Abstract
To deliver nucleic acids including plasmid DNA (pDNA) and short interfering RNA (siRNA), polymeric gene carriers equipped with various functionalities have been extensively investigated. The functionalities of these polymeric vectors have been designed to overcome various extracellular and intracellular hurdles that nucleic acids and their carriers encounter during their journey from injection site to intracellular target site. This review briefly introduces known extracellular and intracellular issues of nucleic acid delivery and their solution strategies. We examine significant yet overlooked factors affecting nucleic acid delivery (e.g., microenvironmental pH, polymer/siRNA complexation, and pharmaceutical formulation) and highlight our reported approaches to solve these problems.
Keywords: Gene delivery, Non-viral gene delivery, Nucleic acid delivery, plasmid DNA, Polymeric gene delivery, short interfering RNA
1. Introduction
Protein expression encoded by plasmid DNA (pDNA) and target mRNA silencing by short interfering RNA (siRNA), parts of gene therapy, are considered attractive methods to control some gene- and protein-related disorders. [1–4] However, when naked nucleic acids are exposed to blood and the extracellular milieu, genes are subject to degradation by serum nucleases. [5, 6] When nucleic acids that survive degradation by digestive enzymes reach their target cells, the intrinsic phosphate-origin negative charge character seriously limits their penetration through the plasma membrane. [7, 8] These problems have been addressed by complexation of nucleic acids with cationic lipids and/or polymers, allowing the resulting complexes to protect the nucleic acids from nucleases and improve cellular internalization.[8–11]
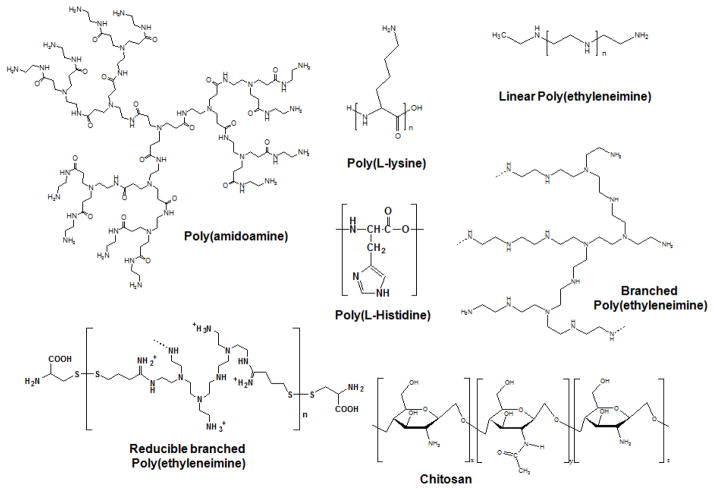
Although Spitnik et al. complexed DNA with polylysine and polyvinylamine as an initial attempt in the 1950s [12], the full potential of cationic polymeric electrolytes for nucleic acid delivery had not been realized until the late 1980s. The earliest investigation of functional polymers (i.e., poly(L-lysine)-grafted asialoorosomucoid and poly(ethyleneimine)) for use in receptor-mediated endocytosis by Wu et al. was reported in the late 1980s [13], and endosomal disruption by Boussif et al. was reported in the mid-1990s [14], respectively. After serious concerns of safety (e.g., cytotoxicity, immunogenicity, and tumorigenicity) and manufacturing (e.g., difficulty of mass production) of viral vectors emerged [2, 4, 9, 15, 16], polymer-based nonviral vectors for gene delivery were extensively researched in the last two decades. Their flexibility in chemistry, size, and structure and acceptable biocompatibility (e.g., less cytotoxicity, less immunogenicity, and no tumorigenicity) have allowed for the extensive design of various functional polymeric materials (Fig. 1). [9, 10, 17, 18]
Fig. 1.
Some examples of polycations for gene delivery
Polymeric gene carriers require a range of functionalities to perform the dual roles of condensing nucleic acids into stable and compact nanoparticles in the extracellular environment and delivering these nanoparticles to the intracellular site of action (the nucleus for pDNA and the cytoplasm for siRNA). On its journey from injection site to intracellular target site of action, a polyplex nanoparticle encounters a series of physicochemical and biological barriers. Most research articles describing pDNA/siRNA delivery report new polycations with different cationic species [10, 17–19], degradability (or reducibility) [20–23], surface modifications (for stealth function [24, 25], target cell interaction [17], and endosomolytic activity [26, 27]), comparative cytotoxicity, in vitro transfection efficiency, and intracellular trafficking [28–30]. Currently known strategies to overcome extracellular and intracellular hurdles during polymeric transfection are summarized in Table 1.
Table 1.
General approaches of polymeric gene carriers to solve extracellular and intracellular environmental issues during polymeric transfection.
