Abstract
BRIDGES is a bioanalytical tool that combines passive sampling with the embryonic zebrafish developmental toxicity bioassay to provide a quantitative measure of the toxicity of bioavailable complex mixtures. Passive sampling devices (PSDs), which sequester and concentrate bioavailable organic contaminants from the environment, were deployed in the Willamette and Columbia Rivers within and outside of the Portland Harbor Superfund site in Portland, Oregon. Six sampling events were conducted in the summer and fall of 2009 and 2010. PSD extracts were analyzed for polycyclic aromatic hydrocarbon (PAH) compounds and screened for 1201 chemicals of concern using deconvolution reporting software. The developmental toxicity of the extracts was analyzed using the embryonic zebrafish bioassay. BRIDGES provided site-specific, temporally resolved information about environmental contaminant mixtures and their toxicity. Multivariate modeling approaches were applied to paired chemical and toxic effects data sets to help unravel chemistry-toxicity associations. Modeling demonstrated a significant correlation between PAH concentrations and the toxicity of the samples and identified a subset of PAH analytes that were the most highly correlated with observed toxicity. Although this research highlights the complexity of discerning specific bioactive compounds in complex mixtures, it demonstrates methods for associating toxic effects with chemical characteristics of environmental samples.
Keywords: Mixture toxicology, Passive sampling, Bioavailability, Superfund
INTRODUCTION
The BRIDGES (Biological Response Indicator Devices Gauging Environmental Stressors) bioanalytical tool provides a quantitative measure of the toxicity of environmentally relevant contaminant mixtures. It pairs passive sampling with the embryonic zebrafish developmental toxicity model to connect environmental chemical exposures to biological effects. This tool responds to three fundamental needs in toxicology research: 1) determining bioavailability in order to assess potential exposure [1]; 2) evaluating the toxicity of complex mixtures of contaminants in the environment [2]; and 3) directly connecting effective environmental sampling with toxicity evaluations [3]. Furthermore, it allows for the determination of the toxicity of environmentally relevant mixtures, even when all of the components of the mixture are not identified [4, 5] and can aid in the identification of bioactive chemicals [5, 6].
The presence of chemicals in the environment is not necessarily indicative of bioavailability [1, 7]; chemicals can only be taken up by organisms and have a biological effect if they are bioavailable [8]. Developing methods for effectively assessing exposure, and integrating these into risk assessment frameworks, has been identified as a priority for ecotoxicology [3] and risk assessment [9]. Although humans and other organisms are exposed to complex mixtures of contaminants, toxicity testing is most often limited to determining the effects of exposure to individual chemicals or classes of chemicals. Models for predicting the effects of complex mixtures can be inadequate because they do not account for antagonistic and synergistic interactions between the components of the mixture [10]. Additionally, environmental exposure assessment is carried out by measuring known pollutants in environmental matrices using analytical chemistry. Chemicals that have not been previously identified, such as unknown substances and breakdown products of known contaminants, can escape detection during chemical analysis; however, these unidentified components of the mixture may be toxicologically significant [5, 6].
Passive sampling devices (PSDs) sequester and concentrate the freely dissolved, and therefore bioavailable, fraction of hydrophobic organic contaminants from aquatic environments [11]. They provide a time integrated measurement of these chemicals in the environment and are ideally suited for determining the bioavailability of chemicals, quantifying chemicals that are present at low concentrations in the water and capturing episodic events [11–13]. Furthermore, samples obtained using PSDs can be applied to in-vitro and in-vivo bioassays [4, 11, 14–20]. Semi-permeable membrane devices (SPMDs) have been extensively utilized in environmental monitoring applications [11]; however, more recently developed PSDs, that do not contain triolein [12, 21–23] may be more suitable for bioassay applications [4]. Unlike SPMDs, samples obtained using lipid-free tubing (LFT) do not contain oleic acid impurities [22] and therefore do not require clean-up prior to use in bioassays.
Bioassays are used to identify the potency and nature of toxic effects elicited by exposure to chemicals or other factors. The embryonic zebrafish is a widely utilized model vertebrate organism for bioassays [24, 25]. Its small size, fecundity, rapid development and early morphology are advantageous and allow for high throughput applications that most vertebrate organisms are not suited for [25]. The developmental morphology of zebrafish has been well documented [26] and genetic and molecular tools have been designed to elucidate mechanisms of action for biological outcomes [27]. A prior study, that laid the foundation for the development of the BRIDGES tool, demonstrated that pairing passive sampling with the embryonic zebrafish developmental model provided spatially and temporally resolved information about the toxicity of bioavailable contaminant mixtures in an industrialized river [4].
The Portland Harbor Superfund site is an example of an area where the application of the BRIDGES tool can provide valuable insight to direct research and remediation goals. The range of chemical contaminants as well as the variety of point and non-point source inputs [23, 28] leads to a situation where understanding the toxicity of environmentally relevant complex mixtures is a priority. Furthermore, significant differences in contaminant levels [23, 28] and toxicity [4] have been observed on reduced spatial and temporal scales. This necessitates the application of methods capable of providing highly resolved, site-specific information, which is a demonstrated advantage of the BRIDGES bioanalytical tool [4].
The present study pairs passive sampling with the embryonic zebrafish developmental toxicity model to examine spatial and temporal differences in the toxicity of bioavailable chemical mixtures obtained from sites within and outside of the Portland Harbor Superfund over the course of two years. A variety of modeling approaches were explored to examine associations between known chemical components of the complex environmental mixtures and the observed developmental toxicity of the samples. One objective of this research is to demonstrate the application of the BRIDGES tool for environmental toxicity assessment and monitoring in a Superfund site. A second objective is to examine multivariate modeling methods that can help unravel associations between the chemical characteristics of environmental samples and their observed toxicity.
MATERIALS AND METHODS
Study area
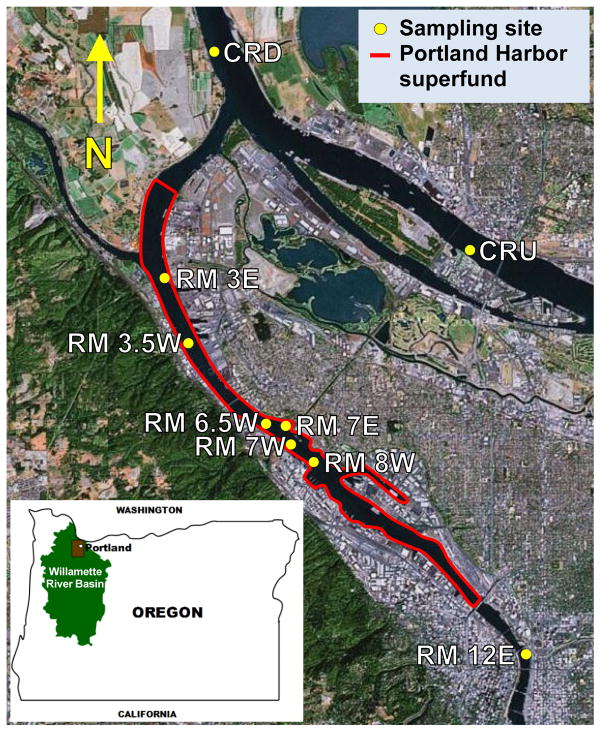
The Willamette River flows north through the Willamette Valley in Oregon before reaching its confluence with the Columbia River. Portland Harbor is a section of the Willamette River that has been heavily industrialized for over a century and continues to be impacted by urban and industrial activities. A 9 mile section of the harbor was designated a Superfund Site in 2000 due to high levels of PAHs, PCBs, dioxins, pesticides and metals in sediments and water [28]. Remediation efforts have been ongoing; most notably at the McCormic and Baxter Superfund site, located on the east bank (E) of river mile (RM) 7 within the larger Portland Harbor Superfund, and the GASCO site at RM 6.3 west (W) [28]. PSDs were deployed at nine sites in 2009–2010. Seven sites were located on the Willamette River: six were within the Portland Harbor Superfund site and one, at RM 12 east, was upstream from the Superfund. Two sampling sites were located on the Columbia River; above and below the confluence with the Willamette River (Fig. 1).
