Abstract
Purpose
We investigated the correlation between body mass index (BMI) and the prognosis of castration-resistant prostate cancer (CRPC) in patients who received docetaxel treatment.
Materials and Methods
A retrospective study was conducted of 55 patients who were diagnosed with CRPC and received docetaxel treatment between 2003 and 2009 at our institution. Patients with a normal or lower BMI (<23.0 kg/m2) were categorized as group I and patients with an overweight or greater BMI (≥23.0 kg/m2) were categorized as group II. Clinicopathological features and survival rates were evaluated by using the Kaplan-Meier method and Cox proportional hazards models.
Results
On the basis of BMI, 27 patients (49.1%) belonged to group I and 28 (50.9%) patients belonged to group II. Mean follow-up periods were 30 months and 34.2 months, respectively (p=0.381). There were no significant differences between the two groups in terms of age, prostate-specific antigen (PSA), Gleason score, Eastern Cooperative Oncology Group Performance Status, hemoglobin level, alkaline phosphatase level, distant metastasis, radiation treatments, or performance of radical prostatectomy (p>0.05). In the univariate analysis for predicting survival rates, BMI (p=0.005; hazard ratio [HR], 0.121), logPSA (p=0.044; HR, 2.878), and alkaline phosphatase level (p=0.039; HR, 8.582) were significant factors for prediction. In the multivariate analysis, BMI (p=0.005; HR, 0.55), logPSA (p=0.008; HR, 7.836), Gleason score (p=0.018; HR, 6.434), hemoglobin (p=0.006; HR, 0.096), alkaline phosphatase level (p=0.005; HR, 114.1), and metastasis to the internal organs (p=0.028; HR, 5.195) were significant factors for prediction.
Conclusions
Better effects on the cancer-specific survival rate were observed in cases with higher BMI.
Keywords: Cancer specific survival, Castration-resistant prostate cancer, Obesity
INTRODUCTION
Obesity is a major health problem throughout the world, including in Korea. According to the Korean Society for the Study of Obesity, about 30% of the Korean population is classified as overweight or obese [1], which has been linked to the development of various malignant diseases, including colorectal cancer and breast cancer [2].
According to data from the Korea Central Cancer Registry in 2008, the onset of prostate cancer has been sharply increasing since 1999. In 2008, a ratio of 26.1 new cases of prostate cancer per 100,000 persons was reported, and by virtue of developments in diagnostic methods such as prostate-specific antigen (PSA) and transrectal ultrasonography, the onset rate is rapidly increasing [3].
Obesity and prostate cancer are highly prevalent in elderly men. However, the relationship between obesity and prostate cancer remains unclear. A recent epidemiologic study reported that obesity may be protective against the development of early stage prostate cancer [4]. On the contrary, other studies have shown that obesity may be associated with an increased risk of advanced disease and death from prostate cancer [5,6]. Amling et al. [7] reported higher rates of positive surgical resection margins and biochemical recurrence in obese patients with localized prostate cancer undergoing radical prostatectomy than in normal-weight patients. That report is considered to be a representative example of an association between obesity and localized prostate cancer. As aforementioned, most studies on the relationship between body mass index (BMI) and the prognosis of prostate cancer patients have focused on localized prostate cancer. To our knowledge, studies are lacking on the relation between BMI and castration-resistant prostate cancer (CRPC). Thus, we proposed to analyze the prognostic value of BMI in Korean patients with CRPC.
MATERIALS AND METHODS
A retrospective study was conducted of 55 patients who were diagnosed with prostate cancer and who received hormonal therapy for local or distant metastasis. All patients received docetaxel chemotherapy owing to the development of CRPC between January 2003 and December 2009 at our institution. This study was approved by the Institutional Review Board of Seoul National University Bundang Hospital.
CRPC was defined as cases of an increased PSA level despite a serum testosterone level in the castrate range, a PSA level that increased 3 consecutive times despite interruption of administrating antiandrogen agents for 4 to 8 weeks or despite secondary hormonal treatment owing to advancement of the metastatic lesion, or the new development of a measurable metastatic lesion as assessed by imaging or an increase in size [8].
BMI was calculated by dividing body weight (kg) by the square of height (m), which was measured before docetaxel treatment. According to the World Health Organization Asian-Pacific Obesity Guidelines, patients with a normal or lower BMI (<23 kg/m2) were categorized as group I and patients with an overweight or greater BMI (≥23 kg/m2) were categorized as group II.
By use of the medical records of the patients, age, time from the diagnosis of prostate cancer, follow-up period, serum PSA level, Gleason score, Eastern Cooperative Oncology Group (ECOG) Performance Status, serum alkaline phosphatase level, serum creatinine level, metastasis to the internal organs, radiation treatments, and performance of radical prostatectomy were investigated retrospectively.
