Abstract
Circadian clocks coordinate behavior and physiology with daily environmental cycles and thereby optimize the timing of metabolic processes such as glucose production and insulin secretion. Such circadian regulation of metabolism provides an adaptive advantage in diverse organisms. Mammalian clocks are primarily based on a transcription and translation feedback loop in which a heterodimeric complex of the transcription factors CLOCK (circadian locomotor output cycles kaput) and BMAL1 (brain and muscle Arnt-like protein 1) activates the expression of its own repressors, the period (PER1-3) and cryptochrome (CRY1,2) proteins. Posttranslational modification of these core clock components is critical for setting clock time or adjusting the speed of the clock. AMP-activated protein kinase (AMPK) is one of several metabolic sensors that have been reported to transmit energy-dependent signals to the mammalian clock. AMPK does so by driving the phosphorylation and destabilization of CRY and PER proteins. In addition, AMPK subunit composition, sub-cellular localization, and substrate phosphorylation are dependent on clock time. Given the well-established role of AMPK in diverse aspects of metabolic physiology, the reciprocal regulation of AMPK and circadian clocks likely plays an important role in circadian metabolic regulation.
1. Introduction
Circadian biological phenomena — from the daily movements of plant leaves to human sleep cycles — have been recognized for centuries, but their underlying physical and biochemical mechanisms remained mysterious for most of that time. A 1972 study (Stephan and Zucker 1972) demonstrated that the suprachiasmatic nucleus (SCN), a collection of approximately 10,000 neurons at the base of the hypothalamus, is required for daily rhythms in animal behavior in response to light stimuli. For a long time mammalian clocks were thought to be confined to this small brain area. However, within the last decade, it has been shown that circadian clocks are widely distributed in mammalian tissues.
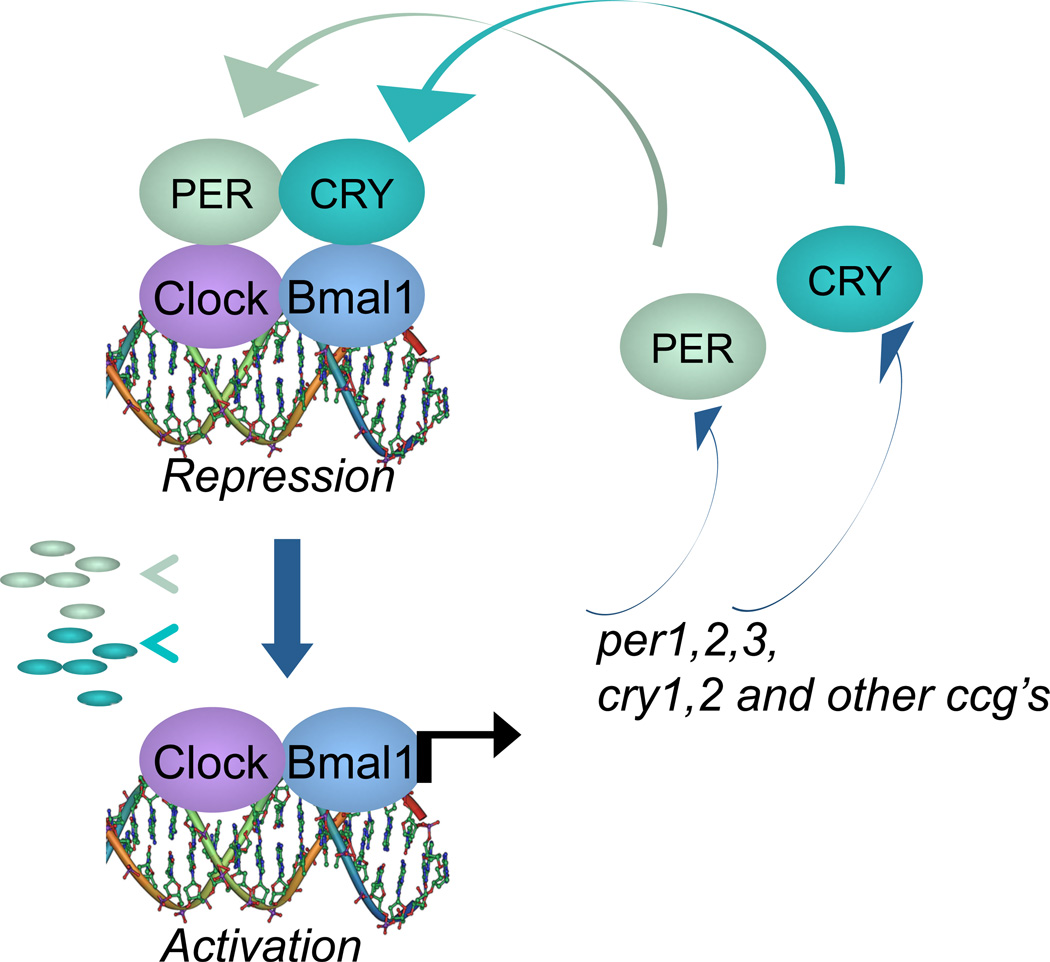
Genetic and biochemical studies in the late 1990s have identified the molecular components of the mammalian circadian clock: a molecular oscillator based on negative feedback, in which the transcription factors CLOCK (or the related neuronal PAS domain-containing protein 2, NPAS2) and its partner BMAL1 work together to drive the expression of many genes, including those encoding their own inhibitors — the period (PER1, PER2 and PER3) and cryptochrome (CRY1 and CRY2) proteins (Green et al. 2008) (Figure 1). Additional loops and layers of control have been described but for the purpose of this review, we will focus on this simplified core clock machinery.
Figure 1. Core circadian clock machinery.
Mammalian circadian clocks are based on a transcription and translation feedback loop in which the transcription factors CLOCK and its heterodimeric partner BMAL1 activate the transcription of genes encoding their own inhibitors, the period (PER1, PER2 and PER3) and cryptochrome (CRY1 and CRY2) repressor proteins.
The ability to predict recurring daily changes in the environment could confer an adaptive advantage on organisms that have circadian clocks. Such a phenomenon has been demonstrated in bacteria and plants: mutants in which the circadian period is genetically altered to be either shorter or longer than 24 hours are less fit than wildtype organisms in a natural environment but they will out-compete their wildtype brethren under environmental conditions engineered to match their altered periods (Dodd et al. 2005,Woelfle et al. 2004). These advantages seem to depend on optimized metabolic function: for example, plant leaves store more chlorophyll when their endogenous period matches the period of the external environment.
Several lines of evidence suggest that mammalian clocks in peripheral organs also optimize the timing of metabolic processes and enable efficient energy storage and utilization. First, the expression of enzymes, transporters, and receptors that regulate metabolism robustly fluctuate throughout the day (Panda et al. 2002). Second, the timing of circadian clocks in tissues outside the SCN is set by feeding time rather than by the light-dark schedule (Damiola et al. 2000,Stokkan et al. 2001). Finally, disruption of clock function in either the liver or the pancreas leads to impaired glucose homeostasis while leaving behavioral rhythms intact (Lamia et al. 2008,Marcheva et al. 2010,Sadacca et al. 2011). Notably, liver-specific ablation of Bmal1 caused lowered blood glucose only during the times of the day when mice are naturally fasting, suggesting that the liver clock predicts recurring daily changes in diet-derived nutrient availability, and increases glucose production during times of expected fasting. Thus, circadian clocks may reduce stress on the systems that mediate response to acute metabolic challenges.