Figure 1. Study area and sampling sites.
The Willamette River flows north, through metropolitan Portland, Oregon to its confluence with the Columbia River, which flows west along the border between Oregon and Washington State. The Portland Harbor Superfund area, on the Willamette River, is outlined in red. The sites where PSDs were deployed in 2009–2010 are indicated by yellow circles. Seven sites along the Willamette River are labeled with the river mile (RM) and the east (E) or west (W) bank that they are located near. Two sites on the Columbia River are located upstream (CRU) and downstream (CRD) of the Willamette River confluence.
Sample collection
Lipid-free tubing PSDs were constructed from low-density polyethylene tubing using methods detailed elsewhere [23]. Briefly, additive free tubing was cleaned with hexanes to remove any potential chemical interferences then heat sealed at both ends, producing a 2.7 × 100 cm, two-layer membrane. PSDs designated for chemical analysis were fortified with perdeuterated performance reference compounds (PRCs) prior to sealing the tubing. PRCs are used for the determination of in situ uptake rates [11, 29, 30]. Samplers designated for bioassay applications were not fortified with PRCs.
Stainless steel cages that contained five PSDs were deployed in the water column, approximately 3 m above the ground using an anchored flotation system described elsewhere [22]. Two cages were deployed at each site; one containing samplers for chemical analysis and one with samplers for bioassay applications. Paired cages were deployed at nine sites (Fig. 1) for six different 30 day deployments in 2009–2010. Deployments were carried out in September and October, 2009 and July, August, September and October, 2010. River flows in July-September were significantly lower than in October for both 2009 and 2010, which has been shown to affect the concentration of organic contaminants in the Portland Harbor Superfund area [23]. Samplers were lost at RM 6.5W in August and October, 2010 and at RM 7W and RM 12E in September 2010.
Following each 30 day deployment, samplers were retrieved from the field, sealed in amber glass jars and transported to the laboratory in coolers. After retrieval, the samplers were cleaned with hydrochloric acid and isopropanol to remove superficial fouling, mineral salts and water. Samplers were stored in glass jars at approximately −20° C until extraction. Samplers for chemical analysis were spiked with perdeuterated PAH surrogate recovery standards prior to extraction to allow for verification of extraction efficiency and recovery correction. The five PSDs from each cage were extracted together by dialysis in n-hexane; 40 mL per PSD for 4 hours, the dialysate was decanted then dialysis was repeated for 2 hours and the dialysates were combined. Samples were quantitatively concentrated to a final volume of 1 mL. Samples for bioassay applications were quantitatively solvent exchanged to dimethylsulfoxide (DMSO).
Chemicals
Solvents used for pre-cleaning, clean-up, extraction and sample preparation were Optima® grade or better (Fisher Scientific, Pittsburgh, PA). The following 33 PAH analytes were included in analyses: naphthalene, 1-methylnaphthalene, 2-methylnaphthalene, 1,2-dimethylnaphthalene, 1,6-dimethylnaphthalene, acenaphthylene, acenaphthene, fluorene, dibenzothiophene, phenanthrene, 1-methylphenanthrene, 2-methylphenanthrene, 3,6-dimethylphenanthrene, anthracene, 2-methylanthracene, 9-methylanthracene, 2,3-dimethylanthracene, 9,10-dimethylanthracene, fluoranthene, pyrene, 1-methylpyrene, retene, benz(a)anthracene, chrysene, 6-methylchrysene, benzo(b)fluoranthene, benzo(k)fluoranthene, benzo(e)pyrene, benzo(a)pyrene, indeno(1,2,3-cd)pyrene, dibenz(ah)anthracene, benzo(ghi)perylene and dibenzo(al)pyrene. The perdeuterated PAH compounds used as PRCs were fluorene-D10, p,p′-DDE-D8and benzo(b)fluoranthene-D10. The following perdeuterated PAHs were used as surrogate recovery standards: naphthalene-D8, acenaphthylene-D8, phenanthrene-D10, fluoranthene-D10, pyrene-D10, benzo(a)pyrene-D12 and benzo(g,h,i)perylene-D12; and perylene-D12 was the internal standard.
Chemical analysis
PSD extracts for chemical analysis were analyzed for 33 PAH compounds; PAHs are one of the principal chemical classes of concern in the Superfund site (see supplemental data for additional details about chemical analysis). Furthermore, the samples were screened for 1201 chemicals of concern using Deconvolution Reporting Software (DRS) (Agilent Technologies) (see supplemental data). Chemicals that were detected in the screening were identified using compiled mass spectral deconvolution and identification system (AMDIS) libraries [31] that included numerous classes of chemicals of concern including pesticides, polychlorinated biphenyls (PCBs), parent and substituted (methyl-, oxy- nitro-) PAHs, pharmaceuticals, phthalates and musks among others. The AMDIS library includes gas chromatography- mass spectrometry (GC-MS) compatible compounds; organic chemicals with at least some degree of hydrophobicity, the same chemical that can be sequestered from an aqueous medium by PSDs.
Over 30% of the total number of samples that were chemically analyzed corresponded to quality control samples, which included laboratory preparation blanks, field and trip blanks for each deployment/retrieval, laboratory clean-up blanks and reagent blanks. All target compounds were below the detection limit in all blank quality control samples. Additional details about chemical analysis, including instrument parameters, information about DRS and quality control can be found in supplemental data.
Zebrafish embryotoxicity assay
1 mL of PSD extract contains the chemicals sequestered by 5 PSDs during a 30 day deployment. The concentration of chemicals of concern in the bioassay samples was determined by analysis of the PSD samples that were co-deployed with the samplers for bioassay applications. Water concentrations were calculated, for comparison to exposure solution concentrations, using the empirical uptake model with PRC derived sampling rates detailed elsewhere [11]. The highest exposure concentration that the zebrafish were exposed to was approximately 1000 times greater than the dissolved concentration in river water; the lowest exposure concentration was approximately 8 times greater. The present study did not intend to mimic environmental exposure, but rather identify differences in the toxicity of environmentally relevant mixtures in a high throughput bioassay, and link the observed toxic outcomes with the characteristics of the mixture.
Zebrafish (Danio rerio), from the Tropical 5D strain, were reared in the Sinnhuber Aquatic Research Laboratory (SARL) at Oregon State University in accordance with approved Institutional Animal Care and Use Committee protocols. Adults were kept at standard laboratory conditions: 14:10 h light:dark photoperiod in polycarbonate tanks on a recirculating system in which the water was maintained at 28 °C and a pH of 7.0. Zebrafish were group spawned and newly fertilized eggs were collected and staged according to previously described methods [26].
Zebrafish embryos were exposed to PSD extract solution using a static waterborne method. PSD extract solutions were prepared in DMSO at four concentrations, corresponding to sequential 5-fold dilutions of the PSD extract: 100x (undiluted extract), 20x, 4x and 0.8x. Exposure solutions were made up by diluting the stock solutions 1:100 directly into embryo medium. The final concentration of DMSO was 1% for all exposure solutions. Embryo medium is made by adding sodium bicarbonate (buffer) and methylene blue (mold growth inhibitor) to reverse osmosis water, creating a solution with a pH of 7.3 that is ideal for rearing embryos until 5 days post fertilization (dpf). 1% DMSO was used as a vehicle control. Trimethyltin (TMT) was used as a positive control; the 5 uM concentration used elicits 100% morphological malformations and less than 20% mortality in exposed zebrafish embryos. Exposures were carried out in 96-well plates, with one embryo in 100 uL of exposure solution in each well. Each plate contained 8 embryos exposed to the vehicle control, 8 embryos exposed to the positive control and 20 embryos exposed to each of the 4 concentrations of a PSD extract. Every set-up was repeated twice meaning that 40 individual embryos were exposed to each concentration of each PSD extract sample. In cases where the vehicle or positive controls were outside of acceptable limits (TMT ≤2/16 zebrafish embryos dead or deformed ≤1% DMSO) or there were significant differences between the observed results for the two plates for the same sample, new exposure solutions were prepared and the set-up was repeated.
