Abstract
Background
Temporal summation of second pain (TSSP) is relevant for the study of central sensitization, and refers to increased pain evoked by repetitive stimuli at a constant intensity. While the literature reports on participants whose pain ratings increase with successive stimuli, response to a TSSP protocol can be variable. The aim of this study was to characterize the full range of responses to a TSSP protocol in pain-free adults.
Method
Three hundred twelve adults received a train of brief, repetitive heat stimuli at a fixed temperature and rated the intensity of second pain after each pulse. TSSP response (Δ in pain ratings) was quantified using the most common methods in the literature, and response groups were formed: TSSP (Δ>0), no change (Δ=0), and temporal decrease in second pain (TDSP) (Δ<0). A cluster analysis was performed on the Δ values to empirically derive response groups.
Results
Depending on how TSSP response was quantified, 61 – 72% of the sample demonstrated TSSP, 11 – 28% had no change in pain ratings, and 0 – 20% demonstrated TDSP. The cluster analysis found that the majority (59%) of participants fell in the no change cluster, 29% clustered into the TSSP group, and 12% in the TDSP cluster.
Conclusions
Using a fixed thermal paradigm, pain-free adults exhibit substantial variability in response to a TSSP protocol not well characterized by group-mean slopes. Studies are needed to determine TSSP response patterns in clinical samples, identify predictors of response, and determine the clinical implications of response variability.
Keywords: temporal summation of pain, wind-up, second pain, quantitative sensory testing, central sensitization
INTRODUCTION
Temporal summation of second pain (TSSP) is a C-fiber mediated process referring to an increase in pain evoked by repetitive noxious stimuli at constant intensities, with an inter-stimulus frequency of 0.33Hz or less (Price, 1972; Price and Dubner, 1977). Thought to represent a psychophysical correlate of excitatory neural activity in the dorsal horn of the spinal cord (i.e., wind-up), TSSP has been found to be mediated by central nervous system mechanisms (Price and Dubner, 1977). Studies have shown that TSSP magnitude is increased in a number of chronic pain populations relative to healthy controls, including patients with fibromyalgia (Staud et al., 2003; Staud et al., 2008), temporomandibular disorders (Maixner et al., 1998), and functional abdominal pain (Dengler-Crish et al., 2011). It is therefore relevant to the study of central sensitization, a mechanism hypothesized to underlie many chronic pain conditions (Yunus, 2007). Furthermore, TSSP magnitude has been demonstrated to be higher in females compared to males (Fillingim et al., 1998) and among the elderly (Edwards and Fillingim, 2001), suggesting potential modulation of endogenous pain processing in these respective groups.
Given the importance of TSSP to the study of chronic pain and age or sex-related differences in pain processing, a review of the literature reveals a surprising lack of standardization in the design of experimental TSSP protocols and how TSSP-related data are reported. This is important given the aforementioned clinical relevance and because the TSSP response is known to be sensitive to alterations in experimental parameters, including stimulus frequency (TSSP is maximally elicited in humans using frequencies of 0.3 – 1.0 Hz) (Nielsen and Arendt-Nielsen, 1998; Price and Dubner, 1977) and intensity (Pedersen et al., 1998). Fixed parameter paradigms (Robinson et al., 2004) have been used in addition to protocols that tailor stimulus intensity to individual pain threshold (Granot et al., 2006; Staud et al., 2004). Quantification of response to a TSSP protocol has also been inconsistent as at least five different methods of calculation have been used in previous studies to evaluate response to a TSSP protocol (Granot et al., 2006; Hastie et al., 2005; Raphael et al., 2009; Robinson et al., 2004; Rolke et al., 2006). The magnitude of TSSP response may vary depending on the method of statistical calculation (Granot et al., 2006; Svendsen et al., 1999).
Similarly, the literature to date has primarily reported on participants in TSSP protocols whose pain ratings increase with the number of stimuli (i.e., temporal summation). Results are often reported for groups whose aggregate response slopes are positive. However, response to a TSSP protocol can vary across individuals, and there is evidence to suggest that some participants demonstrate no change or a temporal decrease in second pain (TDSP) (Rolke et al., 2006). This variability is absent or understated in the literature and currently no study has described the full variability of TSSP response. Accordingly, the aim of the present study was to descriptively characterize the TSSP response in a large sample of healthy young adults using the primary calculation methods found in the literature.
METHODS
This report presents a secondary analysis of data aggregated from 5 previously published studies (Alappattu et al., 2011; Bialosky et al., 2008; Bishop et al., 2011; George et al., 2006a; Robinson et al., 2004). The purpose, design, and findings from these studies are described elsewhere in detail (Alappattu et al., 2011; Bialosky et al., 2008; Bishop et al., 2011; George et al., 2006a; Robinson et al., 2004), and the relevant methods are summarized here. All studies used similar eligibility criteria and quantitative sensory testing procedures, and TSSP response did not differ by data source (see results); thus the studies were deemed appropriate to aggregate by the study authors. The University of Florida Institutional Review Board approved all studies and written informed consent was obtained from all participants before evaluation.