| Issues | Approaches | Mechanisms |
|---|---|---|
| Degradation of nucleic acid by nucleases | Polymeric materials with cationic segments | Polycation complexes with nucleic acids via electrostatic attraction shield nucleic acids from nucleases. |
| Polyplex stability in the blood and extracellular fluid | Surface masking of cationic polyplex using hydrophilic materials (e.g., poly(ethylene glycol) (PEG)) | Electrostatic attraction of cationic polyplex with negatively charged blood components (e.g., red blood cells and serum albumin) forms aggregates. The repellent character of hydrophilic PEG against the blood components prevents the formation of undesired aggregates. |
| Cellular internalization of nucleic acids | Polymeric vectors | Negatively charged nucleic acids are difficult to pass through the plasma membrane with negative surface characteristics. Polymers that carry nucleic acids avoid electrostatic repulsion between nucleic acids and the plasma membrane. |
| Cellular targeting of polyplex | Surface modification of polyplex with cell-targeting ligands | The polyplex may be physically or chemically modified with ligands (e.g., antibody, protein, or small chemicals). The ligands can interact with cell-specific receptors on the plasma membrane of target cells. |
| Endosomal release of polyplex | Introduction of endosomolytic materials in the polyplex | Endosomolytic chemicals, polymers, or peptides incorporated physically or chemically in the polyplex disrupt the endosomal membrane via their fusogenic activity and/or proton buffering capacity. |
| Intracellular release of nucleic acids | Stimuli (pH, glutathione)-triggering degradable polymers | Polymers with functional bonds degraded by acidic pH in the endolysosomes or cytosolic/nuclear glutathione form a stable polyplex with nucleic acids in the extracellular environment, but their stimuli-triggering degradation releases nucleic acid into the intracellular environments. |
| Nuclear import of nucleic acids or polyplex | Nuclear localization signals (NLS) through the nuclear membrane. | NLS dilates energetically nuclear pores and helps nucleic acids or polyplexes pass |
| Cation-mediated cytotoxicity | Degradable polymers | Longer polycations interact more strongly with intracellular vital macromolecules and compartments with negative charges, causing more damage or interference in cellular physiology than shorter polycations. Polymers degraded in the intracellular environments avoid or reduce harmful cellular interactions. |
In vivo disease models, macroscopic biodistribution, and in vivo biological effects have only been occasionally reported. Whereas the microscopic distribution of polyplexes in a target tissue is rarely observed in the literature, it is critical for evaluating its clinical potential. Additional biological factors to be taken into account may include the extracellular microenvironment of the target tissue, the nature of target cells (such as surface marker heterogeneity [31] and mitotic activity [32–34]), and intracellular dynamics. Despite the richness of nonviral vector designs and delivery strategies, the clinical potential of polymeric gene delivery remains to be determined because of gaps in our understanding of in vitro systems and the entire delivery pathway in vivo.
Furthermore, to make gene delivery systems more feasible as therapeutic pharmaceuticals, a range of formulation factors should be addressed, including the inertness of a selected polycation at various biological levels and the stability of the vector in a given dosage form with a defined shelf life.
This review summarizes our recent efforts to address the following issues in polymeric gene delivery: environmental pH effects [29, 30, 35], tuned endosomolytic activity [27], an siRNA/pDNA co-delivery approach [36], and cryopreservation and reconstitution [37].
2. Significant yet overlooked factors in gene delivery
2.1. Environmental pH
Polyplexes form via electrostatic attraction. Polymers, nucleic acids, and the resulting polyplex are exposed to various pHs during different steps from polyplex preparation to transfection. When preparing a polyplex with a basic polyelectrolyte and nucleic acids, the pH of medium used for complexation determines the charge densities of the polymer and the gene, leading to different polyplex compactness. For in vivo disease cases, before the polyplex enters cells of interest, it may face an extracellular microenvironmental pH that is not identical to the normal blood pH of 7.4. For certain diseases, such as ischemia and solid tumors, the extracellular pH is known to be acidic (approximate pH values of 6.4–7.0 for solid tumors [38] and approximate pH values of 6.4–6.8 for ischemia [39, 40]). The endocytosed polyplex encounters dynamic pH microenvironments in the endosomes due to their acidified maturation processes. Although pH dynamics in extracellular and intracellular environments are known and expected, the environmental pH effects on polymeric transfection are lacking. Thus, our group has investigated how environmental pH influences cellular uptake, intracellular trafficking, dissociation kinetics, and transfection efficiency of the polyplex.
2.1.1. Extracellular microenvironmental pH
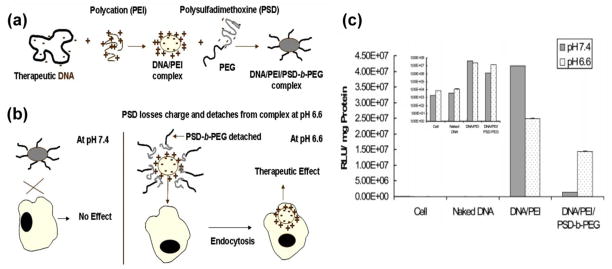
When polyplexes were exposed to extracellular transfection media of various pH values (6.3, 6.7, 7.0, and 7.4), although certain cationic polyplexes (e.g., branched polyethyleneimine- (bPEI) based polyplex) may possess increased positive surface charges with acidification that would lead to slightly higher cellular uptake at lower pH values in some cell lines, the cellular uptake of most cationic polyplexes did not exhibit significant differences.[30] However, when the extracellular pH altered the surface charges of the polyplex from neutral to positive (termed acid-induced deshielding technology, illustrated in Fig. 2), the resulting transfection efficiency from different cellular uptakes was higher at acidic pH than at the normal pH of 7.4.[35] Sethuraman et al. designed poly(methacryloyl sulfadimethoxine)-block-poly(ethylene glycol)- (PSD-b-PEG) shielded PEI/DNA complex via electrostatic attraction. [35] PEG shielding may endow polyplex stability during blood circulation at normal blood pH and limit non-specific interactions with non-target cells, thus minimizing transgene expression in non-target cells. However, acidic extracellular environments (e.g., tumor extracellular environments) trigger a charge alteration from negative at pH 7.4 to neutral at pH 6.6 in PSD, resulting in deshielding of PSD-b-PEG, thus exposing the cationic surface of the PEI/DNA complex. The acidic pH-specific (e.g., tumor-specific) enhanced endocytosis of the designed polyplex may maximize transfection efficiency at the target cells (in this case, tumor cells). Specifically, this concept may be beneficial for anti-tumor gene therapy due to heterogeneity of tumor cells [31].
Fig. 2.
Extracellular acidic pH-induced deshielding approach: (a) Formation of the layer-by-layer nanocomplex through the charge-charge interaction between DNA, polycation (PEI), and PSD-b-PEG, (b) the nanocomplex shielded at a physiological pH of 7.4 and deshielded at an acidic tumor pH of 6.6, and (c) the in vitro transfection efficiency of the nanocomplex exposed to different pH values. [35] (Reproduced with permission)
2.1.2. Intracellular microenvironmental pH
After a polyplex enters a cell via endocytosis, the polyplex is exposed to dynamic pH microenvironments. Endolysosomal compartments are acidified from the extracellular environmental pH of 7.4 to approximately pH 4–5 because ATP-activated proton pumps located in the endosomal membrane promote the influx of cytosolic protons into the endosomes, where acidic lysosomal compartments merge with the acidic matured endosomes. [9] However, most researchers have ignored endosomal pH dynamics and their cell specificities and have instead focused on endosomal release of polyplex.