Preceding exposure, the chorion was removed by pronase treatment to minimize blockage of chemical uptake [32]. All embryos were assessed for viability prior to beginning the exposure and after transfer to the 96 well plates. After all of the embryos were transferred to the exposure solutions, the 96-well plates were sealed, protected from light and maintained at 28 °C for the duration of the exposure. Zebrafish embryo exposures began at approximately 6 hours post fertilization (hpf) and observations were carried out, using a stereo microscope, at 24 hpf and 5 days post fertilization (dpf). At the 24 hpf observation period, each embryos was assessed for mortality, developmental progress and notochord malformations. After 5 days, the embryos were each assessed for mortality and 17 sublethal developmental endpoints: yolk sac edema, pericardial edema, touch response, and malformations of the body axis, eye, snout, jaw, otic vesicle, brain, somites, pectoral fins, caudal fin, pigmentation, circulatory system, trunk, swim bladder and notochord. A malformation, or embryotoxic outcome was considered mortality or any development that deviated from normal morphology described by Kimmel et al [26].
Analysis of toxicity
The incidence of individual toxic outcomes (mortality at 2 time points and 19 sub-lethal endpoints) in embryos exposed to PSD extracts was analyzed. Additionally, a metric was applied to assess the overall toxicity of the PSD extracts to zebrafish embryos. This methodology proved to be effective for assessing general toxicity in prior work, but can overlook subtle differences in toxicity, such as specific outcomes associated with certain samples [4]. Each embryo was assigned a score from 0–1; 0 indicates an embryo that had normal development, 1 indicates death at 24 hours, 0.95 is assigned to death at 5 dpf and each sub-lethal endpoint is 0.045 so that the sum of all 19 sub-lethal outcomes is 0.855. The scores of all of the embryos in a treatment can be used in spatial and temporal comparisons of developmental toxicity.
Statists – Spatial and temporal comparisons
All univariate statistical analyses and graphing were performed using Sigma Plot 11.0 (Systat Software Inc.). Non-parametric tests were used for data that did not pass the Shapiro-Wilk normality test (p<0.05). Mann-Whitney rank sum tests were used for two-way comparisons of chemical characterization and toxic effects. Kruskal-Wallis one way analysis of variance on ranks, followed by the Dunn’s Test for pairwise multiple comparisons when there are unequal treatment group sizes, was used to determine differences in chemical profiles and toxic effects between multiple groups. Comparisons of the incidence of individual endpoints were carried out using maximum likelihood ratios, which allows for the analysis of binary data. Correlations between variables were tested using Spearman rank order.
Multivariate modeling for bridging environmental exposure and toxic effects
A variety of multivariate modeling techniques were explored with the goal of unraveling links between chemical components of environmental mixtures in PSD extracts and associated biological effects in exposed zebrafish embryos. Relationships that are embedded in multivariate data can often be initially explored with data visualization methods then further characterized using, preferentially, robust, easy-to-interpret formal numerical comparison methods [33]. Multivariate analyses were carried out using PRIMER software (version 6, PRIMER-E Ltd., United Kingdom) and included principle component analysis (PCA) with and without loading vectors, and non-metric multiple dimensional scaling (MDS) and MDS using similarity matrices and non-metric comparisons of similarity matrices to investigate significant correlations between two data types.
Principal component analysis (PCA)
When information about a set of samples is in the form of multiple measured quantities (variables), meaningful relationships between samples can be difficult to deduce by direct analysis of these variables. The goal of PCA is to describe relationships between samples by summarizing the information contained in the combination of many variables in terms of a few new variables called principle components (PCs). PCs represent variation in the original data set; the first PC represents the maximum amount of variation possible in one dimension, the second PC represents the maximum amount of the remaining variation in one dimension perpendicular to the first PC, and so on for all remaining PCs. Samples can be plotted as points on a graph with PC pairs as the axes instead of pairs of original variables; this is a non-quantitative visualization tool. Often a small number of PCs explain the majority of the variation in the original data set. In the present study, PCA plots were used to visually show relationships between samples that may not have been obvious when working with the original data [34].
One option available with the PRIMER software package is to overlay loading vectors on PCA plots. The loading vectors depict the original variables in terms of the pair of PCs plotted. The direction of a vector indicated a direction of increasing values for that variable. The vector length represents the importance of the relative contribution of the variable to the plotted PC axes in comparison to all possible PCs. The circle depicted around the loading vectors represents the maximum length of any loading vector; if the vector reaches the perimeter of the circle then it contributes all of its value to one or both of the plotted PCs. The degree to which the vectors are parallel to one another indicated the degree of correlation between the variables in terms of the displayed PCs [35].
Multiple dimensional scaling (MDS)
The goal of MDS is similar to PCA, namely, showing visual relationships between samples in two dimensions. However, while PCA is based on partitioning variation, MDS directly preserves pair-wise relationships between samples in multi-dimensional space, allowing for these relationships to be visualized in two dimensional graphs. For analyses presented here, similarities between samples are presented as a distance in high-dimensional space and these distances are tabulated in a lower triangular matrix, called a similarity matrix, which is a kind of correlation matrix. MDS shows inter-relationships between samples on a graph where the rank of the distances (as opposed to the distances themselves) between samples is preserved. This method is non-parametric and makes no assumptions about multivariate normality [35].
A hierarchical clustering method was another tool that was used for visualizing the results of MDS. Dendograms, that show MDS distances between samples based on similarity matrices, are created by successively fusing samples and groups of samples, based on the distance between them, into larger clusters until all of the samples belong to a single cluster. The distance between the nodes, or connecting lines perpendicular to the distance axis, represents that distance between the samples or groups [35].
Rank correlation coefficient methods were used to quantify the relationship between a set of samples expressed in terms of one set of variables and the same set of samples expressed in terms of a different set of variables. In this case, the environmental samples had a set of chemical variables and another set of toxicity variables associated with them and associations between chemical and toxicity profiles were assess by comparing similarity matrices. Rank correlation coefficients, call Mantel coefficients, were computed by element-to-element matrix comparisons using Spearman’s Rho (ρ) method. Mantel coefficients of ρ=1 indicate perfect correlation while ρ=0 means no correlation. The statistical significance of the ρ value is calculated by randomly permuting one set of sample labels relative to the other many times. With each permutation a ρ value is calculated. A histogram of these randomly generated ρ values represents the null hypothesis that there is no correlation. If, for example, there were 99 permutations and the real ρ value was greater than all 99 randomly generated ρ values then the empirically observed significance, or p value, would be less than 0.01 [36].
Another application of MDS is determining a subset of variables that produces the best correlation with the other corresponding paired variable set. In the present study it was of interest to examine which PAH compounds were most associated with the observed toxic effects. To determine this, every possible similarity matrix of a subset of five PAH variables was compared to a toxic effects results similarity matrix. The subset of PAH variables that produced the highest ρ value and the corresponding statistical significance of the correlation were determined [35].
Data pre-treatment
Data pretreatment was applied to improve the robustness of the statistical methods, meet the assumptions of statistical tests or improve graphical visualization of the data. In this paper, data normalization refers to expressing each variable as its Z-score, which is calculated by subtracting the mean and dividing by the standard deviation for each variable. This assures that the data are on a common scale that does not span orders of magnitude. Analysis with and without data normalization were carried out and are specified in the results.