Participants
Participants were recruited through print advertisements placed around the University of Florida campus and through undergraduate and graduate level classes. Adults (≥18 years of age) not currently experiencing clinical pain were included in the analysis. Participants provided demographic information before undergoing quantitative sensory testing. The pooled sample consisted of 312 participants, 62.3% female. The mean age of the sample was 22.9 years (standard deviation, 3.2 years; age range, 18 – 40 years). Information on race was only available for a subsample of 182 participants; 70.9% reported their race as Caucasian, 6.0% reported as African American, 9.9% were Hispanic, 9.9% were Asian, and 3.3% of participants reported their race as “other.”
Procedure
All participants underwent quantitative sensory testing using thermal heat and a temporal summation of second pain protocol. The thermal stimuli were delivered by a computer-controlled Medoc Neurosensory Analyzer (TSA-2001, Ramat Yishai, Israel) via a 30*30mm Peltier thermode. The TSSP protocols used trains of thermal stimuli consisting of either 5 (Robinson et al., 2004) or 10 (Alappattu et al., 2011; Bialosky et al., 2008; Bishop et al., 2011; George et al., 2006a) consecutive pulses of suprathreshold heat stimuli delivered to either the thenar surface of the palm (N = 195) (Bialosky et al., 2008; George et al., 2006a; Robinson et al., 2004) or the plantar surface of the foot (N = 117) (Alappattu et al., 2011; Bishop et al., 2011). Each stimulus started at a baseline temperature of 39°C – 41°C, peaked at 51°C, then returned to baseline with a rise and decline rate of 10°C/s. The duration of each stimulus was approximately 1 second with a 3 second interval separating the peak of each stimulus (i.e., stimulus frequency = 0.33Hz). Participants were instructed to attend to the delayed pain sensation felt after each heat pulse (i.e., second pain), and verbally rate the intensity of this sensation using a numerical scale from 0 (no pain sensation) to 100 (most intense pain imaginable). Before the test, participants underwent one practice session to acclimate them to the thermal stimuli and pain rating system.
The 78 participants in the Robinson et al. (Robinson et al., 2004) study received a train of 5 heat stimuli and rated pulses 1, 3, and 5, while the 234 participants in the other 4 studies received a train of 10 heat stimuli and rated every pulse. To standardize the evaluation of TSSP response across datasets, only the ratings from pulses 1, 3, and 5 were used in the analyses. It has been demonstrated that A-fiber mediated first pain is significantly diminished by the 4th pulse, leaving only the experience of C-fiber mediated second pain thereafter (Price et al., 1977). Additionally, peak temporal summation occurs in the first 4 stimuli in humans, and response saturates thereafter (Herrero et al., 2000). Thus, the choice of limiting our analysis to the first 5 stimuli pulses is appropriate to evaluate TSSP response and allows for better consistency across the studies.
Data Analysis
The Statistical Package for the Social Sciences (SPSS, version 18.0) was used for all analyses. Descriptive statistics were generated for demographic variables. TSSP response was characterized using the four most common methods of calculation found in the literature. Formula-1 calculated the absolute difference between the 5th pulse pain rating and 1st pulse pain rating (Granot et al., 2006; Pedersen et al., 1998; Robinson et al., 2004). Formula-2 used the difference between the maximum pain rating and the 1st pulse pain rating (Hastie et al., 2005). In Formula-3, hierarchical linear modeling was used to estimate the slope of change in pain ratings for each participant (Dengler-Crish et al., 2011; Raphael et al., 2009). Finally, Formula-4 used a ratio of the 5th pulse pain rating to the 1st pulse pain rating (Rolke et al., 2006; Uhl et al., 2011).
Using the data generated from each of the calculation formulas described above, TSSP response (i.e., Δ in pain ratings) was also characterized categorically to determine the prevalence of temporal summation, no change, and TDSP. Using the data from formulas 1, 2, and 3, a participant showed TSSP if their Δ value was > 0. They were characterized as having no change if their Δ value equaled 0, and they demonstrated TDSP if their Δ value was < 0. Using data from Formula-4, a participant demonstrated TSSP if their Δ value was > 1. They were characterized as having no change if their Δ value equaled 1, and they demonstrated TDSP if their Δ value was < 1. Finally, to establish empirically derived TSSP response groups, a hierarchical cluster analysis (using Ward’s method and a squared Euclidian distance measure interval) was performed on the data from Formula-1 in order to identify homogenous subgroups in the data. The appropriate cluster solution was determined by calculating the percentage change between clusters (i.e., the “step change”). The point at which the percentage increase is significantly larger than that of previous steps indicates that dissimilar clusters have been merged, and thus suggests the optimal solution (Hair et al., 2006).