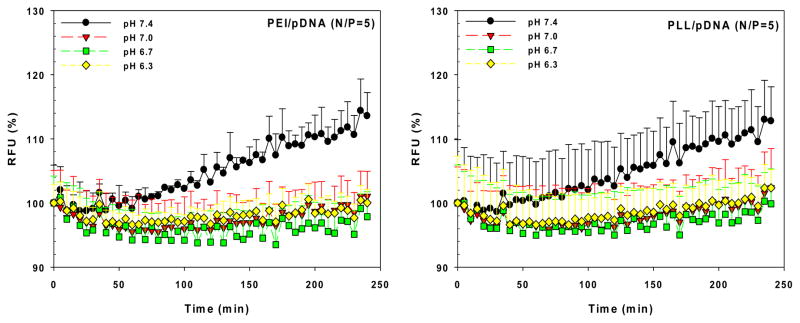
The exposure of the polyplex to the altered endosomal pH may change polyplex stability, especially the degree of decomplexation (or release of nucleic acids). In our recent report, we showed that in acidic environments, the polyplexes (PEI/pDNA and poly(L-lysine) (PLL)/pDNA) may prevent or delay decomplexation compared to neutral pH environments. (Fig. 3) [30] This result may arise from the increasing positive charges of polycations to negative charges of pDNA because the phosphate groups of pDNA (approximately pKa 6.3) contain less negative charge as pH decreases. These findings suggest that the polyplex may be compact or stable in acidic microenvironments (i.e., pathological tumor or ischemic extracellular environments and endocytic compartments) but may be loose or dissociated in neutral pH environments (i.e., the cytoplasm and the nucleus), allowing for the release of nucleic acids.
Fig. 3.
Effects of medium pH on decomplexation of polyplexes. Increasing RFU (%) indicates pDNA exposure in the polyplexes.[30] (Reproduced with permission)
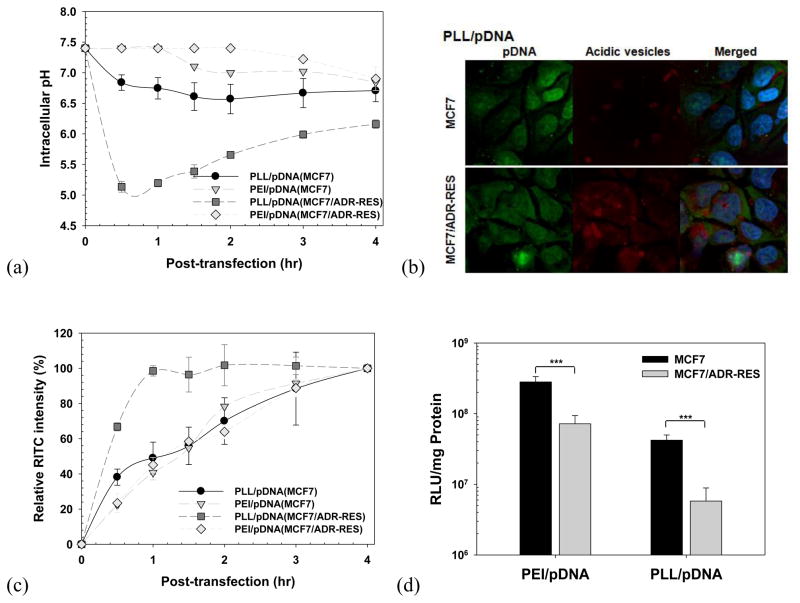
Understanding the cell-specificity of endosomal pH dynamics during polymeric transfection is a very significant task. Nevertheless, except for some studies on polyplex endocytic kinetics using cell lines from different organs [28], a lack of studies exists on cell-specific endosomal pH dynamics for cells derived from same organ. Furthermore, although multidrug resistance (MDR) has become a significant issue in anti-cancer chemotherapeutics, this significance and its endosomal characteristics have been poorly recognized in gene therapeutics. Kang et al. traced the intracellular microenvironmental pH of polyplex (PEI/pDNA and PLL/pDNA) in a drug-sensitive cell line (human breast MCF7 cells) and in a drug-resistant cell line (a subline of doxorubicin-resistant MCF7 cells; MCF7/ADR-RES cells) (Fig. 4). [29] When the polyplex with no endosomal escaping activity (PLL/pDNA) moved into the endolysosomal pathway, the polyplex was slowly exposed to acidified microenvironments in the drug-sensitive cells (Fig. 4(a)). In the drug-resistant cells, more polyplex was trapped in the acidic endosomal/lysosomal compartments for longer periods of time than in the drug-sensitive cells because the drug-resistant cells had faster acidification rates (in the case of PLL/pDNA complexes, approximately pH 5.1 for MCF7/ADR-RES cells vs. approximately pH 6.8 for MCF7 cells at 0.5 hr post-transfection) (Fig. 4(a)). Fig. 4(b) clearly supported sequestration of PLL/pDNA complexes in acidic compartments. Moreover, as shown in Fig. 4(c), the PLL-based polyplex entered the drug-sensitive cells linearly with time, whereas the polyplex was exocytosed by the drug-resistant cells after reaching a certain saturation level. However, this acidic sequestration and exocytosis of the polyplex in the drug-resistant cells may be overcome by the use of the polyplex having endosomal escaping activity (e.g., PEI/pDNA complex). These findings conclusively indicate that the in vitro transfection efficiency of polyplexes in drug-resistant cells is much lower than that in drug-sensitive cells (Fig. 4(d)). This phenomenon of polyplex-transfected drug-resistant cells may be closely related to the well-known characteristics of drug-resistant cells exposed to small chemical anticancer compounds.
Fig. 4.