The primary clinicopathological outcomes according to BMI were compared by using Mann-Whitney U test and chi-square test. Cancer-specific survival was estimated by the Kaplan-Meier method, and differences between the groups were tested by using the log-rank test. The effect of BMI on cancer-specific survival was examined by using a Cox proportional hazards regression model, and hazard ratios (HRs) and 95% confidence intervals (CIs) were computed. All p-values were two-sided, and values <0.05 were considered to indicate statistical significance. Analyses were performed by using the PASW ver. 18.0 (IBM Co., Armonk, NY, USA).
RESULTS
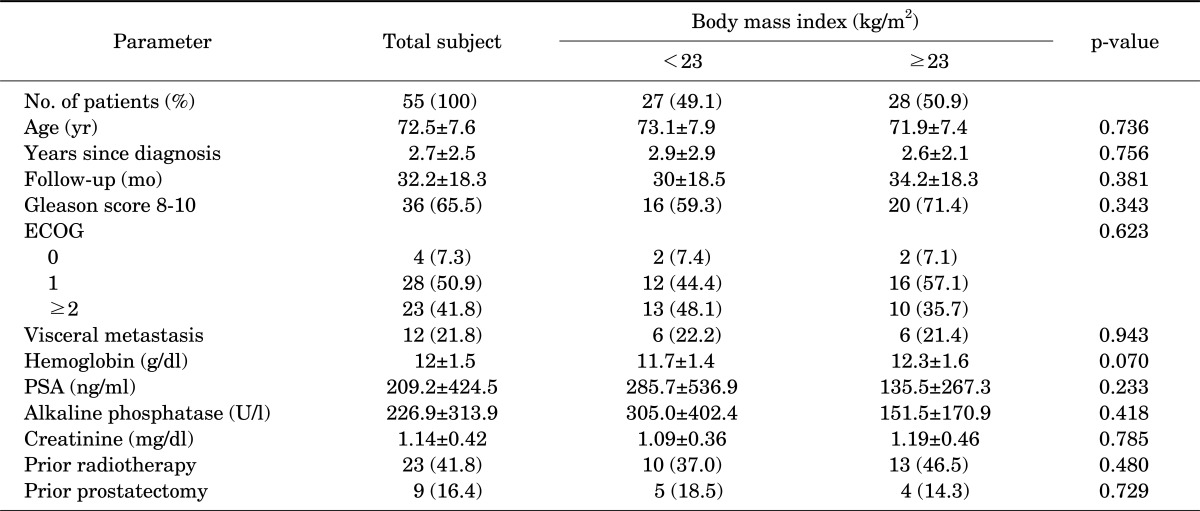
On the basis of BMI, 27 patients (49.1%) belonged to group I (BMI<23 kg/m2) and 28 patients (50.9%) belonged to group II (BMI≥23 kg/m2). Mean follow-up periods were 30 months and 34.2 months, respectively (p=0.381). When subjects were categorized according to BMI, those with a higher BMI had a higher Gleason score (71.4% vs. 59.3%, p=0.343) and lower PSA (285.7±536.9 vs. 135.5±267.3, p=0.233), but the differences were not significant. In addition, there was no significant difference between the two groups in terms of age, ECOG Performance Status, hemoglobin level, alkaline phosphatase level, metastasis to the internal organs, radiation treatments, or performance of radical prostatectomy (p>0.05) (Table 1).
TABLE 1.
Baseline clinical and laboratory variables by body mass index
Values are presented as number (%) or mean±SD.
ECOG, Eastern Cooperative Oncology Group Performance Status; PSA, prostate specific antigen.
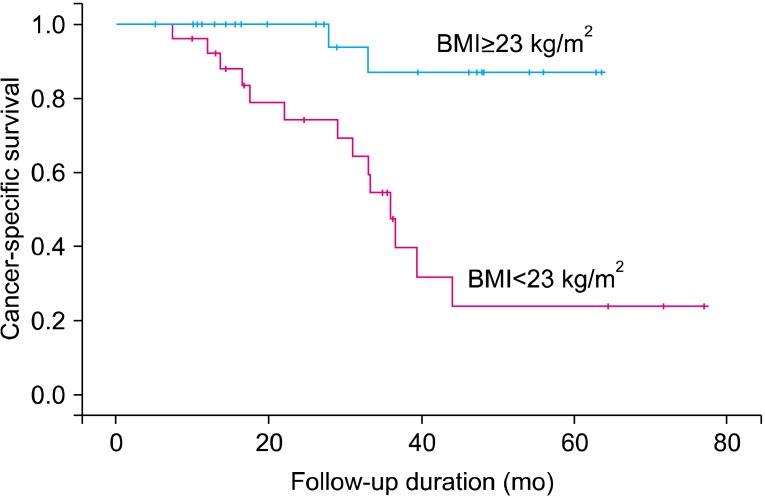
Of 55 patients, 16 patients had died at the time of last follow-up of the current analysis, and the median follow-up among surviving patients was 24.7 months (95% CI, 19.9 to 29.4 months). The median survival times were 17.8 months (95% CI, 12.3 to 23.2 months) for men with a normal BMI and 33.2 months (95% CI, 27.9 to 38.6 months) for overweight men. In the normal-weight group, 14 patients died of prostate cancer. In the overweight group, only 2 patients died of prostate cancer, thus showing a higher prostate cancer-specific death rate in the normal BMI group than in the overweight group (p=0.001) (Fig. 1).