To determine whether site of stimulation (palm vs. foot) or data source (the 5 pooled studies) introduced systematic variance into TSSP response, independent t-tests and analyses of variance were used to compare TSSP response group means. To test for demographic differences in TSSP response, independent t-tests were used to compare to compare mean response in males vs. females, and Pearson r correlations were used to determine the strength of the association between age and TSSP response. Finally, Pearson r correlations were also used to examine the strength of the association between the 4 methods of TSSP response calculation.
RESULTS
Mean TSSP response did not differ by site of stimulation [Tables 1 – 4] or by data source [F(5,306) = .57 – 1.01, p > .05 for all tests] for any of the 4 calculation formulas. Thus, neither location of the test nor the data source introduced systematic variance into the TSSP response analyses, so the following results report on combined data for anatomical location and for data source. TSSP response magnitude did not differ significantly by sex for any of the 4 calculation methods [Tables 1 – 4], and age was not significantly correlated with TSSP response for any of the 4 calculation methods (r = −.06 – .02, p > .05 for all comparisons).
Table 1.
TSSP Response Using Calculation Formula-1 (N = 310)
| Response Group |
Definition | N | % | Mean (SD) | Min - Max |
|---|---|---|---|---|---|
| All | 5th pulse minus 1st pulse | 310 | 100 | 6.8 (16.0) | −70.0 – 75.0 |
|
| |||||
| Male | 5th pulse minus 1st pulse | 117 | 37.7 | 7.6 (15.1) | −40.0 – 50.0 |
| Female | 5th pulse minus 1st pulse | 193 | 62.3 | 6.3 (16.6) | −70.0 – 75.0 |
|
| |||||
| Palm | 5th pulse minus 1st pulse | 195 | 62.9 | 6.3 (16.4) | −70.0 – 75.0 |
| Foot | 5th pulse minus 1st pulse | 117 | 37.1 | 7.6 (15.3) | −30.0 – 60.0 |
|
| |||||
| TSSP | 5th pulse minus 1st pulse > 0 | 192 | 61.9 | 15.2 (12.2) | 2.0 – 75.0 |
| No Change | 5th pulse minus 1st pulse = 0 | 65 | 21.0 | 0 (0) | 0.0 – 0.0 |
| TDSP | 5th pulse minus 1st pulse < 0 | 53 | 17.1 | −15.2 (12.9) | −70.0 – −2.0 |
Mean comparisons by sex or site of stimulation did not reach statistical significance.
Table 4.
*TSSP Response Using Calculation Formula-4 (N = 296)
| Response Group |
Definition | N | % | Mean (SD) | Min - Max |
|---|---|---|---|---|---|
| All | Ratio of 5th pulse to 1st pulse | 296 | 100 | 1.4 (1.0) | 0.0 – 8.0 |
|
| |||||
| Male | Ratio of 5th pulse to 1st pulse | 109 | 36.9 | 1.5 (0.8) | 0.0 – 6.0 |
| Female | Ratio of 5th pulse to 1st pulse | 186 | 63.1 | 1.4 (1.0) | 0.0 – 8.0 |
|
| |||||
| Palm | Ratio of 5th pulse to 1st pulse | 180 | 60.8 | 1.5 (1.1) | 0.0 – 8.0 |
| Foot | Ratio of 5th pulse to 1st pulse | 116 | 39.2 | 1.4 (0.7) | 0.2 – 4.3 |
|
| |||||
| TSSP | Ratio of 5th pulse to 1st pulse > 1 | 179 | 60.5 | 1.8 (1.0) | 1.02 – 8.0 |
| No Change | Ratio of 5th pulse to 1st pulse = 1 | 65 | 22.0 | 1.0 (0.0) | 1.0 – 1.0 |
| TDSP | Ratio of 5th pulse to 1st pulse < 1 | 52 | 17.6 | 0.6 (0.3) | 0.0 – 0.9 |
16 participants had a pain rating of 0 for the 1st pulse. Therefore a ratio could not be calculated for these cases and they were left out of this analysis.
Mean comparisons by sex or site of stimulation did not reach statistical significance.