Polymeric transfection in drug-sensitive cells (MCF7) and drug-resistant cells (MCF7/ADR-RES): (a) intracellular pH, (b) intracellular distribution, (c) cellular uptake, and (d) in vitro transfection efficiency of polyplex. [29] (Reproduced with permission)
2.1.3. Dynamic pH effects of polymeric transfection
The microenvironmental pH is strongly dependent upon the extracellular environment (e.g., blood and extracellular fluid), intracellular compartments (e.g., endosomes, lysosomes, cytoplasm, and nucleus), cell-specificity (e.g., drug-sensitive cells and drug-resistant cells), and disease-specificity (e.g., tumor and ischemia). As mentioned, the microenvironmental pH can influence the cellular uptake and decomplexation of polyplex, resulting in different transfection efficiencies. However, during polymeric transfection, the microenvironmental pH may affect polymer characteristics (e.g., proton buffering capacity and ionization), polyplex characteristics (e.g., size, surface charge, and decomplexation), and as cellular characteristics (e.g., cellular uptake, cell cycle phases, endocytosis, and intracellular pH environment). [30] Negative or positive effects of these complicated factors on polymeric transfection efficiency are summed to represent the gross transfection efficiency.
Kang et al. reported the overall polymeric transfection efficiencies of two representative polymeric vectors (PEI and PLL) after three different cancer cell lines were exposed to different medium pHs (pH 6.3, 6.7, 7.0, and 7.4). [30] However, as summarized in Table 2, when the cells were transfected with polyplex at a fixed extracellular pH, no trend was observed in polymeric transfection efficiency when comparing an acidic extracellular pH (pH 6.3) to extracellular pH 7.4. Realistically, it is difficult to determine how certain microenvironmental pH factors influence overall transfection efficiency due to the dynamic pH changes during polymeric transfection. However, if the polymeric transfection process were performed at a fixed location or at a fixed pH, it may be possible to understand and estimate the environmental effects of certain factors on polymeric transfection. Therefore, a polymeric transfection process was divided into two time frames (a transfection period and an incubation period) as follows: Condition A (4-hr transfection period at different pH values followed by a 44-hr incubation period at pH 7.4), condition B (4 hr transfection period at pH 7.4 followed by a 44-hr incubation period at different pH values) and condition AB (48-hr transfection period and incubation period both at different pH values). Compared to the pH 7.4 medium, the acidic transfection medium resulted in a 1.6–7.7-fold reduction in gene expression, whereas the acidic culture medium pH enhanced transfection efficiency 2.1–2.6-fold. Acidic medium reduced or delayed endocytosis, endosomal acidification, cytosolic release, and decomplexation of polyplex, which may lead to negative effects on gene expression. However, the acidic medium delayed or inhibited mitosis and reduced the dilution of gene expression, resulting in increased transfection efficiency. These findings indicate that culture medium affected overall polymeric transfection more than transfection medium. Therefore, when cells are transfected at a specific extracellular pH, which is similar to clinical situations, the extracellular pH effects on overall transfection efficiency are cell-dependent. To achieve maximum transgene expression, understanding the effects of extracellular pH on polymeric transfection may provide insight into designing effective and safe polymeric gene carriers.
Table 2.
Summary of cell transfection enhancement or reduction with extracellular pH 6.3 compared to extracellular pH 7.4. [30] (Reproduced with permission)
| MCF7 | MCF7/ADR-RES | MES-SA | ||
|---|---|---|---|---|
| PEI/pDNA-mediated transfection | Condition AB | 2-fold ↓ | 2.2-fold ↑ | 2-fold ↑ |
| Condition A | 7.7-fold ↓ | 2.1-fold ↓ | 1.6-fold ↓ | |
| Condition B | 1.6-fold ↑ | 1.9-fold ↑ | 2.6-fold ↑ | |
|
| ||||
| PLL/pDNA-mediated transfection | Condition AB | 1.7-fold ↑ | 1.8-fold ↑ | 1.5-fold ↓ |
| Condition A | 1-fold | 2.4-fold ↓ | 2.7-fold ↓ | |
| Condition B | 1.6-fold ↑ | 1.6-fold ↑ | 1.2-fold ↑ | |
↓ and ↑ indicate lower and higher, respectively. Condition A (4-hr transfection period at different pH values (pH 7.4, 7.0, 6.7, and 6.3) followed by a 44 hr incubation period fixed at pH 7.4), Condition B (4 hr transfection period at pH 7.4 followed by a 44-hr incubation period at different pH values (pH 7.4, 7.0, 6.7, and 6.3)), Condition AB (48-hr transfection period and incubation period both at different pH values (7.4, 7.0, 6.7, and 6.3)).