Root and log transformations were used for data that spanned orders of magnitude or were skewed. These transformations are all increasing functions that tend to deflate the effects of large numbers in the data set. Root transformations are often preferred over log transformations because they do not require adding a one to the argument to guard against taking the log of a small number [35]. Where log transformations were used, log(1+x) was utilized in place of log(x). The main application of transformations in this work was to generate PCA and MDS graphs in which the data was less tightly clustered and easier to visualize. Transformations are noted where applicable in the results.
RESULTS AND DISCUSSION
Chemical characterization of samples used in zebrafish exposures
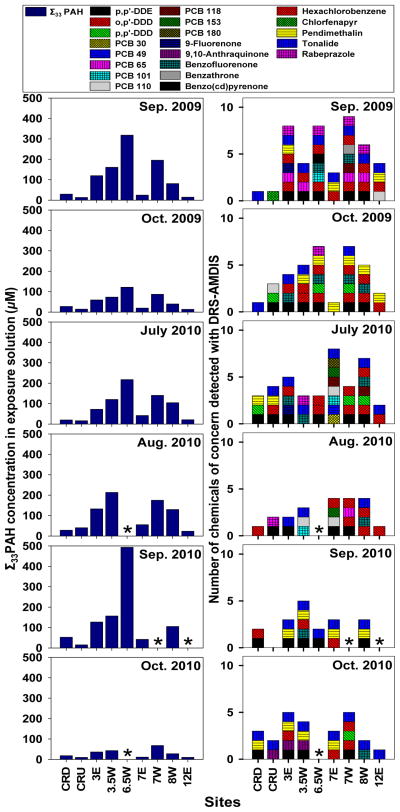
PSDs deployed in the Willamette or Columbia River for 30 days sequestered significantly different amounts of PAHs and other hydrophobic contaminants. The PAH concentrations reported here are for the highest concentration bioassay exposure solutions, which are 1% PSD extract in embryo medium. Samples from RM 6.5W consistently had the highest concentrations of the sum of 33 PAHs (Σ33PAH), except during August and October, 2010, when the samplers were lost from this site. The sample with the greatest Σ33PAH concentration, 1003 pg μL−1, was obtained from RM 6.5W in September 2010. Samples from sites located within the Superfund had significantly higher concentrations of Σ33PAH that the sites located outside of the Superfund (CRU, CRD and RM 12E) (p<0.05). RM 7E, which underwent remediation in 2006, had significantly lower Σ33PAH than the two nearest sites (RM 7W and 6.5W) (p<0.05); however, it was not significantly different from the Superfund sites as a whole or the sites located outside of the Superfund (p>0.05) (Fig. 2). Fluoranthene and pyrene were the most abundant PAH compounds; accounting for 24–36% and 26–39% of the Σ33PAH in every samples respectively. Phenanthrene, retene, benz(a)anthracene and chrysene each composed up to 14% of the Σ33PAH, and other compounds, individually, made up 2% or less of the total PAHs. Three PAH compounds were not detected in any samples: 2,3- and 9,10- dimethylanthracene and 6-methyl-chrysene.
Figure 2. Chemical characterization of PSD extracts from Portland Harbor.
The concentrations of the sum of 33 PAH analytes in the highest dose (100X) exposure solutions used in the zebrafish bioassay is shown on the left. The number of chemicals of concern that were identified in samples from each site and sampling event are shown on the right. The colors/patterns of the blocks indicate which chemicals were identified in the samples. Asteriscs indicate when samples were not obtained.
Significant temporal and seasonal differences in the Σ33PAH were also observed in the samples obtained from the Harbor. Multiple comparison of the data from each sampling period indicated significant differences between events (p=0.038); however, given the variability across sites within each sampling period it was not possible to further characterize these differences. Data were grouped into two seasons based on the flow of the Willamette River. September 2009 and July, August and September 2010 (n=36) were considered ‘dry season’ sampling events because the flow of the Willamette River was significantly lower than the ‘wet season’, which included samples from October 2009 and October 2010 (n=18). The Σ33PAH concentration was significantly greater in samples obtained during the dry season (131 pg uL−1 median) than the wet season (55.7 pg uL−1) (p=0.015). This tendency towards higher concentrations of bioavailable contaminants during the dry season has been previously document in Portland Harbor [23, 37].
DRS screening of the environmental samples for over 1200 chemicals of concern identified twenty-one chemicals, not including the PAH compounds previously quantified. This level of resolution in the chemical characterization of environmental samples lends significant additional depth to the assessment of links between the chemistry and toxicity of the samples. Chemicals identified with DRS included nine polychorinated biphenyls (PCB 30, 49, 65, 101, 110, 118, 153 and 180), p,p′-DDE, o,p′-DDD and p,p′-DDD, which are intermediate breakdown products of the pesticide DDT (1,1,1-trichloro-2,2-di(4-chlorophenyl)ethane), five oxygenated PAH compounds (9-fluorenone, 9,10-anthraquinone, benzofluorenone, benzanthrone and benzo(cd)pyrenone), hexachlorobenzene (fungicide; persistent organic pollutant), chlorfenapyr (pro-insecticide), pendimethalin (herbicide), tonalide (musks found in personal care products) and rabeprazole (antiulcer pharmaceutical). The presence of these chemicals in samples from Portland Harbor showed spatial and temporal variability (Fig. 2). There was a tendency for a greater number of chemicals of concern to be detected in samples obtained from sites within the Superfund area than upstream or on the Columbia River.
Developmental toxicity of PSD extracts
A total of 10944 embryos were exposed to 4 concentrations of 50 different environmental samples, as well as positive and vehicle controls. This highlights the truly high throughput character of the BRIDGES bioanalytical tool. Zebrafish embryos that were exposed to PSD extracts demonstrated a range of responses including normal development, mortality, and a variety of morphological deformities (Fig. 3). Univariate and multivariate statistical and modeling approaches were applied to the data to help unravel associations between the chemical components of environmental mixtures and observed outcomes in zebrafish embryos exposed to the mixtures.
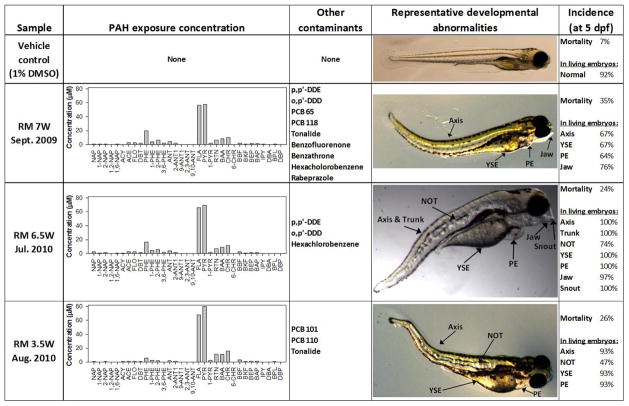
Figure 3. Morphological malformations observed in embryos exposed to chemical mixtures in PSD extracts at 5 dpf.
The concentrations of PAH analytes and presence of other chemicals of concern in exposure solutions composed of 1% PSD extract obtained from sites within the Portland Harbor Superfund are shown. Examples of developmental abnormalities in 5 day post fertilization (dpf) embryos exposed to PSD extract solutions include mortality, yolk sac edema (YSE), pericardial edema (PE) and malformations of the body axis, trunk, notochord (NOT), jaw and snout. Normal development in an embryo exposed to the vehicle control is also pictured. Not all deformities pictured are labeled.