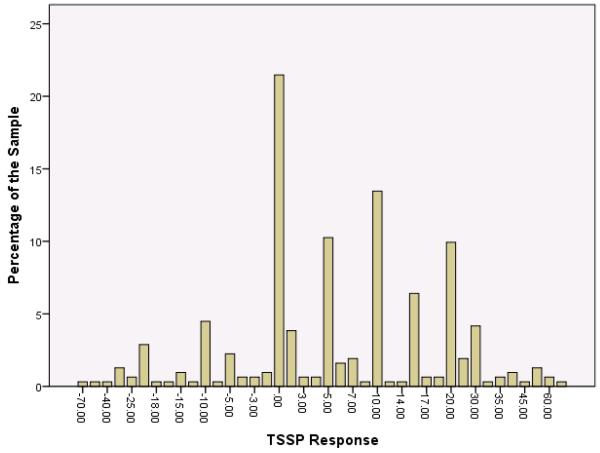
Tables 1 – 4 present the TSSP response means, standard deviations, minimum / maximum values, and prevalence of response groups (i.e., TSSP, no change, and TDSP). Using Formula-1, the mean difference between the 5th and 1st pulse ratings was 6.8±16.0 (range, −70.0 – 75.0), and the prevalence of TSSP, no change, and TDSP was 61.9%, 21.0%, 17.1%, respectively (Table 1). Figure 1 graphically demonstrates the distribution of TSSP response across the sample using Formula-1: a relatively normal distribution with a modal peak at 0. Using Formula-2, the mean difference between the maximal pain rating and the 1st pulse rating was 10.6±12.1 (range, 0.0 – 75.0), and the prevalence of temporal summation, no change, and TDSP was 72.4%, 27.6%, and 0%, respectively (Table 2). Mean slope of change in pain ratings (Formula-3) was 1.5±2.8; (range, −10.3 – 13.1), and the prevalence of temporal summation, no change, and TDSP was 69.2%, 11.2%, and 19.6%, respectively (Table 3). Finally, the mean ratio of 5th pulse rating to 1st pulse rating (Formula-4) was 1.4±1.0 (range, 0.0 – 8.0), and the prevalence of temporal summation, no change, and TDSP was 60.5%, 22.0%, 17.6%, respectively (Table 4).
Figure 1.
The distribution of responses to a fixed parameter TSSP protocol using calculation Formula-1: 1st pulse pain rating subtracted from the 5th pulse pain rating.
Table 2.
TSSP Response Using Calculation Formula-2 (N = 312)
| Response Group |
Definition | N | % | Mean (SD) | Min - Max |
|---|---|---|---|---|---|
| All | Maximal pulse minus 1st pulse | 312 | 100 | 10.6 (12.1) | 0.0 – 75.0 |
|
| |||||
| Male | Maximal pulse minus 1st pulse | 117 | 37.7 | 11.0 (12.0) | 0.0 – 50.0 |
| Female | Maximal pulse minus 1st pulse | 193 | 62.3 | 10.4 (12.2) | 0.0 – 75.0 |
|
| |||||
| Palm | Maximal pulse minus 1st pulse | 195 | 62.9 | 9.9 (12.1) | 0.0 – 75.0 |
| Foot | Maximal pulse minus 1st pulse | 117 | 37.1 | 11.7 (11.9) | 0.0 – 60.0 |
|
| |||||
| TSSP | Maximal pulse minus 1st pulse > 0 | 226 | 72.4 | 14.5 (11.9) | 1.0 – 75.0 |
| No Change | Maximal pulse minus 1st pulse = 0 | 86 | 27.6 | 0.0 (0.0) | 0.0 – 0.0 |
| TDSP | Maximal pulse minus 1st pulse < 0 | 0 | 0 | -- | -- |
Mean comparisons by sex or site of stimulation did not reach statistical significance.
Table 3.
TSSP Response Using Calculation Formula-3 (N = 312)
| Response Group |
Definition | N | % | Mean (SD) | Min - Max |
|---|---|---|---|---|---|
| All | Slope of change in pain ratings | 312 | 100 | 1.5 (2.8) | −10.3 – 13.1 |
|
| |||||
| Male | Slope of change in pain ratings | 117 | 37.7 | 1.6 (2.6) | −6.3 – 9.1 |
| Female | Slope of change in pain ratings | 193 | 62.3 | 1.4 (2.9) | −10.3 – 13.1 |
|
| |||||
| Palm | Slope of change in pain ratings | 195 | 62.9 | 1.4 (2.8) | −10.3 – 13.1 |
| Foot | Slope of change in pain ratings | 117 | 37.1 | 1.6 (2.8) | −6.0 – 12.0 |
|
| |||||
| TSSP | Slope of change in pain ratings > 0 | 216 | 69.2 | 2.7 (2.2) | 0.1 – 13.1 |
| No Change | Slope of change in pain ratings = 0 | 35 | 11.2 | 0.0 (0.0) | 0.0 – 0.0 |
| TDSP | Slope of change in pain ratings < 0 | 61 | 19.6 | −2.0 (2.2) | −10.3 – −0.5 |
Mean comparisons by sex or site of stimulation did not reach statistical significance.