2.1.4. pH-tunable endosomolytic oligomer
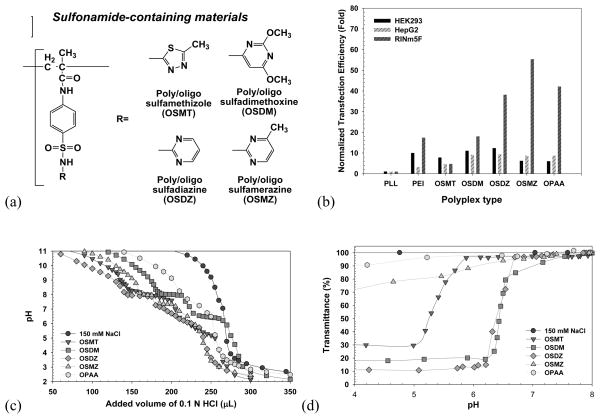
The extracellular microenvironmental pH [30] and endolysosomal pH dynamics, which may be disease- and cell-specific, influence polymeric transfection efficiency [29, 30], prompting the development of cell-customized endosomolytic agents for more effective gene transfection. Kang and Bae described their views on acidic sulfonamides, which possess a broad range of pKa values (3–11), and the hydrophobicity of these compounds is determined by various substituted groups, R (Fig. 5(a)). [27] For feasibility studies, oligomeric sulfonamides (OSAs) were prepared by radical polymerization of sulfamethizole (SMT; pKa 5.45), sulfadimethoxine (SDM; pKa 6.1), sulfadiazine (SDZ; pKa 6.4), and sulfamerazine (SMZ; pKa 7.0), which had pKa values within endolysosomal pH values. The synthesized OSAs (designated OSMT, OSDM, OSDZ, and OSMZ) had Mn values between 1.8 and 2.5 kDa and displayed different proton buffering capacities and aqueous solubility transitions within the endolysosomal pH, which are related to endosomal escaping activity. As shown in Fig. 5(c), OSMT and OSDZ displayed broad proton buffering ranges of pH 5.0–6.4 and 5.7–7.3, respectively, whereas OSDM and OSMZ displayed strong proton buffering at specific pH values of 6.5 and 7.3, respectively. Their apparent pKa values were 5.7 (OSMT), 6.5 (OSDM and OSDZ), and 7.3 (OSMZ). In aqueous solubility transition studies (Fig. 5(d)), the OSMZ solubility slowly changed, and its solubility transition occurred within broad pH ranges. However, other OSAs exhibited relatively sharp changes in solubility within narrow pH ranges (pH 6.2–6.5 for OSDM, pH 6.2–6.7 for OSDZ, and pH 5.1–5.9 for OSMT). Using three different cell lines (HepG2 (human hepatoma cells), HEK293 (human embryonic kidney cells), and RINm5F (rat insulinoma cells)) derived from different organ origins, the incorporated effects of OSAs in the PLL/pDNA complexes were investigated. OSA-containing PLL/pDNA complex (OSA-polyplex) showed 4–55-fold higher gene expression than control polyplex (PLL/pDNA) (Fig. 5(b)). Interestingly, transfecting HEK293 and HepG2 cells with OSA-polyplex, OSDM-polyplex and OSDZ-polyplex displayed more favorable transfection than other OSA-polyplexes, whereas RINm5F cells showed the best transfection results with OSMZ-polyplex. This study verified the need for cell-customized endosomolytic materials to achieve higher rates of transfection.
Fig. 5.
pH-tunable endosomolytic oligomeric sulfonamides (OSAs): (a) chemical structures, (b) in vitro transfection efficiency of OSA-polyplexes, (c) acid titration curve of OSAs for proton buffering capacity, and (d) aqueous solubility transition of OSAs. [27] (Reproduced with permission)
2.2. pDNA/siRNA co-condensation
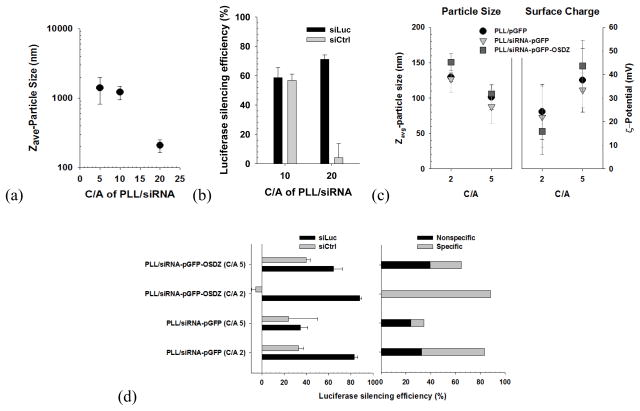
Because of the significance of siRNA therapy, polymeric materials have been applied toward siRNA delivery in the same manner as for pDNA.[8] However, different intrinsic physicochemical characteristics between pDNA and siRNA, such as long and flexible chains compared to short and rigid chains, require different amount of polycations to form nanosized complexes. It is known that long-chain polyanions, such as anionic polysaccharides (e.g., hyaluronic acid), and non-functional DNA (e.g., calf thymus DNA) are beneficial in forming compact siRNA nanoparticles.[41, 42] Similarly, Kang and Bae selected functional pDNA (a plasmid of green fluorescent protein; pGFP) as a helper polyanion because pDNA and siRNA delivery in a single nanovector can endow various therapeutic/diagnostic benefits.[36] As shown in Fig. 6, the short and rigid nature of an siRNA chain resulted in larger and more loosely packed particles (1–2 μm in size at C/A (cation/anion) 5) compared to pGFP (approximately 90 nm in size at C/A 5) after complexing with PLL and, in turn, poor specific silencing effects. However, with pGFP and polycation, siRNA formed compact nanosized polyplex (90–150 nm in size) at C/As of 2 and 5. At C/A 2, the PLL/siRNA-pGFP-OSDZ polyplex improved the specific gene silencing (90%) more dramatically than the PLL/siRNA-pGFP polyplex (50%), demonstrating a potential role for OSDZ. In addition, pGFP in the PLL/siRNA-pGFP polyplex successfully expressed GFP without interfering with the siRNA.
Fig. 6.
PLL-based polyplexes containing siRNA and pDNA: (a) particle size of PLL/siRNA polyplex, (b) luciferase silencing efficiency of PLL/siRNA polyplex, (c) particle size and surface charge of PLL-based siRNA-pDNA polyplex, and (d) luciferase silencing efficiency of PLL-based siRNA-pDNA polyplex.[36] (Reproduced with permission)
2.3. Polyplex as a reconstitutable pharmaceutical
Although polymeric gene vectors are still in an infant stage toward becoming a readily available major therapeutic option, their appropriate formulations for clinical practice should be taken into account. Some considerations for these formulations are optimal concentrations of bioactive components [43], ease of administration [44], and formulation stability during storage [45, 46]. Polymer-based colloidal formulations may be a viable option because polymeric vectors are generally prepared in a liquid form. However, polymers as liquid formulations may be gradually degraded [47, 48], and therapeutics may undergo unwanted release [49] with reduced bioactivity [50] during long-term storage. To overcome the stability limitations of colloidal formulations, an alternative is a powder formulation prepared from liquid product, followed by buffer reconstitution when necessary.