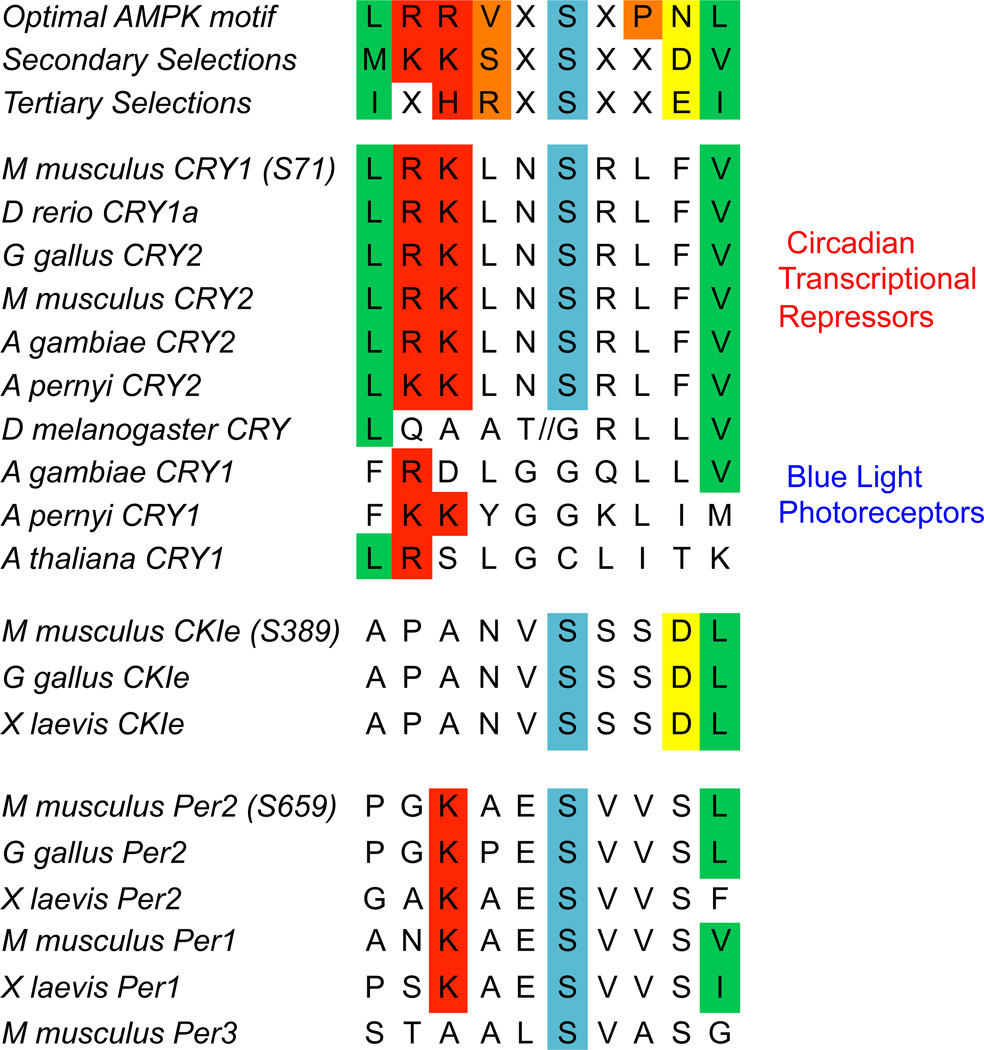
AMPK is a heterotrimeric protein kinase that responds to cellular energy stress by virtue of adenine nucleotides binding to its gamma regulatory subunits (AMPKγ1, AMPKγ2, and AMPKγ3). AMPKγ binds to ATP, ADP or AMP and its AMP- or ADP-bound conformation enables activation of the catalytic alpha subunits (AMPKα1 and AMPKα2) such that AMPK activity increases in response to cellular ATP depletion. The beta regulatory subunits (AMPKβ1 and AMPKβ2) interact with glycogen (Polekhina et al. 2003), are modified by myristoylation (Oakhill et al. 2010), and seem to control the subcellular localization of the heterotrimeric AMPK complex. In the liver, AMPK is activated in response to prolonged fasting (Witters et al. 1994), and twenty-four hour oscillations in the phosphorylation of the AMPK substrates acetyl coA carboxylase (ACC) and Raptor have been observed (Davies et al. 1992,Lamia et al. 2009). AMPK has long been recognized as a central regulator of mammalian metabolic function (Kahn et al. 2005), and as such there has been a great deal of interest in identifying its phosphorylation targets (Mihaylova and Shaw 2011). As part of that effort, two groups have described amino acid sequence motifs that increase the probability of phosphorylation by AMPK (Gwinn et al. 2008,Scott et al. 2002) and several clock components contain potential AMPK target phosphorylation sites (Figure 2). Thus, AMPK has the properties required to act as a clock-resetting signal.
Figure 2. AMPK phosphorylation motifs in clock proteins.
Several proteins that are critical for the functioning of mammalian circadian clocks contain evolutionarily conserved sequences that may be preferentially phosphorylated by AMPK. Intriguingly, the AMPK-dependent phosporylation of cryptochromes seems to represent an evolutionary switch in which cryptochromes gained the ability to sense changes in chemical energy (ATP levels) rather than electromagnetic energy (light).
2. AMPK roles in clock function
2.1 Phosphorylation of Cryptochromes
Mammalian cryptochromes (CRY1 and CRY2) are transcriptional repressors that are required for circadian clock function (Sancar 2004). Fruitflies and plants also have cryptochrome proteins but instead of repressing transcription, they function as blue light photoreceptors that are destabilized upon exposure to sunlight and thus participate in light-induced clock resetting (Sancar et al. 2000). The importance of cryptochrome stability for determining the speed of mammalian clocks became apparent when the most prominent mutants identified in each of two forward genetic screens for circadian rhythm perturbation in mice were alleles of the E3 ligase component FBXL3 (F-box/LRR-repeat protein 3) (Godinho et al. 2007,Siepka et al. 2007) that catalyzes the polyubiquitination of CRY1 and CRY2 and thus stimulates their proteasomal degradation (Busino et al. 2007).
FBXL3 is a member of a large family of F-box proteins, which mediate regulated degradation by targeting Skp-Cullin-F-box (SCF) E3 ligases to phosphorylated substrates (Ho et al. 2006). In the case of cryptochromes and FBXL3, AMPK-mediated phosphorylation of CRY1 and CRY2 stimulates their interaction and the FBXL3-mediated degradation of cryptochromes (Lamia et al. 2009). Interestingly, the serines that are phosphorylated by AMPK are evolutionarily conserved in all cryptochromes that act as transcriptional repressors and are not present in those that function as blue light photoreceptors (Lamia et al. 2009,Yuan et al. 2007) (Figure 2). Furthermore, the presence of AMPK-dependent phosphorylation sites seems to be associated with increased body size, suggesting that AMPK-dependent degradation may have evolved to replace light-induced degradation as a clock-resetting signal in organisms in which light cannot penetrate to all cells.
2.2 Phosphorylation of Casein kinase I
Casein kinases are important modulators of circadian clock function in mammals. A naturally occurring mutation in hamsters (Tau) that causes a long circadian period was determined to be a hypomorphic allele of casein kinase I epsilon (CKIε) (Lowrey et al. 2000). In addition, genetic disruption or pharmacological inhibition of CKIε and/or casein kinase I delta (CKIδ) alters both cellular and behavioral circadian rhythms in mice (Etchegaray et al. 2009,Hirota et al. 2011,Meng et al. 2008). Casein kinases preferentially phosphorylate serines located within negatively charged amino acid sequence motifs and several serines in PER2 (which are conserved in PER1) have been identified as targets of CKI phosphorylation (Vanselow et al. 2006). CKI-mediated phosphorylation of PER proteins is a primary determinant of their stability and circadian period (Lee et al. 2011). Interestingly, the human sleep disorder familial advanced sleep phase syndrome (FASPS), characterized by an extreme “early bird” phenotype, is caused by mutation of serine 662 in human PER2 (S659 in mouse PER2) to glycine (Toh et al. 2001), which appears to prevent the phosphorylation of nearby serines by CKI (Xu et al. 2007). The kinase responsible for phosphorylating PER2 S662 has not yet been identified, but the surrounding amino acid sequence suggests the possibility that AMPK may be involved (Figure 2). Though a possible role of AMPK in directly phosphorylating PER proteins has not been investigated, AMPK was reported to be capable of phosphorylating CKIε at serine 389 and thereby increasing its enzymatic activity, indirectly leading to a destabilization of PER2 (Um et al. 2007).