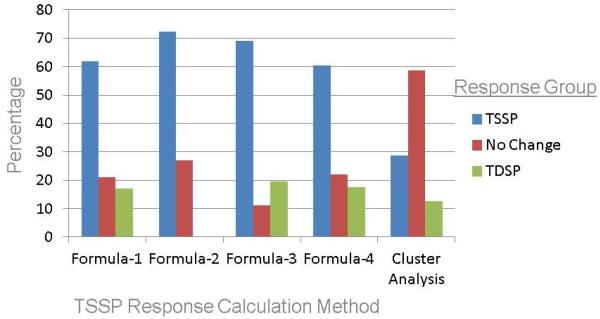
Examination of the agglomeration coefficients for the hierarchical cluster analysis yielded 3 distinct clusters of TSSP response (Table 5). The majority (58.7%) of participants fell in the no change cluster (mean TSSP response, 3.6; standard deviation, 4.5; range −5.0 – 12.0), while 28.7% clustered into the temporal summation group (mean TSSP response, 24.8; standard deviation, 11.8; range 14.0 – 75.0), and 12.6% fell in the TDSP cluster (mean TSSP response, −19.3; standard deviation, 12.8; range, −70.0 – −8.0). Figure 2 graphically presents the percentage of participants in each TSSP response group for the four calculation formulas and the cluster analysis.
Table 5.
Cluster Analysis on TSSP Response Data Using Calculation Formula-1 (N = 310)
| Response Group | N | % | Mean (SD) | Min - Max |
|---|---|---|---|---|
| Cluster 1 (TSSP) | 89 | 28.7 | 24.8 (11.8) | 14.0 – 75.0 |
| Cluster 2 (no change) | 182 | 58.7 | 3.6 (4.5) | −5.0 – 12.0 |
| Cluster 3 (TDSP) | 39 | 12.6 | −19.3 (12.8) | −70.0 – −8.0 |
Figure 2.
Percentage of participants in each TSSP response group for the four calculation formulas and the cluster analysis.
DISCUSSION
The present study is the first to characterize the full variability in responses to a TSSP protocol in a sample of pain-free young adults. It is also the first to explore the various methods of TSSP response calculation found in the literature, and determine the prevalence of categorical response groups depending on the method of quantification. We conclude that a fixed, thermal heat paradigm yields substantial variability in response that is not well characterized by group mean response slopes. Using the four primary calculation methods found in the literature, 28 – 40% of the sample demonstrated a no change or TDSP response, while 60 – 72% showed temporal summation. Alternatively, empirically derived TSSP response groups suggest that the majority (58%) of participants experienced no significant change in pain during the TSSP protocol, while only about one-third of the sample demonstrated temporal summation.
The majority of studies in the TSSP literature report on aggregate results, often in order to compare TSSP response between two or more groups of participants (e.g., healthy vs. clinical). However, several studies have mentioned response variability in their reports on TSSP in healthy adult samples. Using pinprick stimuli on three locations across the body, Rolke et al. (Rolke et al., 2006) noted that “the absence of wind-up is normal, and wind-up ratio has to show clear wind-down to be abnormal.” Our results confirm this finding in a thermal heat paradigm; a temporal summation response, a no change response, and even a slight TDSP response all fell within 1 standard deviation of the mean, suggesting that only extreme TSSP or TDSP responses should be considered abnormal. Using electrical stimuli, Pederson et al. (Pedersen et al., 1998) found a response pattern similar to the present study, and using thermal heat paradigms, Granot et al. (Granot et al., 2003) and Raphael et al. (Raphael et al., 2009) found that temporal summation could be induced in 56% and 50 – 70% of the participants tested, respectively. In sum, the findings from these four studies support our conclusion: a substantial portion of healthy adult participants do not demonstrate temporal summation of pain in response to a TSSP protocol. This variability in response appears to be robust, occurring across different stimulus modalities and durations, and different locations on the body.
Variability in response to a TSSP protocol may, in part, be accounted for by methodological considerations. The literature has defined TSSP response in a variety of ways, and our study demonstrated that the definition used to quantify TSSP response can affect both the magnitude of response and the frequency of individuals meeting criteria for each response category. For example, an individual with a first pulse pain rating of 50 and a fifth pulse pain rating of 45 meets criteria for TDSP using calculation formulas 1, 3, and 4, but would be classified as having no change using formula 2 and the cluster analysis. The response categories determined by the cluster analysis present an empirically-derived alternative to simply using the sign of the number (positive, negative, or 0) to determine the category. It is up for debate whether minimal perceptual changes in pain during the course of a TSSP protocol truly represent the TSSP (or TDSP) phenomenon or just chance fluctuations in pain ratings. If the goal is to isolate participants who exhibit a TSSP or TDSP response, future studies might consider using cut points that specify a minimum increase or decrease in pain necessary to be considered TSSP or TDSP. While our cluster analysis outlines one possible set of cut point values, it is likely that these values are not universal, but rather they are dependent upon experimental parameters like stimulus type and intensity. An alternative strategy is to consider TSSP as a continuous measure and instead study the phenomenon as an individual difference construct, and examine models that predict its variability both within and between individuals.