The average number of developmental endpoints observed in embryos that showed sub-lethal effects at the 5 day observation was 6 of the 17 assessed at that time point. The median number of sublethal endpoints was 3. A tendency for an ‘all-or-nothing’ response in the exposed zebrafish was seen throughout the study, where normal development or complete deformity/mortality were much more common results than an intermediate number of sublethal deformities. This distribution of the number of expressed sub-lethal endpoints is likely due to a biological threshold above which development is completely disrupted, as well as the dosing regimen used in the present study. In future work it may be necessary to perform dose range-finding experiments for each sample prior to high-throughput analysis of developmental toxicity in order to procure exposure dose-response curves that consistently capture sublethal morphological deformities. Given the ‘all-or-nothing’ characteristics of the toxicity data in the present study, further analyses are based on overall toxicity scores (described in materials and methods) as opposed to individual specific toxic endpoints.
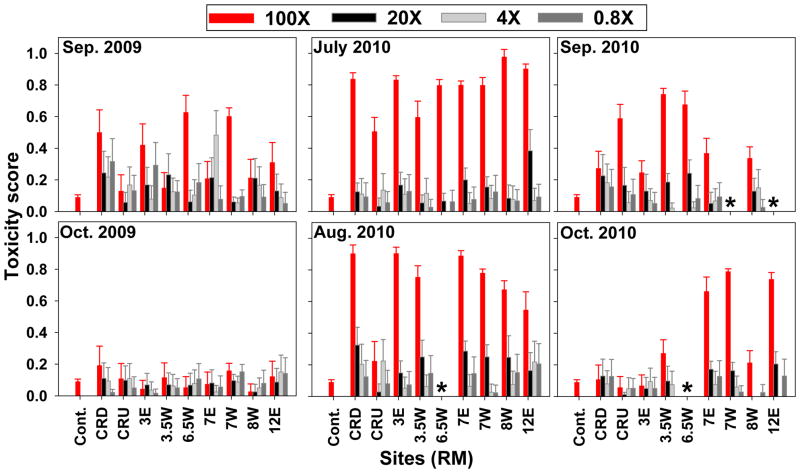
Significant exposure dose-response relationships were observed in some samples. However this was most often seen as a greater toxicity score for the highest exposure dose compared to the other three doses, which were not significantly different from one another (Fig. 4). A significantly greater response at the highest exposure dose, compared to the lower doses was seen in samples from RM 6.5W and 7W in September 2009, all samples in July 2010, all samples except CRU in August 2010, CRU and RMs 3.5W and 6.5W in September 2010 and RMs 7E, 7W and 12E in October 2010 (Fig. 4). Intermediate toxicity, greater than the lowest two exposure doses but less than the highest exposure dose, was seen at the second highest dosing level in 3 samples: 12E in July 2012 and CRD and &W in August 2010. Further comparisons between samples are based on only the highest exposure dose.
Figure 4. Developmental toxicity of PSD extracts.
The developmental toxicity of each sample was scored based on the presence or absence of 21 toxic outcomes. Bars represent the average score of all embryos exposed to the sample (n=40 per treatment) and lines represent the standard deviation. Scores range from 0–1 where 0 is indicative of normal development and 1 represents mortality at the earliest time point. The highest concentration (100X; red bars) was 1% PSD extract and the other exposure concentrations are successive five-fold dilutions of the extract (20X, 4X and 0.8X). The average outcome of the 1% DMSO control (Cont.) is shown on each graph for comparison. Asterisks indicate that samples were not obtained from the field. See Figure 2.1 for the location of the sampling sites.
The developmental toxicity of the samples showed significant spatial variability. All samples from September 2009, except from 3.5W and CRU, were significantly different from the 1% DMSO control (p<0.05). The samples from RMs 6.5W and 7W were significantly more toxic than the sample from CRU, 3.5W, 8W and 7E (p<0.05); CRD and RM 3E were significantly more toxic than CRU, 3.5W and 8W (p<0.05) and 12E was more toxic than CRU (p<0.05). Only the sample obtained from RM 7W was significantly more toxic than the control in October 2009 (p<0.05) and there were no differences between the sites. All samples from July, 2010 were significantly more toxic than the control; however there were no significant differences between the sites. All of the samples obtained in August, 2010, except from CRU, were significantly more toxic than the control (p<0.05) and samples from all other sites were more toxic than CRU. All samples from September, 2010 were more toxic than the control (p<0.05) and the sample from RM 3.5W was more toxic than RM 3E. Samples from RMs 3.5W, 7E, 7W, 8W and 12E from October 2010 showed significantly greater toxicity than the control as well as the samples from CRU, CRD and 3E (p<0.05) (Fig. 4).
Differences in toxicity between the sites, including those that are located very close to one another on the river, were observed in a prior study [4]. The sites at RMs 7W, 7E and 6.5W are all located within 0.5 km of each other. However, samples from RMs 6.5W and 7W had higher Σ33PAHs in all comparable sampling events and were more toxic than RM 7E during two sampling events. This demonstrates the effects of remediation at 7E, the McCormick and Baxter site, on the chemical contamination and toxicity in the overlying water column. These results demonstrate that toxicity can vary significantly within a reduced spatial scale and highlight the importance of using analytical methods that provide highly resolves, site-specific information, such as the BRIDGES tool.
Significant temporal differences in toxicity were observed in the present study. Similar to the Σ33PAH chemical data, the toxicity scores of the samples obtained during the ‘dry season’ (July and August) in 2009 and 2010 were significantly greater (n=1440; 0.72 median toxicity on a scale of 0–1) than those obtained during the ‘wet season’ (September and October) (n=840; 0.0 median toxicity) (p<0.001). Samples from July, 2010 and August, 2010 (0.810 median score for both) were significantly more toxic than samples from all other months included in the present study (0.23, 0.00, 0.36 and 0.18 median scores in September and October 2009 and October 2010 respectively) (p<0.001). PSD extracts from September, 2009 and 2010 were more toxic than those from October 2009 and 2010 and samples from October 2010 had greater toxicity than those from October 2009 (p<0.001).
Temporal changes in the concentration of bioavailable contaminants in the Willamette River have been recorded in previous studies [23]. These results demonstrate that those seasonal differences in contamination translate into differences in the toxicity of PSD extracts obtained from the environment. This is not a foregone conclusion considering that PAHs are a class of compounds with widely variable solubility and toxicity. Therefore, changes in dissolved Σ33PAH levels are not necessarily indicative of greater toxicity. As with the results that demonstrate significant spatial differences in toxicity, these data provide further support for the importance of applying environmental monitoring tools that are capable of capturing temporal changes in chemical concentrations and toxicity.
One aspect of this BRIDGES method that is a subject of current research is the determination of uptake rates of chemicals by the embryonic zebrafish. The present study uses quantitative exposure doses based on the concentration of chemicals in the PSD extract and the concentration of the extract in the exposure solution. Knowledge of uptake rates for the bioassay organism or improved methodology for estimating dose could refine observed associations between environmental mixtures sequestered by PSDs and the toxicity of these mixtures to organisms and substantiate the comparability of PSD based chemical analysis and bioanalysis.
Associations between chemistry and biological effects- Univariate analyses
Two characteristics of the data limited the ability to link specific chemicals present in the environmental mixtures to the toxic outcomes that they elicited: the ‘all-or-nothing’ tendency in the number of toxic effects seen in the zebrafish embryos exposed to PSD extracts and the significant correlations between the concentrations of individual analytes in a sample and the total concentration of contaminants in the sample. The chemical profiles of all of the samples were similar and diagnostic ratios between pairs of PAHs were not significantly different in any of the samples obtained from Portland Harbor. As a result, the magnitude of the concentration of analytes in the samples was variable, but the composition was similar. This was not the case in past studies in Portland Harbor [23], however remediation efforts have had an apparent effect on the magnitude and chemical characteristics of contamination at former ‘hot-spots’.