2.3 AMPK in Entrainment
The ability of AMPK to respond to metabolic cues and to directly modify circadian clock components suggests that it may be an important mediator of metabolic entrainment in peripheral clocks. The results of preliminary investigations into this question are consistent with such a role but not conclusive. Pharmacological activation of AMPK by intraperitoneal injection of either AICAR (5-Aminoimidazole-4-carboxyamide ribonucleoside) (Lamia et al. 2009) or metformin (Um et al. 2007) caused a phase shift of the liver clock in mice, which suggests a possible ability to entrain the liver clock. In addition, acute AICAR stimulation altered the expression of clock genes in skeletal muscle in wildtype mice but not mice lacking the AMPKγ3 regulatory subunit, suggesting that AMPK activation may also play a role in circadian entrainment of muscle clocks (Vieira et al. 2008). However, glucocorticoids are similarly capable of causing phase shifts upon acute exposure in vivo but have subsequently been found to be dispensible for metabolic entrainment (Balsalobre et al. 2000,Le Minh et al. 2001). Further study is required to decipher the precise role of AMPK in the entrainment of circadian clocks in peripheral organs. It seems likely that metabolic entrainment occurs through a multitude of pathways and may not absolutely require any single signal.
3. Other metabolic sensors implicated in clock regulation
3.1 SIRT1
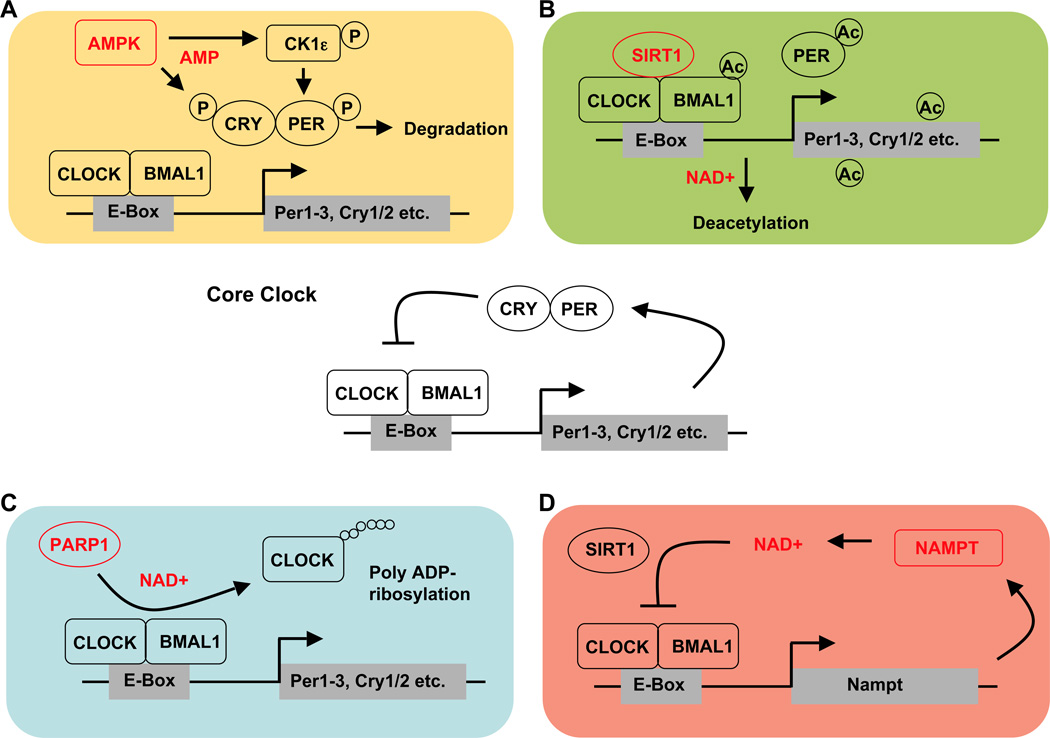
Another fuel-sensing molecule positioned at the crossroads of nutritional status and circadian regulation is SIRT1 (silent mating type information regulation 2 homolog 1). Like AMPK, SIRT1 has emerged as a key metabolic sensor that directly links nutrient signals to metabolic homeostasis. As a member of the sirtuin protein family, SIRT1 is a class III histone deacetylase (HDAC) that, in addition to histones, deacetylates numerous transcription factors and co-regulators (Imai et al. 2000,Landry et al. 2000,Smith et al. 2000). Class III HDACS are structurally distinct from other HDACs and require the coenzyme NAD+ (nicotinamide adenine dinucleotide). The NAD(P)+/NAD(P)H ratio serves as a readout for the cellular redox state, thereby enabling SIRT1 to dynamically sense cellular energy metabolism (Bordone and Guarente 2005,Haigis and Guarente 2006). The circadian transcription factor CLOCK has been reported to have histone acetyltransferase (HAT) activity, and SIRT1 was identified as the HDAC that counteracts the HAT activity of CLOCK (Asher et al. 2008,Nakahata et al. 2008) (Figure 3).
Figure 3. Metabolic inputs to the circadian core clock.
A) AMPK phosphorylates CRY1 and CRY2, and targets them for degradation. AMPK has also been reported to phosphorylate CK1ε thereby increasing CK1ε activity and ultimately phosphorylation-mediated PER degradation. B) SIRT1 deacetylates BMAL1, PER2 and histone3 in a NAD+-dependent manner thus counteracting the histone-acetylase function of CLOCK. C) PARP1 poly ADP-ribosylates CLOCK in a NAD+-dependent reaction, which inhibits DNA binding by CLOCK-BMAL1. D) Nampt encodes for the rate-limiting enzyme in NAD+-synthesis and is regulated by CLOCK-BMAL1. The resulting circadian oscillations of NAD+ levels feed back on CLOCK-BMAL1 activity by affecting SIRT1 activity.
The finding that both AMPK and SIRT1 are implicated in the crosstalk of circadian and metabolic regulation is of special interest because they also regulate each other. AMPK enhances SIRT1 activity by increasing cellular NAD+ levels (Canto et al. 2009,Fulco et al. 2008) and activation of SIRT1 may cause AMPK phosphorylation via deacetylation-dependent activation of the AMPK-activating kinase liver kinase B1 (LKB1) (Hou et al. 2008,Lan et al. 2008). Therefore, AMPK and SIRT1 not only regulate each other but their metabolic actions often converge (Ruderman et al. 2010) suggesting that their close interrelationship might also play a role in circadian clock regulation.