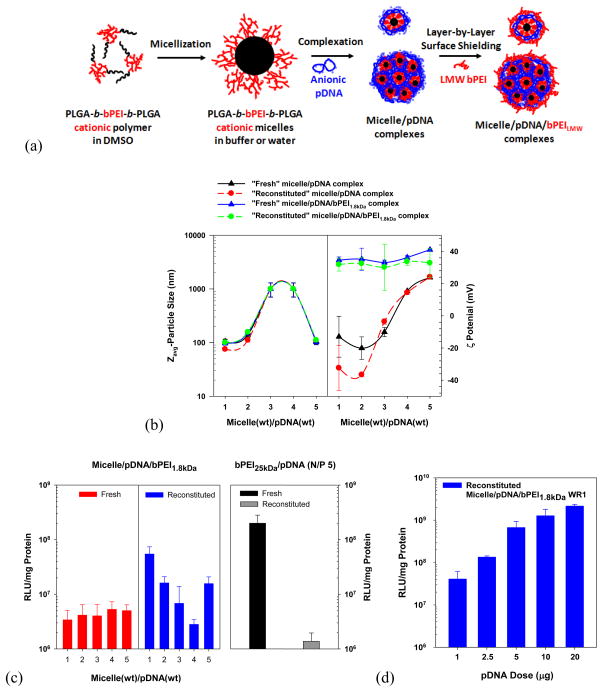
Mishra et al. synthesized poly(lactide-co-glycolide)36kDa (PLGA36kDa)-b-bPEI25kDa-b-PLGA36kDa ((PLGA)36kDa)2-b-bPEI25kDa) and constructed its cationic micelle.[37] The micelle/pDNA polyplex was prepared and was then coated with a low molecular weight bPEI1.8kDa (Fig. 7(a)). The resulting micelle/pDNA/bPEI1.8kDa polyplex retained the physicochemical characteristics of particle size (100–150 nm) and surface charge (30–40 mV), which were similar before lyophilization and after reconstitution of the lyophilized powder (Fig. 7(b)). Unlike the bPEI25kDa/pDNA polyplex, which exhibited reduced transfection efficiency after lyophilization, the designed micelle-based polyplex retained or improved in vitro transfection efficiency after lyophilization/reconstitution. Specifically, the reconstituted micelle/pDNA/bPEI1.8kDa polyplex (weight ratio (WR) of 1 micelle to pDNA) showed a 16-fold higher gene expression than its fresh counterpart and also exhibited a 39-fold higher transfection efficiency than the reconstituted bPEI25kDa/pDNA polyplex (N/P 5) (Fig. 7(c)). Interestingly, the micelle-based polyplex (WR 1) with pDNA doses up to 20 μg increased its transfection levels linearly and had very low cytotoxicity (Fig. 7(d)). This study indicates that the designed PLGA-b-bPEI micelle and its gene complexes represent a potential pharmaceutical formulation for genetic therapeutics.
Fig. 7.
Reconstitutable (PLGA36kDa)2-b-bPEI micelle-based pDNA polyplex: (a) schematic representation of the structures of (PLGA36kDa)2-b-bPEI25kDa micelles, micelle/pDNA complexes, and micelle/pDNA/bPEILMW complexes, (b) particle sizes and surface charges of micelle/pDNA complexes and micelle/pDNA/bPEI1.8kDa complexes before and after reconstitution, (c) in vitro transfection efficiency of fresh and reconstituted micelle/pDNA/bPEI1.8kDa complexes (1 μg of pDNA) in MCF7 cells (5×105 cells seeded), and (d) pDNA-dose dependent transfection efficiency of “reconstituted” micelle/pDNA/bPEI1.8kDa (WR 1) complexes in MCF7 cells. When using 1 μg of pDNA, its concentration was 0.5 μg/mL. [37] (Reproduced with permission)
3. Concluding Remarks
It is not yet a viable option to create a simple and universal polymeric carrier system that can meet pharmaceutical requirements and perform all of the functions necessary for both transport to and transfection of every target cell to elicit the intended biological effects. Each disease presents a unique pathophysiological environment and biological barriers to delivery. The drug delivery vehicles used to treat specific diseases should be specifically designed considering such factors, similar to viruses that have evolved to infect living bodies in a species/organ/tissue/cell-specific manner. These carriers should thus possess a minimum number of constituting components.
Acknowledgments
This work was supported by NIH GM82866 (Y.H. Bae), Basic Science Research Program through National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (M-2012-A0154-00010, H.C. Kang), and Research Fund of The Catholic University of Korea (M-2011-B0014-00002, H.C. Kang).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bumcrot D, Manoharan M, Koteliansky V, Sah DW. RNAi therapeutics: a potential new class of pharmaceutical drugs. Nat Chem Biol. 2006 Dec;2(12):711–719. doi: 10.1038/nchembio839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Glover DJ, Lipps HJ, Jans DA. Towards safe, non-viral therapeutic gene expression in humans. Nat Rev Genet. 2005 Apr;6(4):299–310. doi: 10.1038/nrg1577. [DOI] [PubMed] [Google Scholar]
- 3.Kim DH, Rossi JJ. Strategies for silencing human disease using RNA interference. Nat Rev Genetics. 2007;8:173–184. doi: 10.1038/nrg2006. [DOI] [PubMed] [Google Scholar]
- 4.Ferber D. Gene therapy: safer and virus-free? Science. 2001;294:1638–1642. doi: 10.1126/science.294.5547.1638. [DOI] [PubMed] [Google Scholar]
- 5.Watts JK, Deleavey GF, Damha MJ. Chemically modified siRNA: tools and applications. Drug Discov Today. 2008 Jul 7;13:842–855. doi: 10.1016/j.drudis.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 6.Brus C, Petersen H, Aigner A, Czubayko F, Kissel T. Efficiency of polyethylenimines and polyethylenimine-graft-poly (ethylene glycol) block copolymers to protect oligonucleotides against enzymatic degradation. Eur J Pharm Biopharm. 2004;57:427–430. doi: 10.1016/j.ejpb.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 7.Takahashi Y, Nishikawa M, Takakura Y. Nonviral vector-mediated RNA interference: its gene silencing characteristics and important factors to achieve RNAi-based gene therapy. Adv Drug Deliv Rev. 2009 Jul 25;61(9):760–766. doi: 10.1016/j.addr.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 8.Kim WJ, Kim SW. Efficient siRNA Delivery with Non-viral Polymeric Vehicles. Pharm Res. 2009 Mar;26(3):657–666. doi: 10.1007/s11095-008-9774-1. [DOI] [PubMed] [Google Scholar]
