Vital Statistics Reports. 2011 at http://www.cdc.gov/nchs/data/nvsr/nvsr59/nvsr59_04.pdf.
- 2.Mitchell SL, Kiely DK, Hamel MB. Dying with advanced dementia in the nursing home. Arch Intern Med. 2004;164:321–6. doi: 10.1001/archinte.164.3.321. [DOI] [PubMed] [Google Scholar]
- 3.Morrison RS, Siu AL. Survival in end-stage dementia following acute illness. JAMA. 2000;284:47–52. doi: 10.1001/jama.284.1.47. [DOI] [PubMed] [Google Scholar]
- 4.Meier DE, Ahronheim JC, Morris J, Baskin-Lyons S, Morrison RS. High short-term mortality in hospitalized patients with advanced dementia: lack of benefit of tube feeding. Arch Intern Med. 2001;161:594–9. doi: 10.1001/archinte.161.4.594. [DOI] [PubMed] [Google Scholar]
- 5.Ahronheim JC, Morrison RS, Baskin SA, Morris J, Meier DE. Treatment of the dying in the acute care hospital. Advanced dementia and metastatic cancer. Arch Intern Med. 1996;156:2094–100. [PubMed] [Google Scholar]
- 6.Sachs GA, Shega JW, Cox-Hayley D. Barriers to excellent end-of-life care for patients with dementia. J Gen Intern Med. 2004;19:1057–63. doi: 10.1111/j.1525-1497.2004.30329.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mitchell SL, Teno JM, Kiely DK, et al. The clinical course of advanced dementia. N Engl J Med. 2009;361:1529–38. doi: 10.1056/NEJMoa0902234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mitchell SL, Black BS, Ersek E, et al. Advanced dementia: State-of-the-art and priorities for the next decade. Ann Intern Med. 2012;156:45–51. doi: 10.1059/0003-4819-156-1-201201030-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.D'Agata E, Mitchell SL. Patterns of antimicrobial use among nursing home residents with advanced dementia. Arch Intern Med. 2008;168:357–62. doi: 10.1001/archinternmed.2007.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Luchins DJ, Hanrahan P. What is appropriate health care for end-stage dementia? J Am Geriatr Soc. 1993;41:25–30. doi: 10.1111/j.1532-5415.1993.tb05943.x. [DOI] [PubMed] [Google Scholar]
- 11.Loeb M, Simor AE, Landry L, et al. Antibiotic use in Ontario facilities that provide chronic care. J Gen Intern Med. 2001;16:376–83. doi: 10.1046/j.1525-1497.2001.016006376.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Warren JW, Palumbo FB, Fitterman L, Speedie SM. Incidence and characteristics of antibiotic use in aged nursing home patients. J Am Geriatr Soc. 1991;39:963–72. doi: 10.1111/j.1532-5415.1991.tb04042.x. [DOI] [PubMed] [Google Scholar]
- 13.Zimmer JG, Bentley DW, Valenti WM, Watson NM. Systemic antibiotic use in nursing homes. A quality assessment. J Am Geriatr Soc. 1986;34:703–10. doi: 10.1111/j.1532-5415.1986.tb04301.x. [DOI] [PubMed] [Google Scholar]
- 14.Pickering TD, Gurwitz JH, Zaleznik D, Noonan JP, Avorn J. The appropriateness of oral fluoroquinolone-prescribing in the long-term care setting. J Am Geriatr Soc. 1994;42:28–32. doi: 10.1111/j.1532-5415.1994.tb06069.x. [DOI] [PubMed] [Google Scholar]
- 15.Jones SR, Parker DF, Liebow ES, Kimbrough RC, 3rd, Frear RS. Appropriateness of antibiotic therapy in long-term care facilities. Am J Med. 1987;83:499–502. doi: 10.1016/0002-9343(87)90761-3. [DOI] [PubMed] [Google Scholar]
- 16.Yoshikawa TT. Antimicrobial resistance and aging: beginning of the end of the antibiotic era? J Am Geriatr Soc. 2002;50:S226–9. doi: 10.1046/j.1532-5415.50.7s.2.x. [DOI] [PubMed] [Google Scholar]