No significant correlation between the Σ33PAH in the samples and the developmental toxicity score was observed for the highest exposure-dose data. This could be partially explained by the extremeness of the toxicity results and the number of samples, with a wide range of PAH concentrations, that elicited mortality in the majority of the exposed embryos. Tests of correlations between incidences of specific developmental outcomes and Σ33PAH showed no significant association between analyte concentrations and the incidence of any developmental endpoint. The presence/absence of chemicals of concern that were identified using DRS did not show a significant correlation with observed toxic outcomes in exposed embryos. However, the greatest number of compounds identified by DRS were detected in samples from RM 6.5W and this site consistently showed elevated toxicity. The ability to screen for a large number of compounds of concern in environmental samples could help explain observed toxicity [5], but in this case the relative similarity in the chemicals that were present at sites in the Superfund area made it difficult to define specific links to toxic outcomes.
Unraveling associations between exposures and effects- Multivariate modeling
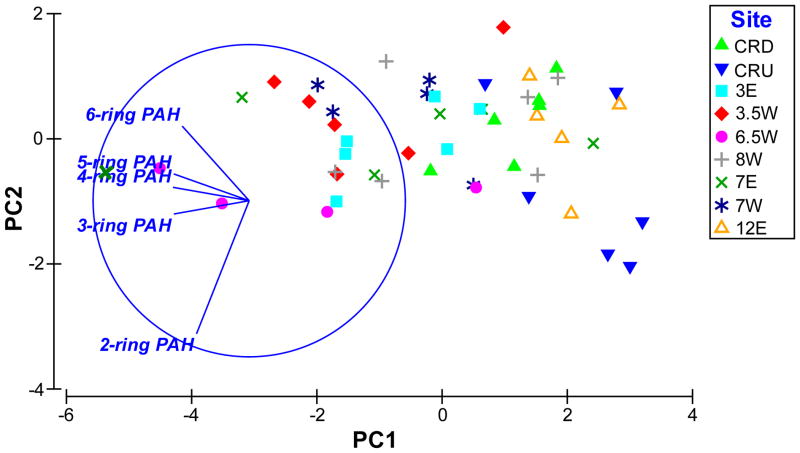
As noted, the PAH chemical profiles for samples obtained from the lower Willamette River in the present study were similar in spite of significant differences in the magnitudes of the concentrations. PCA was used to elucidate trends in the variability of the chemical profiles. When the concentrations of the PAH analytes quantified in the present study were grouped based on the number of rings in their molecular structure and analyzed using PCA, spatial trends that were not apparent in earlier analyses were visible (Fig. 5). PC1 explains 80.1% of the variability in the data set and there is a clear spatial trend in the variability of the PAH profiles along this axis; there is a gradient from superfund site samples to samples from outside of the superfund from left to right along the PC1 axis. As seen in Figure 5, the 4-ring and 5-ring PAHs are highly correlated (parallel loading vectors) and tend to increase from non-superfund sites to superfund sites. They also show a tendency to increase from 12E and CUS to CDS. The 3-ring PAHs increase from non-superfund to superfund sites and 6-ring PAHs show a similar tendency but to a lesser extent. In terms of PC1 and PC2, the 2-ring PAH variable shows constant values for samples from RM 6.5W and little association with spatial trends in other sites (Fig. 5). These trends in the chemical composition of the samples are relevant to understanding the biological outcomes of exposure to the environmental mixtures because previous research has demonstrated clear links between certain classes of PAHs and specific toxic endpoints. Although the nature of the chemical and toxicity data obtained in the present study limits our ability to make direct connections between specific compounds, a higher incidence of craniofacial, body axis and cardiac malformations in embryos exposed to extracts from superfund sites, which showed higher relative concentrations of 3-, 4- and 5- ring PAHs is consistent with research that demonstrates causative links between 3- and 4- ring PAHs and these specific endpoints [38].
Figure 5. PCA of PAH data.
A plot of PC1 and PC2 for PAH concentrations by ring size visually demonstrates variability in the chemical characteristics of PSD samples from different sites and sampling events along the lower Willamette River. See Figure 1 for the location of the sampling sites. PC1 and PC2 represent 80.1% and 14.3% of the variability in the data set, respectively. Data was normalized to achieve a common scale and forth root transformed to reduce data clustering and facilitate visualization. The vectors point in the direction of increasing variable values in terms of PC1 and PC2 and their length represents relative contribution of the variable to the plotted axis where the circle indicates maximum contribution.
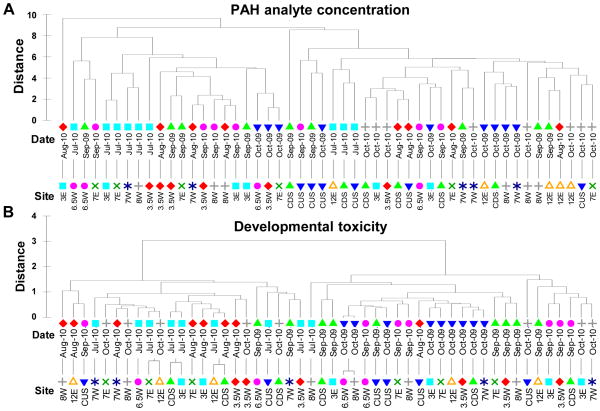
MDS was used to examine other relationships between the chemical characteristics and toxicity of the samples. Dendograms, showing the results of a hierarchical clustering method, were used to visualize the results of MSD (Fig. 6). MDS demonstrated spatial and temporal trends in both chemical and biological data, although the trends were not the same in both groups of data. Based on the concentration of PAH analytes, the samples fell into two groups with one notable outlier (RM 3E from August, 2010) and one group outlier (CDS from September, 2009). In general, samples from unremediated sites within the superfund, from most sampling events, were similar to each other, while samples obtained outside of the superfund site formed a separate group. Additionally, 12 samples that were obtained from sites within the superfund during the wet season (September and October) showed more similarity with the non-superfund site samples than with the superfund group (Fig. 6A).
Figure 6. Dendograms of MDS results for chemical and toxicity characteristics of environmental samples.
Dendograms show the similarity of samples to one another in terms of PAH analyte concentrations (A) and developmental toxicity (B). Distance is a high-dimensional measure of similarity between samples with multiple variable measurements. PAH similarity matrices were constructed using the concentrations of all quantified analytes at the highest exposure concentration and data were normalized prior to analysis. The developmental toxicity similarity matrices were constructed from the percent incidence of each observed developmental endpoint in embryos exposed to the highest concentration PSD extract solution. The sampling date and site labels for each sample are shown; symbols are a visualization guide only and repetition of symbols between date and site labels does not indicate an association.
The similarity of the developmental toxicity of the samples showed two distinct, distant groups defined principally by sampling date as opposed to sampling site. The greater degree of separation between the two groups of samples, in terms of toxicity, is likely a reflection of the “all or nothing” tendency for developmental effects discussed previously. With a few exceptions, samples taken in July and August (dry season) formed one group and samples taken in September and October, 2009 formed another group. Based on toxicity, samples from October, 2010 fell into both groups with no obvious site-based tendency (Fig. 6B). Together, this visual analysis of MDS results suggests that while PAHs are likely contributing to the observed toxicity, they may not be the principal driver or observed effects. The PAH chemical profile of the samples is dependent on both spatial and temporal factors, whereas the toxicity of the samples is apparently more driven by temporal changes.
Analysis of a dendogram of the results of the DRS screening for chemicals of concern did not provide additional insight into the connection between the chemical characteristics of the samples and their toxicity. Based on other chemicals identified in the PSD extracts, the samples did not cluster into well defined groups, with the exception of 4 samples from September, 2009. It was not possible to define a clear spatial or temporal trend in the DRS data based on this MDS analysis (Supplemental data, Fig. S1).
In addition to using MDS to examine similarities between samples based on chemical and toxicity variables, it was applied to examine correlations between sets of variables. In this case, the correlation between the similarity matrices of the subset of PAH variables and the subset of toxicity endpoints was examined. Three PAH analytes (9-methylanthracene, 9,10-dimethylanthracene and dibenzo(a,l)pyrene) were removed from this analysis because they were detected a low levels in less the 10% of the samples and tended to skew the outcome of the analysis. A significant correlation between the PAHs in the samples and the observed toxicity was detected (p=0.001) with a ρ value of 0.208. This demonstrates that the PAHs are a significant contributor to observed toxicity, though not the only contributing factor.