- 9.Kang HC, Lee M, Bae YH. Polymeric gene carriers. Crit Rev Eukaryot Gene Expr. 2005;15:317–342. doi: 10.1615/critreveukargeneexpr.v15.i4.30. [DOI] [PubMed] [Google Scholar]
- 10.Christie RJ, Nishiyama N, Kataoka K. Delivering the code: polyplex carriers for deoxyribonucleic acid and ribonucleic acid interference therapies. Endocrinology. 2010 Feb;151(2):466–473. doi: 10.1210/en.2009-1045. [DOI] [PubMed] [Google Scholar]
- 11.Bruno K. Using drug-excipient interactions for siRNA delivery. Adv Drug Deliv Rev. 2011 Oct;63(13):1210–1226. doi: 10.1016/j.addr.2011.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Spitnik P, Lipshitz R, Chargaff E. Studies in nucleoproteins. III. Deoxyribonucleic acid complexes with basic polyelectrolytes and their fractional extraction. J Biol Chem. 1954;215:765–775. [PubMed] [Google Scholar]
- 13.Wu G, Wu C. Receptor-mediated in vitro gene transformation by a soluble DNA carrier system. J Biol Chem. 1987;262:4429–4432. [PubMed] [Google Scholar]
- 14.Boussif O, Lezoualc’h F, Zanta MA, Mergny MD, Scherman D, Demeneix B, et al. A versatile vector for gene and oligonucleotide transfer into cells in culture and in vivo: polyethylenimine. Proc Natl Acad Sci USA. 1995;92:7297–7301. doi: 10.1073/pnas.92.16.7297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Putnam D. Polymers for gene delivery across length scales. Nat Mater. 2006 Jun;5(6):439–451. doi: 10.1038/nmat1645. [DOI] [PubMed] [Google Scholar]
- 16.Kay MA. AAV vectors and tumorigenicity. Nat Biotechnol. 2007 Oct;25(10):1111–1113. doi: 10.1038/nbt1007-1111. [DOI] [PubMed] [Google Scholar]
- 17.Wagner E. Polymers for siRNA Delivery: Inspired by Viruses to be Targeted, Dynamic, and Precise. Acc Chem Res. 2012 Dec 22; doi: 10.1021/ar2002232. In press. [DOI] [PubMed] [Google Scholar]
- 18.Edinger D, Wagner E. Bioresponsive polymers for the delivery of therapeutic nucleic acids. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2011 Jan-Feb;3(1):33–46. doi: 10.1002/wnan.97. [DOI] [PubMed] [Google Scholar]
- 19.Schaffert D, Wagner E. Gene therapy progress and prospects: synthetic polymer-based systems. Gene Ther. 2008 Aug;15(16):1131–1138. doi: 10.1038/gt.2008.105. [DOI] [PubMed] [Google Scholar]
- 20.Kang HC, Kang HJ, Bae YH. A reducible polycationic gene vector derived from thiolated low molecular weight branched polyethyleneimine linked by 2-iminothiolane. Biomaterials. 2011;32:1193–1203. doi: 10.1016/j.biomaterials.2010.08.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meng F, Hennink WE, Zhong Z. Reduction-sensitive polymers and bioconjugates for biomedical applications. Biomaterials. 2009 Feb 4;30:2180–2198. doi: 10.1016/j.biomaterials.2009.01.026. [DOI] [PubMed] [Google Scholar]
- 22.Ou M, Xu R, Kim SH, Bull DA, Kim SW. A family of bioreducible poly(disulfide amine)s for gene delivery. Biomaterials. 2009 Oct;30(29):5804–5814. doi: 10.1016/j.biomaterials.2009.06.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim YH, Park JH, Lee M, Kim YH, Park TG, Kim SW. Polyethylenimine with acid-labile linkages as a biodegradable gene carrier. J Control Release. 2005 Mar 2;103(1):209–219. doi: 10.1016/j.jconrel.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 24.Burke RS, Pun SH. Extracellular barriers to in Vivo PEI and PEGylated PEI polyplex-mediated gene delivery to the liver. Bioconjug Chem. 2008 Mar;19(3):693–704. doi: 10.1021/bc700388u. [DOI] [PubMed] [Google Scholar]
- 25.Lee M, Kim SW. Polyethylene glycol-conjugated copolymers for plasmid DNA delivery. Pharm Res. 2005;22:1–10. doi: 10.1007/s11095-004-9003-5. [DOI] [PubMed] [Google Scholar]
- 26.Cho YW, Kim JD, Park K. Polycation gene delivery systems: escape from endosomes to cytosol. J Pharm Pharmacol. 2003;55:721–734. doi: 10.1211/002235703765951311. [DOI] [PubMed] [Google Scholar]
- 27.Kang HC, Bae YH. pH-Tunable endosomolytic oligomers for enhanced nucleic acid delivery. Adv Funct Mater. 2007;17:1263–1272. [Google Scholar]
- 28.Forrest ML, Pack DW. On the kinetics of polyplex endocytic trafficking: implications for gene delivery vector design. Mol Ther. 2002;6:57–66. doi: 10.1006/mthe.2002.0631. [DOI] [PubMed] [Google Scholar]
- 29.Kang HC, Samsonova O, Bae YH. Trafficking microenvironmental pH of gene vector polycation in drug-sensitive and multidrug-resistant MCF7 breast cancer cell. Biomaterials. 2010;31:3071–3078. doi: 10.1016/j.biomaterials.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kang HC, Samsonova O, Kang SW, Bae YH. The effect of environmental pH on polymeric transfection efficiency. Biomaterials. 2012 Feb;33(5):1651–1662. doi: 10.1016/j.biomaterials.2011.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bae YH, Park K. Targeted drug delivery to tumors: myths, reality and possibility. J Control Release. 2011 Aug 10;153(3):198–205. doi: 10.1016/j.jconrel.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rebuffat A, Bernasconi A, Ceppi M, Wehrli H, Verca SB, Ibrahim M, et al. Selective enhancement of gene transfer by steroid-mediated gene delivery. Nat Biotechnol. 2001 Dec;19(12):1155–1161. doi: 10.1038/nbt1201-1155. [DOI] [PubMed] [Google Scholar]