3.2 PARP1
Recently, a second NAD+-dependent protein was identified to act as both a nutrient sensor and a modulator of clock function. Poly (ADP-ribose) polymerase 1 (PARP1) uses NAD+ to synthesize ADP-ribose polymers and adds them to itself and other proteins in a process called poly ADP-ribosylation. Like other posttranslational modifications, ADP-ribosylation affects protein function, such as ability to bind DNA (D'Amours et al. 1999). In the liver, PARP1 activity was found to be daytime dependent and probably driven by feeding rhythms rather than the local circadian clock since its cyclic activity persists in the absence of a functional liver clock. Further supporting this idea, the time of peak PARP1 activity was shifted in response to altering the time of food availability. Interestingly, PARP1 interacts with CLOCK and BMAL1, and was shown to ribosylate CLOCK in a rhythmic pattern (Asher et al. 2010) (Figure 3). PARP1−/− mice have altered circadian rhythms of locomotor activity and liver gene expression, clearly establishing a link between PARP1 and clock function. Two studies have suggested a link between PARP1 and AMPK, but they disagree about whether PARP1 lies upstream or downstream of AMPK (Shin et al. 2009,Walker et al. 2006). A functional interplay between PARP1 and SIRT1 has also been established (Bai et al. 2011a, Bai et al. 2011b, Kolthur-Seetharam et al. 2006, Pang et al. 2011) suggesting that both NAD+-dependent proteins might act in concert to link metabolic cues to circadian clock regulation.
3.3 NAMPT
Since two NAD+ sensing proteins have been linked to the circadian clock, a direct connection between the NAD+ salvage pathway and clock regulation seems likely. Indeed, in vitro studies have revealed that high NAD+ (and NADP+) levels decrease the binding capacity of CLOCK-BMAL1 heterodimers to their E-box targets, while high levels of NADH (and NADPH) increase binding (Rutter et al. 2001). Nicotinamide phosphoribosyltransferase (NAMPT) is the rate-limiting enzyme in NAD+ biosynthesis and CLOCK and BMAL1 directly activate the Nampt promoter, leading to circadian oscillation of NAMPT activity and, ultimately, of cellular NAD+ levels (Nakahata et al. 2009,Ramsey et al. 2009). Furthermore, there seems to be a circadian feedback loop involving NAMPT and SIRT1. SIRT1 is recruited to the Nampt promoter thereby contributing to the circadian synthesis of its own co-factor via inhibition of CLOCK-BMAL1 transcriptional activity (Figure 3).
Thus, NAD+ levels may be both a metabolic input signal for clock regulation and a clock output signal to regulate metabolism. However, the extent to which clock-regulated Nampt gene expression contributes to the regulation of NAD+ sensor protein activity remains to be determined. There is compelling evidence that other mechanisms such as protein-protein interactions might underlie the circadian regulation of PARP-1 activity (Asher et al. 2010,D'Amours et al. 1999) and SIRT1 is also regulated by phosphorylation and protein-protein interactions (Schug and Li 2011). Interestingly, in myoblasts as well as muscle tissue, AMPK activation is accompanied by increased Nampt gene expression and consequently NAD+ levels (Costford et al. 2009,Fulco et al. 2008). Whether AMPK-mediated degradation of CRY and thus increased CLOCK-BMAL1 transcriptional activity is involved in this process remains to be determined.
4. Outstanding Questions
4.1 Diurnal regulation of AMPK
The ability of AMPK to phosphorylate cryptochromes and to stimulate their proteasome-dependent degradation suggests that AMPK may be an important mediator of metabolism-dependent clock resetting in mammalian peripheral organs. In order to serve that function, the ability of AMPK to phosphorylate cryptochromes must be stimulated by a fasting- or feeding-dependent signal at approximately the same time each day. Such a signal could involve an increase in the cellular (AMP or ADP)/ATP ratio, an increase in the expression of AMPK subunits or its upstream activating kinase LKB1 (Shaw et al. 2004), a decrease in the expression or activity of a phosphatase that removes the activating phosphorylation such as protein phosphatase 2 (PP2) a or PP2c (Davies et al. 1995), a change in the posttranslational modification of AMPK subunits (Oakhill et al. 2010), or a change in the nuclear localization of AMPK, or a combination of these mechanisms.
In rodent livers, diurnal rhythms in the phosphorylation of the AMPK substrates ACC and Raptor have been observed (Davies et al. 1992,Lamia et al. 2009) but the time of peak phosphorylation of these cytoplasmic proteins does not coincide with the time of minimum cryptochrome protein abundance. In contrast, the expression of AMPKβ2 in mouse liver exhibits a daily increase in expression at the time when nuclear cryptochrome protein is minimal (Lamia et al. 2009). While most of the transcripts encoding the seven subunits of mammalian AMPK (prkaa1, prkaa2, prkab1, prkab2, prkag1, prkag2, prkag3) as well as the upstream activating kinase LKB1 (stk11) exhibit no time-dependent variation in mouse livers, the expression of prkab2 is eight times higher in the middle of the day than it is at night. The β2 subunit of AMPK has been implicated in nuclear localization of the heterotrimeric AMPK complex (Suzuki et al. 2007) and indeed, the nuclear localization of the AMPKα1 subunit was observed to increase at the same time of day when AMPKβ2 expression peaks (Lamia et al. 2009). It remains to be determined whether a particular combination of AMPK subunits is required for catalyzing the phosphorylation of cryptochromes, but complexes containing the AMPKβ2 subunit may be preferentially involved.
At the time of the original discovery of AMPKβ2, the ratio of expression of AMPKβ2 to AMPKβ1 was found to be high in skeletal muscle and low in the liver (Thornton et al. 1998). However, those experiments were performed at a single unknown time of day so it is unclear how that ratio may depend on day-time given the strongly diurnal expression of AMPKβ2. In addition, the catalytic activity of AMPKα1 (but not AMPKα2) was approximately doubled when in complex with AMPKβ2 instead of AMPKβ1 (Thornton et al. 1998), indicating an even greater possible effect of diurnal changes in subunit availability. Because AMPK is a critical regulator of a multitude of mammalian metabolic pathways (Mihaylova and Shaw 2011), the observation that its activity and complex composition vary in a daytime-dependent manner suggests that circadian input to those pathways via diurnal regulation of AMPK may be an important and understudied mechanism by which clocks impinge on mammalian metabolism.
4.2 Central vs. peripheral clocks
Though the original motivation for studying the role of AMPK in peripheral clocks came from the observation that the timing of those clocks depends on metabolic signals (Damiola et al. 2000,Stokkan et al. 2001), AMPK could also play a role in transmitting light cues to the central SCN clock. The SCN receives light signals from the retina via neurons of the retinohypothalamic tract. However, it is unclear how the neuronal signal is converted to an intracellular cue to the core clock machinery. Diurnal rhythms in intracellular ATP concentration in SCN neurons have been observed (Womac et al. 2009); the circadian phase of SCN neurons can be shifted by glucose deprivation (Hall et al. 1997), which can activate AMPK; and light pulses acutely stimulate the expression of Per1 and Per2 genes (Challet et al. 2003), which would be expected downstream of cryptochrome degradation. Consistent with this possibility, genetic disruption of either AMPKα1 or AMPKα2 changed the free running period of locomotor activity in mice (Um et al. 2011). Further investigation is needed to determine whether this reflects a role of AMPK-dependent phosphorylation of clock components in the SCN.
4.3 Role of cryptochromes in AMPK-dependent metabolic pathways
The role of AMPK in the regulation of cryptochrome stability combined with several recent findings connecting cryptochromes to the regulation of glucose homeostasis (Boesgaard et al. 2010,Dupuis et al. 2010,Hu et al. 2010,Lamia et al. 2011,Zhang et al. 2010) suggests that cryptochromes may be important and previously unappreciated mediators of AMPK-dependent metabolic regulation.