- 17.Yoshikawa TT. Resistant pathogens: considerations in geriatrics and infectious disease. J Am Geriatr Soc. 2002;50:S225. doi: 10.1046/j.1532-5415.50.7s.1.x. [DOI] [PubMed] [Google Scholar]
- 18.Toubes E, Singh K, Yin D, et al. Risk factors for antibiotic-resistant infection and treatment outcomes among hospitalized patients transferred from long-term care facilities: does antimicrobial choice make a difference? Clin Infect Dis. 2003;36:724–30. doi: 10.1086/368081. [DOI] [PubMed] [Google Scholar]
- 19.Trick WE, Weinstein RA, DeMarais PL, et al. Colonization of skilled-care facility residents with antimicrobial-resistant pathogens. J Am Geriatr Soc. 2001;49:270–6. doi: 10.1046/j.1532-5415.2001.4930270.x. [DOI] [PubMed] [Google Scholar]
- 20.Pop-Vicas AE, D'Agata EM. The rising influx of multidrug-resistant gram-negative bacilli into a tertiary care hospital. Clin Infect Dis. 2005;40:1792–8. doi: 10.1086/430314. [DOI] [PubMed] [Google Scholar]
- 21.Mody L, Bradley SF, Strausbaugh LJ, Muder RR. Prevalence of ceftriaxone- and ceftazidime-resistant gram-negative bacteria in long-term-care facilities. Infect Control Hosp Epidemiol. 2001;22:193–4. doi: 10.1086/503397. [DOI] [PubMed] [Google Scholar]
- 22.Lee DC, Barlas D, Ryan JG, Ward MF, Sama AE, Farber BF. Methicillin-resistant Staphylococcus aureus and vancomycin-resistant enterococci: prevalence and predictors of colonization in patients presenting to the emergency department from nursing homes. J Am Geriatr Soc. 2002;50:1463–5. doi: 10.1046/j.1532-5415.2002.50377.x. [DOI] [PubMed] [Google Scholar]
- 23.Pop-Vicas A, Mitchell SL, Kandel R, Schreiber R, D'Agata EM. Multidrug-resistant gram-negative bacteria in a long-term care facility: prevalence and risk factors. J Am Geriatr Soc. 2008;56:1276–80. doi: 10.1111/j.1532-5415.2008.01787.x. [DOI] [PubMed] [Google Scholar]
- 24.D'Agata EM. Rapidly rising prevalence of nosocomial multidrug-resistant, Gram-negative bacilli: a 9-year surveillance study. Infect Control Hosp Epidemiol. 2004;25:842–6. doi: 10.1086/502306. [DOI] [PubMed] [Google Scholar]
- 25.Mitchell SL, Kiely DK, Jones RN, Prigerson H, Volicer L, Teno JM. Advanced dementia research in the nursing home: the CASCADE study. Alzheimer Dis Assoc Disord. 2006;20:166–75. doi: 10.1097/00002093-200607000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mitchell SL, Miller SC, Teno JM, Kiely DK, Davis RB, Shaffer ML. Prediction of 6-month survival of nursing home residents with advanced dementia using ADEPT vs hospice eligibility guidelines. JAMA. 2010;304:1929–35. doi: 10.1001/jama.2010.1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. [Accessed January 31, 2011]; LTCFocUS.org: Long-term Care: Facts on Care in the US. 2012 LTCFocUS.org. at http://ltcfocus.org/
- 28. [Accessed January 31, 2012];Nursing Home Compare, US Department of Health and Human Service, Centers for Medicare and Medicaid Services. at http://www.medicare.gov/NHCompare/
- 29.Reisberg B, Ferris SH, de Leon MJ, Crook T. The Global Deterioration Scale for assessment of primary degenerative dementia. Am J Psychiatry. 1982;139:1136–9. doi: 10.1176/ajp.139.9.1136. [DOI] [PubMed] [Google Scholar]