An operation of the PRIMER software was used to identify subsets of PAHs that show the best correlation with all of the toxicity data. The ten best subsets of 5 or less PAHs were identified; all produced significant correlations (p<0.05) and ρ values between 0.324–0.334, which is greater than the correlation between the similarity matrices of all of the PAH and toxicity variables. The subset of PAH analytes that was most highly correlated with the toxicity of the samples (ρ=0.324, p=0.01) was naphthalene, retene and benzo(a)pyrene. Other PAH analytes that were identified in one or more of the ten subsets most highly correlated with toxicity were acenaphthylene and benzo(b)fluoranthene. It is important to note that the ρ values used to quantify correlations between similarity matrices are not equivalent to r2 values used in univariate correlations. This analysis defined the PAH variables that are most associated with the observed developmental toxicity of the samples. Future work could focus on controlled exposures to these individual and sets of PAHs in order to quantify their toxicity without the influence of other chemicals present in the complex environmental mixture.
Correlating chemical fingerprints to toxic outcomes has been demonstrated in the past [39]; however this approach may require additional refinement before it can be applied to a data set of this size, with a large number of analytes and samples with similar chemical fingerprints. Future research should focus on fractionating the samples to reduce the number of potential analytes [5, 40], and testing individual analytes that showed significant associations with specific endpoints to further refine the understanding of chemical components and biological effects. Obtaining samples from sites with different chemical contaminants may also help elucidate patterns in the biological responses that are masked by the similarity in the chemical profiles of the samples obtained for the present study. The multivariate modeling used demonstrates powerful methods for unraveling spatial and temporal trends in bioavailable chemical contaminants and the toxicity of environmental mixtures. Additionally, it provides tools for elucidating links between complex chemical mixtures and multi-endpoint developmental toxicity assessments.
CONCLUSION
BRIDGES proved to be a sensitive, high throughput bioanalytical tool that was capable of detecting highly resolved spatial and temporal differences in bioavailable chemicals in the environment and the toxicity of those environmental mixtures. PAHs, one of the principal contaminants of concern in the Portland Harbor Superfund site, showed a significant correlation with the toxicity of the samples; however, there was evidence that other chemical components of the mixtures were also significant contributors to toxicity. A number of other contaminants were detected in the samples in the screening for 1201 chemicals of concern, however, the non-quantitative nature of this data made it unfeasible to unravel clear associations between specific chemicals and toxic effects. Having a tool that provides spatially and temporally resolved information about bioavailable contaminants and toxicity is of standalone value, however, refining the methodology used to define exposure doses in future work with the embryonic zebrafish model could provide a more refined understanding of links between components of chemical mixtures and toxic effects. Additionally, molecular tools could be used to detect specific effects at very low doses and may provide greater insight into toxicity than observations of gross embryonic morphology [27]. Multivariate modeling methods applied in the present study illustrated spatial and temporal trends that were not clear in initial analyses. Additionally, MDS proved to be a valuable tool for examining associations between paired sets of variables, such as the chemistry and toxicity data for each sample in the present study. It was also valuable for identifying small subsets of PAH variables that were most strongly correlated with the paired set of toxicity variables. The BRIDGES tool, pairing passive sampling with the embryonic zebrafish developmental toxicity assay, has great potential to be broadly applied to a wide range of environmental monitoring projects and could be refined and modified to meet specific study objectives.
Supplementary Material
Acknowledgments
This project was supported in part by award numbers P42 ES016465 and the associated Analytical Chemistry Facility Core; P30 ES000210 and the associated Aquatic Biomedical Models Facility Core from the National Institute of Environmental Health Sciences. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIEHS or the National Institutes of Health. We appreciate valuable help in the field from Bob Grove, Vaughn Tidwell, Lane Tidwell and Steven O’Connell. Additionally, we are grateful for analytic expertise from Glenn Wilson and assistance with zebrafish rearing and analysis from Lisa Truong and Gregory Gonnerman.
Footnotes
Supplemental data includes detailed information about chemical analyses and Figure S1, which shows a dendogram of the MSD results for chemicals of concern that were identified in the PSD samples.
BIBLIOGRAPHY
- 1.Huckins JN, Tubergen M, Manuweera G. Semipermeable membrane devices containing model lipid: a new approach to monitoring the bioavailability of lipophilic contaminants and estimating their bioconcentration potential. Chemosphere. 1990;20:533–552. [Google Scholar]
- 2.Vijver MG, Peijnenburg WJGM, De Snoo GR. Toxicological Mixture Models are Based on Inadequate Assumptions. Environ Sci Technol. 2010;44:4841–4842. doi: 10.1021/es1001659. [DOI] [PubMed] [Google Scholar]
- 3.Eggen RIL, Behra R, Burkhardt-Holm P, Escher BI, Schweigert N. Challenges in Ecotoxicology. Environ Sci Technol. 2004;38:58A–64A. doi: 10.1021/es040349c. [DOI] [PubMed] [Google Scholar]
- 4.Hillwalker WE, Allan SE, Tanguay RL, Anderson KA. Exploiting lipid-free tubing passive samplers and embryonic zebrafish to link site specific contaminant mixtures to biological responses. Chemosphere. 2010;79:1–7. doi: 10.1016/j.chemosphere.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Legler J, van Velzen M, Cenijn PH, Houtman CJ, Lamoree MH, Wegener JW. Effect-Directed Analysis of Municipal Landfill Soil Reveals Novel Developmental Toxicants in the Zebrafish Danio rerio. Environ Sci Technol. 2011:null–null. doi: 10.1021/es201099s. [DOI] [PubMed] [Google Scholar]
- 6.Brack W. Effect-directed analysis: a promising tool for the identification of organic toxicants in complex mixtures? Anal Bioanal Chem. 2003;377:397–407. doi: 10.1007/s00216-003-2139-z. [DOI] [PubMed] [Google Scholar]
- 7.Hamelink JL, Landrum PF, Bergman HL, Benson WH, editors. Bioavailability: physical chemical, and biological interactions. CRC Press Inc; Boca Raton, FL: 1994. [Google Scholar]
- 8.Connell DW. Bioaccumulation of xenobiotic compounds. CRC Press Inc; Boca Raton, FL: 1990. [Google Scholar]
- 9.Birnbaum LS. Applying Research to Public Health Questions: Biologically Relevant Exposures. Environ Health Perspect. 2010;118 doi: 10.1289/ehp.1002015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dardenne F, Nobels I, De Coen W, Blust R. Mixture toxicity and gene inductions: can we predict the outcome? Environ Toxicol Chem. 2008;27:509–518. doi: 10.1897/07-303.1. [DOI] [PubMed] [Google Scholar]