As transcriptional repressors, cryptochromes are likely to mediate their effects on metabolism by modulating the activity of multiple sequence-specific DNA-binding transcription factors. Indeed, in addition to their long-established repression of CLOCK and BMAL1, they have recently been determined to modulate gluconeogenesis by repressing both CREB (cAMP response element-binding) (Zhang et al. 2010) and the glucocorticoid receptor (Lamia et al. 2011). In each case, cryptochromes were found to oppose glucose production by limiting the expression of gluconeogenic enzymes. CLOCK and BMAL1 activate glucose secretion from the liver by increasing the transcription of rate-limiting enzymes and transporters in a circadian pattern (Lamia et al. 2008). AMPK opposes glucose production by phosphorylating other transcriptional regulators including CREB regulated transcription coactivator 2 (CRTC2) (Koo et al. 2005,Shaw et al. 2005) and class II HDACs (Mihaylova et al. 2011). Thus, AMPK-dependent degradation of cryptochromes would be expected to counteract the glucose-lowering effects of AMPK activation. Such a phenomenon may cause the glucose responsiveness to AMPK activation to vary with the time of day. It will be particularly important to determine whether the therapeutic benefit of the widely prescribed diabetes drug metformin, which lowers blood glucose at least in part by activating AMPK in the liver (Shaw et al. 2005), is altered by circadian clock function and/or day-time.
5. Conclusions
Accumulating evidence suggests that circadian clocks play an important and underappreciated role in the regulation of mammalian metabolic physiology. In humans this is supported by epidemiological studies showing that disturbance of circadian rhythms by sleep deprivation or shift work is associated with metabolic dysregulation and increased risk for obesity, type 2 diabetes and cardiovascular disease (Karlsson et al. 2005). The recent demonstrations that AMPK and other chemical energy sensors modulate clock function via posttranslational modification of core clock components suggest specific pathways that may be useful for the pharmacological control of circadian metabolic functions. The reciprocal regulation of AMPK and circadian clocks suggests that the effectiveness of widely prescribed drugs (e.g. metformin) and/or new therapies that ameliorate glucose homeostasis by activating AMPK might be improved by altering the timing of treatment. However, it remains to be determined whether AMPK acts as a metabolic sensor in human circadian clocks. Finally, future studies are needed to address whether the core clock proteins that are modified by AMPK and other enzymes with well-known roles in metabolic physiology play a role in the established physiological effects of those enzymes.
Highlights.
Circadian clocks regulate mammalian metabolic physiology.
AMPK contributes to clock time setting by phosphorylating clock component proteins.
AMPK activity, subunit composition, and localization depend on clock time.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Asher G, Gatfield D, Stratmann M, Reinke H, Dibner C, Kreppel F, Mostoslavsky R, Alt FW, Schibler U. SIRT1 regulates circadian clock gene expression through PER2 deacetylation. Cell. 2008;134:317–328. doi: 10.1016/j.cell.2008.06.050. [DOI] [PubMed] [Google Scholar]
- 2.Asher G, Reinke H, Altmeyer M, Gutierrez-Arcelus M, Hottiger MO, Schibler U. Poly(ADP-ribose) polymerase 1 participates in the phase entrainment of circadian clocks to feeding. Cell. 2010;142:943–953. doi: 10.1016/j.cell.2010.08.016. [DOI] [PubMed] [Google Scholar]
- 3.Bai P, Canto C, Brunyanszki A, Huber A, Szanto M, Cen Y, Yamamoto H, Houten SM, Kiss B, Oudart H, Gergely P, Menissier-de Murcia J, Schreiber V, Sauve AA, Auwerx J. PARP-2 regulates SIRT1 expression and whole-body energy expenditure. Cell Metab. 2011a;13:450–460. doi: 10.1016/j.cmet.2011.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bai P, Canto C, Oudart H, Brunyanszki A, Cen Y, Thomas C, Yamamoto H, Huber A, Kiss B, Houtkooper RH, Schoonjans K, Schreiber V, Sauve AA, Menissier-de Murcia J, Auwerx J. PARP-1 inhibition increases mitochondrial metabolism through SIRT1 activation. Cell Metab. 2011b;13:461–468. doi: 10.1016/j.cmet.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Balsalobre A, Brown SA, Marcacci L, Tronche F, Kellendonk C, Reichardt HM, Schutz G, Schibler U. Resetting of circadian time in peripheral tissues by glucocorticoid signaling. Science. 2000;289:2344–2347. doi: 10.1126/science.289.5488.2344. [DOI] [PubMed] [Google Scholar]
- 6.Boesgaard TW, Grarup N, Jorgensen T, Borch-Johnsen K, Hansen T, Pedersen O. Variants at DGKB/TMEM195, ADRA2A, GLIS3 and C2CD4B loci are associated with reduced glucose-stimulated beta cell function in middleaged Danish people. Diabetologia. 2010;53:1647–1655. doi: 10.1007/s00125-010-1753-5. [DOI] [PubMed] [Google Scholar]
- 7.Bordone L, Guarente L. Calorie restriction, SIRT1 and metabolism: understanding longevity. Nat Rev Mol Cell Biol. 2005;6:298–305. doi: 10.1038/nrm1616. [DOI] [PubMed] [Google Scholar]
- 8.Busino L, Bassermann F, Maiolica A, Lee C, Nolan PM, Godinho SI, Draetta GF, Pagano M. SCFFbxl3 controls the oscillation of the circadian clock by directing the degradation of cryptochrome proteins. Science. 2007;316:900–904. doi: 10.1126/science.1141194. [DOI] [PubMed] [Google Scholar]
- 9.Canto C, Gerhart-Hines Z, Feige JN, Lagouge M, Noriega L, Milne JC, Elliott PJ, Puigserver P, Auwerx J. AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature. 2009;458:1056–1060. doi: 10.1038/nature07813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Challet E, Caldelas I, Graff C, Pevet P. Synchronization of the molecular clockwork by light- and food-related cues in mammals. Biol Chem. 2003;384:711–719. doi: 10.1515/BC.2003.079. [DOI] [PubMed] [Google Scholar]
- 11.Costford SR, Bajpeyi S, Pasarica M, Albarado DC, Thomas SC, Xie H, Church TS, Jubrias SA, Conley KE, Smith SR. Skeletal muscle NAMPT is induced by exercise in humans. Am J Physiol Endocrinol Metab. 2009;298:E117–E126. doi: 10.1152/ajpendo.00318.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.D'Amours D, Desnoyers S, D'Silva I, Poirier GG. Poly(ADPribosyl) ation reactions in the regulation of nuclear functions. Biochem J. 1999;342(Pt 2):249–268. [PMC free article] [PubMed] [Google Scholar]
- 13.Damiola F, Le Minh N, Preitner N, Kornmann B, Fleury-Olela F, Schibler U. Restricted feeding uncouples circadian oscillators in peripheral tissues from the central pacemaker in the suprachiasmatic nucleus. Genes Dev. 2000;14:2950–2961. doi: 10.1101/gad.183500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davies SP, Carling D, Munday MR, Hardie DG. Diurnal rhythm of phosphorylation of rat liver acetyl-CoA carboxylase by the AMP-activated protein kinase, demonstrated using freeze-clamping. Effects of high fat diets. Eur J Biochem. 1992;203:615–623. doi: 10.1111/j.1432-1033.1992.tb16591.x. [DOI] [PubMed] [Google Scholar]