- 30.Volicer L, Hurley AC, Lathi DC, Kowall NW. Measurement of severity in advanced Alzheimer's disease. J Gerontol. 1994;49:M223–6. doi: 10.1093/geronj/49.5.m223. [DOI] [PubMed] [Google Scholar]
- 31.Albert M, Cohen C. The Test for Severe Impairment: an instrument for the assessment of patients with severe cognitive dysfunction. J Am Geriatr Soc. 1992;40:449–53. doi: 10.1111/j.1532-5415.1992.tb02009.x. [DOI] [PubMed] [Google Scholar]
- 32.O'Fallon E, Kandel R, Schreiber R, D'Agata EM. Acquisition of multidrug-resistant gram-negative bacteria: incidence and risk factors within a long-term care population. Infect Control Hosp Epidemiol. 2010;31:1148–53. doi: 10.1086/656590. [DOI] [PubMed] [Google Scholar]
- 33.Performance standards for antimicrobial disk susceptibility tests. Clinical and Laboratory Standards Institute; Wayne, PA: 2006. [Google Scholar]
- 34.Identification of bacteria and yeast . M35-A. Clinical and Laboratory Standards Institute; Wayne, PA: 2007. Approved guidelines. [Google Scholar]
- 35.D'Agata E, Venkataraman L, DeGirolami P, Samore M. Molecular epidemiology of acquisition of ceftazidime-resistant gram-negative bacilli in a nonoutbreak setting. J Clin Microbiol. 1997;35:2602–5. doi: 10.1128/jcm.35.10.2602-2605.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tenover FC, Arbeit RD, Goering RV, et al. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J Clin Microbiol. 1995;33:2233–9. doi: 10.1128/jcm.33.9.2233-2239.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barry MJ, Cherkin DC, YuChiao C, Fowler FJ, Skates S. A randomized trial of a multimedia shared decision-making program for men facing a treatment decision for benign prostatic hypertrophy. Dis Manage Clin Outcomes. 1997;1:5–14. [Google Scholar]
- 38.Loeb M, Bentley DW, Bradley S, et al. Development of minimum criteria for the initiation of antibiotics in residents of long-term-care facilities: results of a consensus conference. Infect Control Hosp Epidemiol. 2001;22:120–4. doi: 10.1086/501875. [DOI] [PubMed] [Google Scholar]
- 39.Hanson LC, Carey TS, Caprio AJ, et al. Improving decision-making for feeding options in advanced dementia: a randomized, controlled trial. J Am Geriatr Soc. 2011;59:2009–16. doi: 10.1111/j.1532-5415.2011.03629.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Laird NM, Ware JH. Random-effects models for longitudinal data. Biometrics. 1982;38:963–74. [PubMed] [Google Scholar]
- 41.Liang KY, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73:13–22. [Google Scholar]
- 42.McCullagh P, Nelder JA. Generalized linear models. Second ed. Chapman & Hal; London: 1989. [Google Scholar]
- 43.O'Fallon E, Pop-Vicas A, D'Agata E. The emerging threat of multidrug-resistant gram-negative organisms in long-term care facilities. J Gerontol A Biol Sci Med Sci. 2009;64:138–41. doi: 10.1093/gerona/gln020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.O'Fallon E, Schreiber R, Kandel R, D'Agata EM. Multidrug-resistant gram-negative bacteria at a long-term care facility: assessment of residents, healthcare workers, and inanimate surfaces. Infect Control Hosp Epidemiol. 2009;30:1172–9. doi: 10.1086/648453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Little RJA, Rubin DB. Statistical Analysis with Missing Data. Second ed. John Wiley & Sons; New York: 2002. [Google Scholar]