Alterations in experimental parameters have been shown to affect the magnitude of TSSP. TSSP requires a stimulus repetition of at least once every 3 seconds, and higher intensity stimuli generally provoke a greater magnitude of TSSP response than lower intensity stimuli (Nielsen and Arendt-Nielsen, 1998; Pedersen et al., 1998). However, the relation between TSSP response and stimulus intensity may be dependent on pain threshold such that a positive correlation exists unless a ceiling is reached on the first stimulus. In other words, if the input stimulus yields an elevated pain rating (e.g. 100) then subsequent ratings can only stay the same or decrease and temporal summation cannot occur. Regarding stimulus modality, TSSP has been demonstrated using heat (Edwards and Fillingim, 2001; Granot et al., 2003), electrical (Pedersen et al., 1998), cold (Price et al., 2002), pinprick (Rolke et al., 2006; Uhl et al., 2011), and mechanical pressure stimuli (Nie et al., 2005; Staud et al., 2003). However, no study to date has directly compared TSSP response magnitude between two modalities, so it is unknown whether one type of stimulus confers an advantage over another in eliciting temporal summation, or whether there is intra-individual specificity of TSSP to stimuli. Similarly, studies examining the effect of stimulus duration on TSSP response are sparse. Granot and colleagues (Granot et al., 2006) found that a single, tonic thermal stimulus results in a TSSP response magnitude similar to that of repetitive phasic stimuli.
Fixed parameter protocols like the one used in the present study employ an unchanging set of experimental parameters that are expected to induce temporal summation. However, as shown in our study, a substantial portion of participants do not demonstrate temporal summation of pain in response to a fixed paradigm, or they do not do so to the same degree. In hopes of maximizing the frequency and magnitude of temporal summation response, recent studies have developed TSSP protocols tailored to the individual by altering the stimulus intensity to match an individual’s pain threshold, and thereby avoiding ceiling effects (Granot et al., 2006; Pedersen et al., 1998; Raphael et al., 2009; Staud et al., 2003; Staud et al., 2006). This individualized design has resulted in more pronounced temporal summation compared to a fixed design in at least one of the studies (Staud et al., 2006). The ceiling effect could potentially explain why 28 - 70% of our sample did not demonstrate temporal summation. However, only 29% of participants who exhibited no change or a decrease in pain ratings had an elevated first pulse pain rating (≥60), suggesting that the ceiling effect did not apply to 71% of those who fail to demonstrate TSSP. While individually tailored protocols may help to optimize TSSP, 27 – 50% of the samples still fail to achieve temporal summation (Pedersen et al., 1998; Raphael et al., 2009) and the ability to compare results across studies is diminished due to the variation in experimental parameters.
Physiological differences in pain processing as well as psychosocial factors may also help to explain the variability in response to a TSSP protocol. Sex (Fillingim et al., 1998; Sarlani et al., 2004) and age (Edwards and Fillingim, 2001; Lautenbacher et al., 2005) differences have been demonstrated as women and older adults have shown enhanced temporal summation relative to men and younger adults, respectively. Furthermore, Robinson et al (Robinson et al., 2004) found that gender role expectations predicted TSSP response in healthy adults, suggesting that social learning factors help account for the sex-related differences in pain perception. The present study failed to find age or sex-related differences in TSSP response, perhaps due to the restricted age range of the sample (primarily undergraduates). Finally, emotion may also influence spinal mechanisms of pain via modulation of descending inhibitory functioning, as it has been demonstrated that anxiety and fear is associated with greater TSSP response (George et al., 2006b; Robinson et al., 2004).
Conclusion and Future Directions
Healthy adults exhibit substantial variability in response to a fixed parameter TSSP protocol. Potentially, this variability in response may be accounted for by methodological considerations (how one quantifies TSSP response), alterations in experimental parameters, psychosocial factors, or by physiological differences in pain processing. Future studies are needed to identify the predictors of response to a TSSP protocol, information that can be used to create response profiles or subgroups, and potentially identify those at risk for central sensitization. Additional work to determine individual differences in stimulus parameters to optimize temporal summation may also prove fruitful.
In this sample of pain-free adults, 11 – 58% of participants had no change in pain ratings, and up to 20% demonstrated a TDSP response. Future studies are needed to examine TSSP response patterns in clinical samples, and to determine the clinical implications of variability in TSSP. More broadly, consistency and standardization in the design of the TSSP protocol and the calculation of TSSP response are lacking in the literature, and our study reveals the variability that this inconsistency can introduce. Development of a standardized TSSP protocol and a normed database of responses has begun in Germany (Rolke et al., 2006), and continued work in this area is necessary to establish the clinical utility of this assessment.
1) What is already known about this topic?
- Temporal summation of second pain (TSSP) is a C-fiber mediated process thought to be relevant to the study of central sensitization.
2) What does this study add?
- Reporting on within-group variability in TSSP response is largely absent in the literature.
- This study is the first to characterize the full variability in response to a TSSP protocol, and explore the various methods of response quantification found in the literature.
Acknowledgments
This work was supported by grants from the National Institutes of Health to Dr. Robinson (R01 DE013208), to Dr. George (AT002796), and to Dr. Bishop (AR54331-4). Support provided to Dr. Bialosky by the National Institutes of Health T-32 Neural Plasticity Research Training Fellowship (T32HD043730).