- 15.Davies SP, Helps NR, Cohen PT, Hardie DG. 5'-AMP inhibits dephosphorylation, as well as promoting phosphorylation, of the AMP-activated protein kinase. Studies using bacterially expressed human protein phosphatase- 2C alpha and native bovine protein phosphatase-2AC. FEBS Lett. 1995;377:421–425. doi: 10.1016/0014-5793(95)01368-7. [DOI] [PubMed] [Google Scholar]
- 16.Dodd AN, Salathia N, Hall A, Kevei E, Toth R, Nagy F, Hibberd JM, Millar AJ, Webb AA. Plant circadian clocks increase photosynthesis, growth, survival, and competitive advantage. Science. 2005;309:630–633. doi: 10.1126/science.1115581. [DOI] [PubMed] [Google Scholar]
- 17.Dupuis J, Langenberg C, Prokopenko I, Saxena R, Soranzo N, Jackson AU, Wheeler E, Glazer NL, Bouatia-Naji N, Gloyn AL, Lindgren CM, Magi R, Morris AP, Randall J, Johnson T, Elliott P, Rybin D, Thorleifsson G, Steinthorsdottir V, Henneman P, Grallert H, Dehghan A, Hottenga JJ, Franklin CS, Navarro P, Song K, Goel A, Perry JR, Egan JM, Lajunen T, Grarup N, Sparso T, Doney A, Voight BF, Stringham HM, Li M, Kanoni S, Shrader P, Cavalcanti-Proenca C, Kumari M, Qi L, Timpson NJ, Gieger C, Zabena C, Rocheleau G, Ingelsson E, An P, O'Connell J, Luan J, Elliott A, McCarroll SA, Payne F, Roccasecca RM, Pattou F, Sethupathy P, Ardlie K, Ariyurek Y, Balkau B, Barter P, Beilby JP, Ben-Shlomo Y, Benediktsson R, Bennett AJ, Bergmann S, Bochud M, Boerwinkle E, Bonnefond A, Bonnycastle LL, Borch-Johnsen K, Bottcher Y, Brunner E, Bumpstead SJ, Charpentier G, Chen YD, Chines P, Clarke R, Coin LJ, Cooper MN, Cornelis M, Crawford G, Crisponi L, Day IN, de Geus EJ, Delplanque J, Dina C, Erdos MR, Fedson AC, Fischer-Rosinsky A, Forouhi NG, Fox CS, Frants R, Franzosi MG, Galan P, Goodarzi MO, Graessler J, Groves CJ, Grundy S, Gwilliam R, Gyllensten U, Hadjadj S, et al. New genetic loci implicated in fasting glucose homeostasis and their impact on type 2 diabetes risk. Nat Genet. 2010;42:105–116. doi: 10.1038/ng.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Etchegaray JP, Machida KK, Noton E, Constance CM, Dallmann R, Di Napoli MN, DeBruyne JP, Lambert CM, Yu EA, Reppert SM, Weaver DR. Casein kinase 1 delta regulates the pace of the mammalian circadian clock. Mol Cell Biol. 2009;29:3853–3866. doi: 10.1128/MCB.00338-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fulco M, Cen Y, Zhao P, Hoffman EP, McBurney MW, Sauve AA, Sartorelli V. Glucose restriction inhibits skeletal myoblast differentiation by activating SIRT1 through AMPK-mediated regulation of Nampt. Dev Cell. 2008;14:661–673. doi: 10.1016/j.devcel.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Godinho SI, Maywood ES, Shaw L, Tucci V, Barnard AR, Busino L, Pagano M, Kendall R, Quwailid MM, Romero MR, O'Neill J, Chesham JE, Brooker D, Lalanne Z, Hastings MH, Nolan PM. The after-hours mutant reveals a role for Fbxl3 in determining mammalian circadian period. Science. 2007;316:897–900. doi: 10.1126/science.1141138. [DOI] [PubMed] [Google Scholar]
- 21.Green CB, Takahashi JS, Bass J. The meter of metabolism. Cell. 2008;134:728–742. doi: 10.1016/j.cell.2008.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gwinn DM, Shackelford DB, Egan DF, Mihaylova MM, Mery A, Vasquez DS, Turk BE, Shaw RJ. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol Cell. 2008;30:214–226. doi: 10.1016/j.molcel.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haigis MC, Guarente LP. Mammalian sirtuins--emerging roles in physiology, aging, and calorie restriction. Genes Dev. 2006;20:2913–2921. doi: 10.1101/gad.1467506. [DOI] [PubMed] [Google Scholar]
- 24.Hall AC, Hoffmaster RM, Stern EL, Harrington ME, Bickar D. Suprachiasmatic nucleus neurons are glucose sensitive. J Biol Rhythms. 1997;12:388–400. doi: 10.1177/074873049701200501. [DOI] [PubMed] [Google Scholar]
- 25.Hirota T, Lee JW, Lewis WG, Zhang EE, Breton G, Liu X, Garcia M, Peters EC, Etchegaray JP, Traver D, Schultz PG, Kay SA. Highthroughput chemical screen identifies a novel potent modulator of cellular circadian rhythms and reveals CKIalpha as a clock regulatory kinase. PLoS Biol. 2011;8:e1000559. doi: 10.1371/journal.pbio.1000559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ho MS, Tsai PI, Chien CT. F-box proteins: the key to protein degradation. J Biomed Sci. 2006;13:181–191. doi: 10.1007/s11373-005-9058-2. [DOI] [PubMed] [Google Scholar]
- 27.Hou X, Xu S, Maitland-Toolan KA, Sato K, Jiang B, Ido Y, Lan F, Walsh K, Wierzbicki M, Verbeuren TJ, Cohen RA, Zang M. SIRT1 regulates hepatocyte lipid metabolism through activating AMP-activated protein kinase. J Biol Chem. 2008;283:20015–20026. doi: 10.1074/jbc.M802187200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hu C, Zhang R, Wang C, Wang J, Ma X, Hou X, Lu J, Yu W, Jiang F, Bao Y, Xiang K, Jia W. Variants from GIPR, TCF7L2, DGKB, MADD, CRY2, GLIS3, PROX1, SLC30A8 and IGF1 are associated with glucose metabolism in the Chinese. PLoS One. 2010;5:e15542. doi: 10.1371/journal.pone.0015542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Imai S, Armstrong CM, Kaeberlein M, Guarente L. Transcriptional silencing and longevity protein Sir2 is an NAD-dependent histone deacetylase. Nature. 2000;403:795–800. doi: 10.1038/35001622. [DOI] [PubMed] [Google Scholar]
- 30.Kahn BB, Alquier T, Carling D, Hardie DG. AMP-activated protein kinase: ancient energy gauge provides clues to modern understanding of metabolism. Cell Metab. 2005;1:15–25. doi: 10.1016/j.cmet.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 31.Karlsson B, Alfredsson L, Knutsson A, Andersson E, Toren K. Total mortality and cause-specific mortality of Swedish shift- and dayworkers in the pulp and paper industry in 1952–2001. Scand J Work Environ Health. 2005;31:30–35. doi: 10.5271/sjweh.845. [DOI] [PubMed] [Google Scholar]
- 32.Kolthur-Seetharam U, Dantzer F, McBurney MW, de Murcia G, Sassone-Corsi P. Control of AIF-mediated cell death by the functional interplay of SIRT1 and PARP-1 in response to DNA damage. Cell Cycle. 2006;5:873–877. doi: 10.4161/cc.5.8.2690. [DOI] [PubMed] [Google Scholar]
- 33.Koo SH, Flechner L, Qi L, Zhang X, Screaton RA, Jeffries S, Hedrick S, Xu W, Boussouar F, Brindle P, Takemori H, Montminy M. The CREB coactivator TORC2 is a key regulator of fasting glucose metabolism. Nature. 2005;437:1109–1111. doi: 10.1038/nature03967. [DOI] [PubMed] [Google Scholar]