- 33.Rebuffat AG, Nawrocki AR, Nielsen PE, Bernasconi AG, Bernal-Mendez E, Frey BM, et al. Gene delivery by a steroid-peptide nucleic acid conjugate. Faseb J. 2002 Sep;16(11):1426–1428. doi: 10.1096/fj.01-0706fje. [DOI] [PubMed] [Google Scholar]
- 34.Gruneich JA, Price A, Zhu J, Diamond SL. Cationic corticosteroid for nonviral gene delivery. Gene Ther. 2004 Apr;11(8):668–674. doi: 10.1038/sj.gt.3302214. [DOI] [PubMed] [Google Scholar]
- 35.Sethuraman VA, Na K, Bae YH. pH-responsive sulfonamide/PEI system for tumor specific gene delivery: In vitro study. Biomacromolecules. 2006;7:64–70. doi: 10.1021/bm0503571. [DOI] [PubMed] [Google Scholar]
- 36.Kang HC, Bae YH. Co-delivery of small interfering RNA and plasmid DNA using a polymeric vector incorporating endosomolytic oligomeric sulfonamide. Biomaterials. 2011 Jul;32(21):4914–4924. doi: 10.1016/j.biomaterials.2011.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mishra D, Kang HC, Bae YH. Reconstitutable charged polymeric (PLGA)2-b-PEI micelles for gene therapeutics delivery. Biomaterials. 2011 Feb 25;32:3845–3854. doi: 10.1016/j.biomaterials.2011.01.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Volk T, Jahde E, Fortmeyer HP, Glusenkamp KH, Rajewsky MF. pH in human tumour xenografts: effect of intravenous administration of glucose. Br J Cancer. 1993 Sep;68(3):492–500. doi: 10.1038/bjc.1993.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sarantopoulos C, McCallum B, Sapunar D, Kwok WM, Hogan Q. ATP-sensitive potassium channels in rat primary afferent neurons: the effect of neuropathic injury and gabapentin. Neurosci Lett. 2003 Jun 12;343(3):185–189. doi: 10.1016/s0304-3940(03)00383-5. [DOI] [PubMed] [Google Scholar]
- 40.Hunjan S, Mason RP, Mehta VD, Kulkarni PV, Aravind S, Arora V, et al. Simultaneous intracellular and extracellular pH measurement in the heart by 19F NMR of 6-fluoropyridoxol. Magn Reson Med. 1998 Apr;39(4):551–556. doi: 10.1002/mrm.1910390407. [DOI] [PubMed] [Google Scholar]
- 41.Li SD, Huang L. Targeted delivery of antisense oligodeoxynucleotide and small interference RNA into lung cancer cells. Mol Pharmaceutics. 2006 Sep-Oct;3(5):579–588. doi: 10.1021/mp060039w. [DOI] [PubMed] [Google Scholar]
- 42.Chono S, Li SD, Conwell CC, Huang L. An efficient and low immunostimulatory nanoparticle formulation for systemic siRNA delivery to the tumor. J Control Release. 2008 Oct 6;131(1):64–69. doi: 10.1016/j.jconrel.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mishra V, Gupta U, Jain NK. Biowaiver: an alternative to in vivo pharmacokinetic bioequivalence studies. Pharmazie. 2010;65(3):155–161. [PubMed] [Google Scholar]
- 44.Fuchs GS, Mikkelsen S, Knudsen TK, Kappelgaard A-M. Ease of use and acceptability of a new pen device for the administration of growth hormone therapy in pediatric patients: An open-label, uncontrolled usability test. Clin Ther. 2009;31(12):2906–2914. doi: 10.1016/j.clinthera.2009.12.014. [DOI] [PubMed] [Google Scholar]
- 45.Collier J, Shah R, Gupta A, Sayeed V, Habib M, Khan M. Influence of Formulation and Processing Factors on Stability of Levothyroxine Sodium Pentahydrate. AAPS PharmSciTech. 2010;11(2):818–825. doi: 10.1208/s12249-010-9434-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McCluskey SV, Graner KK, Kemp J, Aloumanis V, Ben M, Kupiec T, et al. Stability of fentanyl 5 {micro}g/mL diluted with 0.9% sodium chloride injection and stored in polypropylene syringes. Am J Health Syst Pharm. 2009 May 1;66(9):860–863. doi: 10.2146/ajhp080255. [DOI] [PubMed] [Google Scholar]
- 47.Bulmus V, Chan Y, Nguyen Q, Tran HL. Synthesis and Characterization of Degradable p(HEMA) Microgels: Use of Acid-Labile Crosslinkers. Macromol Biosci. 2007;7(4):446–455. doi: 10.1002/mabi.200600258. [DOI] [PubMed] [Google Scholar]
- 48.Molina MdC, Allison SD, Anchordoquy TJ. Maintenance of nonviral vector particle size during the freezing step of the lyophilization process is insufficient for preservation of activity: Insight from other structural indicators. J Pharm Sci. 2001;90(10):1445–1455. doi: 10.1002/jps.1096. [DOI] [PubMed] [Google Scholar]
- 49.Thompson C, Hansford D, Higgins S, Rostron C, Hutcheon G, Munday D. Preparation and evaluation of microspheres prepared from novel polyester-ibuprofen conjugates blended with non-conjugated ibuprofen. J Microencapsul. 2009;26(8):676–683. doi: 10.3109/02652040802656333. [DOI] [PubMed] [Google Scholar]
- 50.Miene C, Klenow S, Veeriah S, Richling E, Glei M. Impact of apple polyphenols on GSTT2 gene expression, subsequent protection of DNA and modulation of proliferation using LT97 human colon adenoma cells. Mol Nutr Food Res. 2009;53(10):1254–1262. doi: 10.1002/mnfr.200800444. [DOI] [PubMed] [Google Scholar]