Footnotes
Authors have no conflicts of interest to declare.
REFERENCES
- Alappattu MJ, Bishop MD, Bialosky JE, George SZ, Robinson ME. Stability of behavioral estimates of activity-dependent modulation of pain. J Pain Res. 2011;4:151–157. doi: 10.2147/JPR.S18105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bialosky JE, Bishop MD, Robinson ME, Barabas JA, George SZ. The influence of expectation on spinal manipulation induced hypoalgesia: an experimental study in normal subjects. BMC Musculoskelet Disord. 2008;9:19. doi: 10.1186/1471-2474-9-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop MD, Horn ME, George SZ, Robinson ME. Self-reported pain and disability outcomes from an endogenous model of muscular back pain. BMC Musculoskelet Disord. 2011;12:35. doi: 10.1186/1471-2474-12-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dengler-Crish CM, Bruehl S, Walker LS. Increased wind-up to heat pain in women with a childhood history of functional abdominal pain. Pain. 2011;152:802–808. doi: 10.1016/j.pain.2010.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards RR, Fillingim RB. Effects of age on temporal summation and habituation of thermal pain: Clinical relevance in healthy older and younger adults. The Journal of Pain. 2001;2:307–317. doi: 10.1054/jpai.2001.25525. [DOI] [PubMed] [Google Scholar]
- Fillingim RB, Maixner W, Kincaid S, Silva S. Sex differences in temporal summation but not sensory-discriminative processing of thermal pain. Pain. 1998;75:121–127. doi: 10.1016/S0304-3959(97)00214-5. [DOI] [PubMed] [Google Scholar]
- George SZ, Bishop MD, Bialosky JE, Zeppieri G, Jr., Robinson ME. Immediate effects of spinal manipulation on thermal pain sensitivity: an experimental study. BMC Musculoskelet Disord. 2006a;7:68. doi: 10.1186/1471-2474-7-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George SZ, Wittmer VT, Fillingim RB, Robinson ME. Fear-avoidance beliefs and temporal summation of evoked thermal pain influence self-report of disability in patients with chronic low back pain. J Occup Rehabil. 2006b;16:95–108. doi: 10.1007/s10926-005-9007-y. [DOI] [PubMed] [Google Scholar]
- Granot M, Granovsky Y, Sprecher E, Nir RR, Yarnitsky D. Contact heat-evoked temporal summation: tonic versus repetitive-phasic stimulation. Pain. 2006;122:295–305. doi: 10.1016/j.pain.2006.02.003. [DOI] [PubMed] [Google Scholar]
- Granot M, Sprecher E, Yarnitsky D. Psychophysics of phasic and tonic heat pain stimuli by quantitative sensory testing in healthy subjects. Eur J Pain. 2003;7:139–143. doi: 10.1016/S1090-3801(02)00087-3. [DOI] [PubMed] [Google Scholar]
- Hair JF, Black WC, Babin BJ, Anderson RE, Tatham RL. Multivariate Data Analysis. Macmillion Publishing Company; New York: 2006. [Google Scholar]
- Hastie BA, Riley JL, 3rd, Robinson ME, Glover T, Campbell CM, Staud R, Fillingim RB. Cluster analysis of multiple experimental pain modalities. Pain. 2005;116:227–237. doi: 10.1016/j.pain.2005.04.016. [DOI] [PubMed] [Google Scholar]
- Herrero JF, Laird JMA, Lopez-Garcia JA. Wind-up of spinal cord neurones and pain sensation: much ado about something? Progress in Neurobiology. 2000;61:169–203. doi: 10.1016/s0301-0082(99)00051-9. [DOI] [PubMed] [Google Scholar]
- Lautenbacher S, Kunz M, Strate P, Nielsen J, Arendt-Nielsen L. Age effects on pain thresholds, temporal summation and spatial summation of heat and pressure pain. Pain. 2005;115:410–418. doi: 10.1016/j.pain.2005.03.025. [DOI] [PubMed] [Google Scholar]
- Maixner W, Fillingim R, Sigurdsson A, Shelley K, Silva S. Sensitivity of patients with painful temporomandibular disorders to experimentally evoked pain: evidence for altered temporal summation of pain. Pain. 1998;76:71–81. doi: 10.1016/s0304-3959(98)00028-1. [DOI] [PubMed] [Google Scholar]
- Nie H, Arendt-Nielsen L, Andersen H, Graven-Nielsen T. Temporal summation of pain evoked by mechanical stimulation in deep and superficial tissue. J Pain. 2005;6:348–355. doi: 10.1016/j.jpain.2005.01.352. [DOI] [PubMed] [Google Scholar]