- 34.Lamia KA, Papp SJ, Yu RT, Barish GD, Uhlenhaut NH, Jonker JW, Downes M, Evans RM. Cryptochromes mediate rhythmic repression of the glucocorticoid receptor. Nature. 2011;480:552–556. doi: 10.1038/nature10700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lamia KA, Sachdeva UM, DiTacchio L, Williams EC, Alvarez JG, Egan DF, Vasquez DS, Juguilon H, Panda S, Shaw RJ, Thompson CB, Evans RM. AMPK regulates the circadian clock by cryptochrome phosphorylation and degradation. Science. 2009;326:437–440. doi: 10.1126/science.1172156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lamia KA, Storch KF, Weitz CJ. Physiological significance of a peripheral tissue circadian clock. Proc Natl Acad Sci U S A. 2008;105:15172–15177. doi: 10.1073/pnas.0806717105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lan F, Cacicedo JM, Ruderman N, Ido Y. SIRT1 modulation of the acetylation status, cytosolic localization, and activity of LKB1. Possible role in AMP-activated protein kinase activation. J Biol Chem. 2008;283:27628–27635. doi: 10.1074/jbc.M805711200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Landry J, Sutton A, Tafrov ST, Heller RC, Stebbins J, Pillus L, Sternglanz R. The silencing protein SIR2 and its homologs are NADdependent protein deacetylases. Proc Natl Acad Sci U S A. 2000;97:5807–5811. doi: 10.1073/pnas.110148297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Le Minh N, Damiola F, Tronche F, Schutz G, Schibler U. Glucocorticoid hormones inhibit food-induced phase-shifting of peripheral circadian oscillators. EMBO J. 2001;20:7128–7136. doi: 10.1093/emboj/20.24.7128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee HM, Chen R, Kim H, Etchegaray JP, Weaver DR, Lee C. The period of the circadian oscillator is primarily determined by the balance between casein kinase 1 and protein phosphatase 1. Proc Natl Acad Sci U S A. 2011;108:16451–16456. doi: 10.1073/pnas.1107178108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lowrey PL, Shimomura K, Antoch MP, Yamazaki S, Zemenides PD, Ralph MR, Menaker M, Takahashi JS. Positional syntenic cloning and functional characterization of the mammalian circadian mutation tau. Science. 2000;288:483–492. doi: 10.1126/science.288.5465.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Marcheva B, Ramsey KM, Buhr ED, Kobayashi Y, Su H, Ko CH, Ivanova G, Omura C, Mo S, Vitaterna MH, Lopez JP, Philipson LH, Bradfield CA, Crosby SD, JeBailey L, Wang X, Takahashi JS, Bass J. Disruption of the clock components CLOCK and BMAL1 leads to hypoinsulinaemia and diabetes. Nature. 2010;466:627–631. doi: 10.1038/nature09253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Meng QJ, Logunova L, Maywood ES, Gallego M, Lebiecki J, Brown TM, Sladek M, Semikhodskii AS, Glossop NR, Piggins HD, Chesham JE, Bechtold DA, Yoo SH, Takahashi JS, Virshup DM, Boot-Handford RP, Hastings MH, Loudon AS. Setting clock speed in mammals: the CK1 epsilon tau mutation in mice accelerates circadian pacemakers by selectively destabilizing PERIOD proteins. Neuron. 2008;58:78–88. doi: 10.1016/j.neuron.2008.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mihaylova MM, Shaw RJ. The AMPK signalling pathway coordinates cell growth, autophagy and metabolism. Nat Cell Biol. 2011;13:1016–1023. doi: 10.1038/ncb2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mihaylova MM, Vasquez DS, Ravnskjaer K, Denechaud PD, Yu RT, Alvarez JG, Downes M, Evans RM, Montminy M, Shaw RJ. Class IIa histone deacetylases are hormone-activated regulators of FOXO and mammalian glucose homeostasis. Cell. 2011;145:607–621. doi: 10.1016/j.cell.2011.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nakahata Y, Kaluzova M, Grimaldi B, Sahar S, Hirayama J, Chen D, Guarente LP, Sassone-Corsi P. The NAD+-dependent deacetylase SIRT1 modulates CLOCK-mediated chromatin remodeling and circadian control. Cell. 2008;134:329–340. doi: 10.1016/j.cell.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nakahata Y, Sahar S, Astarita G, Kaluzova M, Sassone-Corsi P. Circadian control of the NAD+ salvage pathway by CLOCK-SIRT1. Science. 2009;324:654–657. doi: 10.1126/science.1170803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Oakhill JS, Chen ZP, Scott JW, Steel R, Castelli LA, Ling N, Macaulay SL, Kemp BE. beta-Subunit myristoylation is the gatekeeper for initiating metabolic stress sensing by AMP-activated protein kinase (AMPK) Proc Natl Acad Sci U S A. 2010;107:19237–19241. doi: 10.1073/pnas.1009705107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Panda S, Antoch MP, Miller BH, Su AI, Schook AB, Straume M, Schultz PG, Kay SA, Takahashi JS, Hogenesch JB. Coordinated transcription of key pathways in the mouse by the circadian clock. Cell. 2002;109:307–320. doi: 10.1016/s0092-8674(02)00722-5. [DOI] [PubMed] [Google Scholar]
- 50.Pang J, Gong H, Xi C, Fan W, Dai Y, Zhang TM. Poly(ADP-ribose) polymerase 1 is involved in glucose toxicity through SIRT1 modulation in HepG2 hepatocytes. J Cell Biochem. 2011;112:299–306. doi: 10.1002/jcb.22919. [DOI] [PubMed] [Google Scholar]
- 51.Polekhina G, Gupta A, Michell BJ, van Denderen B, Murthy S, Feil SC, Jennings IG, Campbell DJ, Witters LA, Parker MW, Kemp BE, Stapleton D. AMPK beta subunit targets metabolic stress sensing to glycogen. Curr Biol. 2003;13:867–871. doi: 10.1016/s0960-9822(03)00292-6. [DOI] [PubMed] [Google Scholar]
- 52.Ramsey KM, Yoshino J, Brace CS, Abrassart D, Kobayashi Y, Marcheva B, Hong HK, Chong JL, Buhr ED, Lee C, Takahashi JS, Imai S, Bass J. Circadian clock feedback cycle through NAMPT-mediated NAD+ biosynthesis. Science. 2009;324:651–654. doi: 10.1126/science.1171641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ruderman NB, Xu XJ, Nelson L, Cacicedo JM, Saha AK, Lan F, Ido Y. AMPK and SIRT1: a long-standing partnership? Am J Physiol Endocrinol Metab. 2010;298:E751–E760. doi: 10.1152/ajpendo.00745.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rutter J, Reick M, Wu LC, McKnight SL. Regulation of clock and NPAS2 DNA binding by the redox state of NAD cofactors. Science. 2001;293:510–514. doi: 10.1126/science.1060698. [DOI] [PubMed] [Google Scholar]
- 55.Sadacca LA, Lamia KA, Delemos AS, Blum B, Weitz CJ. An intrinsic circadian clock of the pancreas is required for normal insulin release and glucose homeostasis in mice. Diabetologia. 2011 doi: 10.1007/s00125-010-1920-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sancar A. Regulation of the mammalian circadian clock by cryptochrome. J Biol Chem. 2004;279:34079–34082. doi: 10.1074/jbc.R400016200. [DOI] [PubMed] [Google Scholar]
- 57.Sancar A, Thompson C, Thresher RJ, Araujo F, Mo J, Ozgur S, Vagas E, Dawut L, Selby CP. Photolyase/cryptochrome family blue-light photoreceptors use light energy to repair DNA or set the circadian clock. Cold Spring Harb Symp Quant Biol. 2000;65:157–171. doi: 10.1101/sqb.2000.65.157. [DOI] [PubMed] [Google Scholar]