- 11.Huckins JN, Petty JD, Booij K. Monitors of organic chemicals in the environment: semipermeable membrane devices. Springer; New York: 2006. [Google Scholar]
- 12.Adams RG, Lohmann R, Fernandez LA, Macfarlane JK, Gschwend PM. Polyethylene devices: Passive samplers for measuring dissolved hydrophobic organic compounds in aquatic environments. Environ Sci Technol. 2007;41:1317–1323. doi: 10.1021/es0621593. [DOI] [PubMed] [Google Scholar]
- 13.Carls MG, Holland LG, Short JW, Heintz RA, Rice SD. Monitoring polynuclear aromatic hydrocarbons in aqueous environments with passive low-density polyethylene membrane devices. Environ Toxicol Chem. 2004;23:1416–1424. doi: 10.1897/03-395. [DOI] [PubMed] [Google Scholar]
- 14.Bonetta S, Carraro E, Bonetta S, Pignata C, Pavan I, Romano C, Gilli G. Application of semipermeable membrane device (SPMD) to assess air genotoxicity in an occupational environment. Chemosphere. 2009;75:1446–1452. doi: 10.1016/j.chemosphere.2009.02.039. [DOI] [PubMed] [Google Scholar]
- 15.Ke R, Li J, Qiao M, Xu Y, Wang Z. Using semipermeable membrane devices, bioassays, and chemical analysis for evaluation of bioavailable polycyclic aromatic hydrocarbons in water. Arch Environ Contam Toxicol. 2007;53:313–320. doi: 10.1007/s00244-006-0158-4. [DOI] [PubMed] [Google Scholar]
- 16.Parrott JL, Backus SM, Borgmann AI, Swyripa M. The use of semipermeable membrane devices to concentrate chemicals in oil refinery effluent on the Mackenzie river. Arctic. 1999;52:125–138. [Google Scholar]
- 17.Parrott JL, Tillitt DE. The Use of Semipermeadble Membrane Devices (SPMDs) to Concentrate Inducers of Fish Hepatic Mixed Function Oxygenase (MFO) In: Zelikoff JT, editor. Ecotoxicology: Responses, Biomarkers and Risk Assessment, an OECD workshop. SOS Publications; Fair Haven, NJ: 1997. pp. 185–196. [Google Scholar]
- 18.Shaw M, Negri A, Fabricius K, Mueller JF. Predicting water toxicity: pairing passive sampling with bioassays on the Great Barrier Reef. Aquat Toxicol. 2009;95:108–116. doi: 10.1016/j.aquatox.2009.08.007. [DOI] [PubMed] [Google Scholar]
- 19.Shaw M, Tibbetts IR, Müller JF. Monitoring PAHs in the Brisbane River and Moreton Bay, Australia, using semipermeable membrane devices and EROD activity in yellowfin bream, Acanthopagrus australis. Chemosphere. 2004;56:237–246. doi: 10.1016/j.chemosphere.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 20.Springman KR, Short JW, Lindeberg MR, Maselko JM, Khan C, Hodson PV, Rice SD. Semipermeable membrane devices link site-specific contaminants to effects: Part 1 -Induction of CYP1A in rainbow trout from contaminants in Prince William Sound, Alaska. Mar Environ Res. 2008;66:477–486. doi: 10.1016/j.marenvres.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 21.Allan IJ, Booij K, Paschke A, Vrana B, Mills GA, Greenwood R. Field Performance of Seven Passive Sampling Devices for Monitoring of Hydrophobic Substances. Environ Sci Technol. 2009;43:5383–5390. doi: 10.1021/es900608w. [DOI] [PubMed] [Google Scholar]
- 22.Anderson KA, Sethajintanin D, Sower G, Quarles L. Field trial and modeling of uptake rates on in situ lipid-free polyethylene membrane passive sampler. Environ Sci Technol. 2008;42:4486–4493. doi: 10.1021/es702657n. [DOI] [PubMed] [Google Scholar]
- 23.Sower GJ, Anderson KA. Spatial and Temporal Variation of Freely Dissolved Polycyclic Aromatic Hydrocarbons in an Urban River Undergoing Superfund Remediation. Environ Sci Technol. 2008;42:9065–9071. doi: 10.1021/es801286z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dooley K, Zon LI. Zebrafish: a model system for the study of human disease. Current Opinion in Genetics & Development. 2000;10:252–256. doi: 10.1016/s0959-437x(00)00074-5. [DOI] [PubMed] [Google Scholar]
- 25.Hill AJ, Teraoka H, Heideman W, Peterson RE. Zebrafish as a model vertebrate for investigating chemical toxicity. Toxicol Sci. 2005;86:6–19. doi: 10.1093/toxsci/kfi110. [DOI] [PubMed] [Google Scholar]
- 26.Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF. Stages of Embryonic Development of the Zebrafish. Dev Dyn. 1995;203:253–310. doi: 10.1002/aja.1002030302. [DOI] [PubMed] [Google Scholar]
- 27.Ekker SC, Stemple DL, Clark M, Chien C-B, Rasooly RS, Javois LC. Zebrafish Genome Project: Bringing New Biology to the Vertebrate Genome Field. Zebrafish. 2007;4:239–251. doi: 10.1089/zeb.2007.9979. [DOI] [PubMed] [Google Scholar]
- 28.Integral, Windward, Kennedy/Jenks, Anchor-QEA. Portland Harbor RI/FS Remedial Investigation Report. IC09–0003. 1–14. Prepared for the Lower Willamette Group; Portland OR: Integral Consulting Inc; Portland, OR: Windward Environmental LLC, Inc; Seattle, WA: Kennedy/Jenks Consultants; Portland, OR: Anchor QEA LLC; Seattle, WA, Portland Oregon: 2009. [Google Scholar]
- 29.Booij K, Smedes F. An Improved Method for Estimating in Situ Sampling Rates of Nonpolar Passive Samplers. Environ Sci Technol. 2010;44:6789–6794. doi: 10.1021/es101321v. [DOI] [PubMed] [Google Scholar]
- 30.Huckins JN, Petty JD, Lebo JA, Almeida FV, Booij K, Alvarez DA, Cranor WL, Clark RC, Mogensen BB. Development of the Permeability/Performance Reference Compound Approach for In Situ Calibration of Semipermeable Membrane Devices. Environ Sci Technol. 2001;36:85–91. doi: 10.1021/es010991w. [DOI] [PubMed] [Google Scholar]
- 31.Mallard WG, Reed J. Automated Mass spectral Deconvolution & Identification System -User Guide. US Department of Commerce, National Institute of Standards and Technology; Gaithersburg, MD: 1997. p. 62. [Google Scholar]
- 32.Mizell M, Romig ES. The aquatic vertebrate embryo as a sentinel for toxins: zebrafish embryo dechorionation and perivitelline space microinjection. Int J Dev Biol. 1997;41:411–423. [PubMed] [Google Scholar]
- 33.Clarke KR, Somerfield PJ, Airoldi L, Warwick RM. Exploring interactions by second-stage community analyses. J Exp Mar Biol Ecol. 2006;338:179–192. [Google Scholar]
- 34.Shaw PJA. Multivariate Statistics for the Environmental Sciences. 2. Wiley, John & Sons Inc; Chincester: 2003. [Google Scholar]
- 35.Clarke K. Primer v6: User Manual/Tutorial. PRIMER-E; Plymouth: 2006. [Google Scholar]
- 36.Clarke KR. Non-parametric multivariate analyses of changes in community structure. Aust J Ecol. 1993;18:117–143. [Google Scholar]
- 37.Allan SE, Sower GJ, Anderson KA. Estimating risk at a Superfund site using passive sampling devices as biological surrogates inhuman health risk models. Chemosphere. 2011;85:920–927. doi: 10.1016/j.chemosphere.2011.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Incardona JP, Collier TK, Scholz NL. Defects in cardiac function precede morphological abnormalities in fish embryos exposed to polycyclic aromatic hydrocarbons. Toxicol Appl Pharmacol. 2004;196:191–205. doi: 10.1016/j.taap.2003.11.026. [DOI] [PubMed] [Google Scholar]
- 39.Eide I, Neverdal G, Thorvaldsen B, Grung B, Kvalheim OM. Toxicological evaluation of complex mixtures by pattern recognition: Correlating chemical fingerprints of mutagenicity. Environ Health Perspect. 2002;110:985–988. doi: 10.1289/ehp.02110s6985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sundberg H, Ishaq R, Akerman G, Tjarnlund U, Zebuhr Y, Linderoth M, Broman D, Balk L. A Bio-Effect Directed Fractionation Study for Toxicological and Chemical Characterization of Organic Compounds in Bottom Sediment. Toxicol Sci. 2005;84:63–72. doi: 10.1093/toxsci/kfi067. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.