- Nielsen J, Arendt-Nielsen L. The importance of stimulus configuration for temporal summation of first and second pain to repeated heat stimuli. Eur J Pain. 1998;2:329–341. doi: 10.1016/s1090-3801(98)90031-3. [DOI] [PubMed] [Google Scholar]
- Pedersen JL, Andersen OK, Arendt-Nielsen L, Kehlet H. Hyperalgesia and temporal summation of pain after heat injury in man. Pain. 1998;74:189–197. doi: 10.1016/s0304-3959(97)00162-0. [DOI] [PubMed] [Google Scholar]
- Price DD. Characteristics of second pain and flexion reflexes indicative of prolonged central summation. Experimental Neurology. 1972;37:371–387. doi: 10.1016/0014-4886(72)90081-7. [DOI] [PubMed] [Google Scholar]
- Price DD, Dubner R. Mechanisms of first and second pain in the peripheral and central nervous systems. J Invest Dermatol. 1977;69:167–171. doi: 10.1111/1523-1747.ep12497942. [DOI] [PubMed] [Google Scholar]
- Price DD, Hu JW, Dubner R, Gracely RH. Peripheral suppression of first pain and central summation of second pain evoked by noxious heat pulses. Pain. 1977;3:57–68. doi: 10.1016/0304-3959(77)90035-5. [DOI] [PubMed] [Google Scholar]
- Price DD, Staud R, Robinson ME, Mauderli AP, Cannon R, Vierck CJ. Enhanced temporal summation of second pain and its central modulation in fibromyalgia patients. Pain. 2002;99:49–59. doi: 10.1016/s0304-3959(02)00053-2. [DOI] [PubMed] [Google Scholar]
- Raphael KG, Janal MN, Ananthan S, Cook DB, Staud R. Temporal Summation of Heat Pain in Temporomandibular Disorder Patients. J. Orofac. Pain. 2009;23:54–64. [PMC free article] [PubMed] [Google Scholar]
- Robinson ME, Wise EA, Gagnon C, Fillingim RB, Price DD. Influences of gender role and anxiety on sex differences in temporal summation of pain. The Journal of Pain. 2004;5:77–82. doi: 10.1016/j.jpain.2003.11.004. [DOI] [PubMed] [Google Scholar]
- Rolke R, Baron R, Maier C, Tolle TR, Treede RD, Beyer A, Binder A, Birbaumer N, Birklein F, Botefur IC, et al. Quantitative sensory testing in the German Research Network on Neuropathic Pain (DFNS): standardized protocol and reference values. Pain. 2006;123:231–243. doi: 10.1016/j.pain.2006.01.041. [DOI] [PubMed] [Google Scholar]
- Sarlani E, Grace EG, Reynolds MA, Greenspan JD. Sex differences in temporal summation of pain and aftersensations following repetitive noxious mechanical stimulation. Pain. 2004;109:115–123. doi: 10.1016/j.pain.2004.01.019. [DOI] [PubMed] [Google Scholar]
- Staud R, Cannon RC, Mauderli AP, Robinson ME, Price DD, Vierck CJ. Temporal summation of pain from mechanical stimulation of muscle tissue in normal controls and subjects with fibromyalgia syndrome. Pain. 2003;102:87–95. doi: 10.1016/s0304-3959(02)00344-5. [DOI] [PubMed] [Google Scholar]
- Staud R, Craggs JG, Perlstein WM, Robinson ME, Price DD. Brain activity associated with slow temporal summation of C-fiber evoked pain in fibromyalgia patients and healthy controls. Eur J Pain. 2008;12:1078–1089. doi: 10.1016/j.ejpain.2008.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staud R, Price DD, Fillingim RB. Advanced continuous-contact heat pulse design for efficient temporal summation of second pain (windup) J Pain. 2006;7:575–582. doi: 10.1016/j.jpain.2006.02.005. [DOI] [PubMed] [Google Scholar]
- Staud R, Price DD, Robinson ME, Mauderli AP, Vierck CJ. Maintenance of windup of second pain requires less frequent stimulation in fibromyalgia patients compared to normal controls. Pain. 2004;110:689–696. doi: 10.1016/j.pain.2004.05.009. [DOI] [PubMed] [Google Scholar]
- Svendsen F, Hole K, Tjolsen A. Some problems with wind-up and its calculation. Pain. 1999;83:109–112. [PubMed] [Google Scholar]
- Uhl I, Krumova EK, Regeniter S, Bar KJ, Norra C, Richter H, Assion HJ, Westermann A, Juckel G, Maier C. Association between wind-up ratio and central serotonergic function in healthy subjects and depressed patients. Neurosci Lett. 2011;504:176–180. doi: 10.1016/j.neulet.2011.09.033. [DOI] [PubMed] [Google Scholar]
- Yunus MB. Fibromyalgia and overlapping disorders: the unifying concept of central sensitivity syndromes. Semin Arthritis Rheum. 2007;36:339–356. doi: 10.1016/j.semarthrit.2006.12.009. [DOI] [PubMed] [Google Scholar]