- 58.Schug TT, Li X. Sirtuin 1 in lipid metabolism and obesity. Ann Med. 2011;43:198–211. doi: 10.3109/07853890.2010.547211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Scott JW, Norman DG, Hawley SA, Kontogiannis L, Hardie DG. Protein kinase substrate recognition studied using the recombinant catalytic domain of AMP-activated protein kinase and a model substrate. J Mol Biol. 2002;317:309–323. doi: 10.1006/jmbi.2001.5316. [DOI] [PubMed] [Google Scholar]
- 60.Shaw RJ, Kosmatka M, Bardeesy N, Hurley RL, Witters LA, DePinho RA, Cantley LC. The tumor suppressor LKB1 kinase directly activates AMP-activated kinase and regulates apoptosis in response to energy stress. Proc Natl Acad Sci U S A. 2004;101:3329–3335. doi: 10.1073/pnas.0308061100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shaw RJ, Lamia KA, Vasquez D, Koo SH, Bardeesy N, Depinho RA, Montminy M, Cantley LC. The kinase LKB1 mediates glucose homeostasis in liver and therapeutic effects of metformin. Science. 2005;310:1642–1646. doi: 10.1126/science.1120781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shin SM, Cho IJ, Kim SG. Resveratrol protects mitochondria against oxidative stress through AMP-activated protein kinase-mediated glycogen synthase kinase-3beta inhibition downstream of poly(ADPribose) polymerase-LKB1 pathway. Mol Pharmacol. 2009;76:884–895. doi: 10.1124/mol.109.058479. [DOI] [PubMed] [Google Scholar]
- 63.Siepka SM, Yoo SH, Park J, Song W, Kumar V, Hu Y, Lee C, Takahashi JS. Circadian mutant Overtime reveals F-box protein FBXL3 regulation of cryptochrome and period gene expression. Cell. 2007;129:1011–1023. doi: 10.1016/j.cell.2007.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Smith JS, Brachmann CB, Celic I, Kenna MA, Muhammad S, Starai VJ, Avalos JL, Escalante-Semerena JC, Grubmeyer C, Wolberger C, Boeke JD. A phylogenetically conserved NAD+-dependent protein deacetylase activity in the Sir2 protein family. Proc Natl Acad Sci U S A. 2000;97:6658–6663. doi: 10.1073/pnas.97.12.6658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Stephan FK, Zucker I. Circadian rhythms in drinking behavior and locomotor activity of rats are eliminated by hypothalamic lesions. Proc Natl Acad Sci U S A. 1972;69:1583–1586. doi: 10.1073/pnas.69.6.1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Stokkan KA, Yamazaki S, Tei H, Sakaki Y, Menaker M. Entrainment of the circadian clock in the liver by feeding. Science. 2001;291:490–493. doi: 10.1126/science.291.5503.490. [DOI] [PubMed] [Google Scholar]
- 67.Suzuki A, Okamoto S, Lee S, Saito K, Shiuchi T, Minokoshi Y. Leptin stimulates fatty acid oxidation and peroxisome proliferator-activated receptor alpha gene expression in mouse C2C12 myoblasts by changing the subcellular localization of the alpha2 form of AMP-activated protein kinase. Mol Cell Biol. 2007;27:4317–4327. doi: 10.1128/MCB.02222-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Thornton C, Snowden MA, Carling D. Identification of a novel AMP-activated protein kinase beta subunit isoform that is highly expressed in skeletal muscle. J Biol Chem. 1998;273:12443–12450. doi: 10.1074/jbc.273.20.12443. [DOI] [PubMed] [Google Scholar]
- 69.Toh KL, Jones CR, He Y, Eide EJ, Hinz WA, Virshup DM, Ptacek LJ, Fu YH. An hPer2 phosphorylation site mutation in familial advanced sleep phase syndrome. Science. 2001;291:1040–1043. doi: 10.1126/science.1057499. [DOI] [PubMed] [Google Scholar]
- 70.Um JH, Pendergast JS, Springer DA, Foretz M, Viollet B, Brown A, Kim MK, Yamazaki S, Chung JH. AMPK regulates circadian rhythms in a tissue- and isoform-specific manner. PLoS One. 2011;6:e18450. doi: 10.1371/journal.pone.0018450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Um JH, Yang S, Yamazaki S, Kang H, Viollet B, Foretz M, Chung JH. Activation of 5'-AMP-activated kinase with diabetes drug metformin induces casein kinase Iepsilon (CKIepsilon)-dependent degradation of clock protein mPer2. J Biol Chem. 2007;282:20794–20798. doi: 10.1074/jbc.C700070200. [DOI] [PubMed] [Google Scholar]
- 72.Vanselow K, Vanselow JT, Westermark PO, Reischl S, Maier B, Korte T, Herrmann A, Herzel H, Schlosser A, Kramer A. Differential effects of PER2 phosphorylation: molecular basis for the human familial advanced sleep phase syndrome (FASPS) Genes Dev. 2006;20:2660–2672. doi: 10.1101/gad.397006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Vieira E, Nilsson EC, Nerstedt A, Ormestad M, Long YC, Garcia-Roves PM, Zierath JR, Mahlapuu M. Relationship between AMPK and the transcriptional balance of clock-related genes in skeletal muscle. Am J Physiol Endocrinol Metab. 2008;295:E1032–E1037. doi: 10.1152/ajpendo.90510.2008. [DOI] [PubMed] [Google Scholar]
- 74.Walker JW, Jijon HB, Madsen KL. AMP-activated protein kinase is a positive regulator of poly(ADP-ribose) polymerase. Biochem Biophys Res Commun. 2006;342:336–341. doi: 10.1016/j.bbrc.2006.01.145. [DOI] [PubMed] [Google Scholar]
- 75.Witters LA, Gao G, Kemp BE, Quistorff B. Hepatic 5'-AMPactivated protein kinase: zonal distribution and relationship to acetyl-CoA carboxylase activity in varying nutritional states. Arch Biochem Biophys. 1994;308:413–419. doi: 10.1006/abbi.1994.1058. [DOI] [PubMed] [Google Scholar]
- 76.Woelfle MA, Ouyang Y, Phanvijhitsiri K, Johnson CH. The adaptive value of circadian clocks: an experimental assessment in cyanobacteria. Curr Biol. 2004;14:1481–1486. doi: 10.1016/j.cub.2004.08.023. [DOI] [PubMed] [Google Scholar]
- 77.Womac AD, Burkeen JF, Neuendorff N, Earnest DJ, Zoran MJ. Circadian rhythms of extracellular ATP accumulation in suprachiasmatic nucleus cells and cultured astrocytes. Eur J Neurosci. 2009;30:869–876. doi: 10.1111/j.1460-9568.2009.06874.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Xu Y, Toh KL, Jones CR, Shin JY, Fu YH, Ptacek LJ. Modeling of a human circadian mutation yields insights into clock regulation by PER2. Cell. 2007;128:59–70. doi: 10.1016/j.cell.2006.11.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yuan Q, Metterville D, Briscoe AD, Reppert SM. Insect cryptochromes: gene duplication and loss define diverse ways to construct insect circadian clocks. Mol Biol Evol. 2007;24:948–955. doi: 10.1093/molbev/msm011. [DOI] [PubMed] [Google Scholar]
- 80.Zhang EE, Liu Y, Dentin R, Pongsawakul PY, Liu AC, Hirota T, Nusinow DA, Sun X, Landais S, Kodama Y, Brenner DA, Montminy M, Kay SA. Cryptochrome mediates circadian regulation of cAMP signaling and hepatic gluconeogenesis. Nat Med. 2010;16:1152–1156. doi: 10.1038/nm.2214. [DOI] [PMC free article] [PubMed] [Google Scholar]