FIG. 1.
Kaplan-Meier survival curves demonstrating patients' cancer-specific survival for castration-resistant prostate cancer on the basis of body mass index. BMI, body mass index.
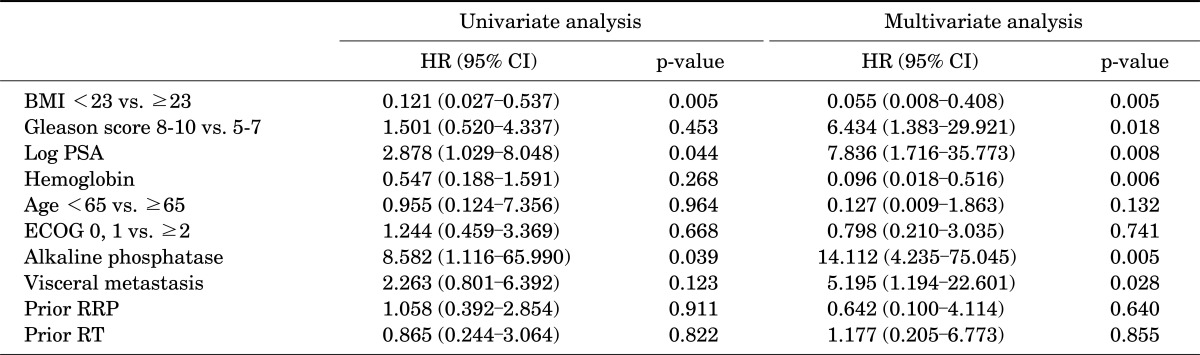
In the univariate analysis for predicting prostate cancer-specific survival rates, BMI (p=0.005; HR, 0.121), logPSA (p=0.044; HR, 2.878), and alkaline phosphatase level (p=0.039; HR, 8.582) were significant factors for prediction. In the multivariate analysis, BMI (p=0.005; HR, 0.55), logPSA (p=0.008; HR, 7.836), Gleason score (p=0.018; HR, 6.434), hemoglobin (p=0.006; HR, 0.096), alkaline phosphatase level (p=0.005; HR, 114.1), and metastasis to the internal organs (p=0.028; HR, 5.195) were significant factors for prediction (Table 2).
TABLE 2.
Univariate and multivariate analyses of factors influencing cancer-specificsurvival in patients with castration-resistant prostate cancer
HR, hazard ratio; CI, confidence interval; BMI, body mass index; PSA, prostate specific antigen; ECOG, Eastern Cooperative Oncology Group Performance Status; RRP, retropublicradical prostatectomy; RT, radiation therapy.
DISCUSSION
According to the medical check-up data in 2008 from the National Health Insurance Corporation, 3.24 million persons had a BMI>25 kg/m2, comprising 32.8% of a total of 9.88 million people who received the exam [9]. High BMI is a problem not only of Korea, which is experiencing rapid industrialization, but also of most industrialized countries, including Europe and America. In the United States (US), about 48 to 66 billion US dollars are invested annually in fighting obesity [10]. The severity of obesity is its association with various systemic diseases, such as hypertension, diabetes, circulatory diseases, and eye diseases, as reported in many studies [11-13].
In this study, high BMI was observed to positively affect the patients with CRPC who received docetaxel treatment. In the multivariate analysis, improvement in the cancer-specific survival rate was observed in the group of patients with BMI≥23 kg/m2. In addition, logPSA, Gleason score, hemoglobin level, alkaline phosphatase level, and metastasis to internal organs were reported as significant prognostic factors.
The above results contradict those of a previous study that reported an adverse clinical effect of obesity on localized prostate cancer. Magheli et al. [14] reported worse pathological results of obese patients compared with normal-weight patients in a study of 1,877 patients who underwent radical prostatectomy. Irani et al. [15] reported that obesity not only raised the onset of prostate cancer with an odds ratio of 2.47 but significantly increased the prevalence rate in the resection area in the case of radical prostatectomy. According to Rodriguez et al. [2], obese patients have a high risk of prostate cancer death. Adams et al. [16] reported a linear relationship between BMI increase and the prostate cancer-specific death rate. According to the study conducted by Freedland et al. [17] using Shared Equal Access Regional Cancer Hospital data targeting a total of 1,106 patients, obesity raised biochemical recurrence rates after radical prostatectomy. These studies show contradictory results with those of the present study. However, most of the preceding studies targeted patients with localized prostate cancer. According to the study conducted by Halabi et al. [18] of a total of 1,226 patients with CRPC, the overall survival rate and cancer-specific survival rate of overweight and obese patients were higher than those of the normal-weight patients, which agrees with the results of the present study. Moreover, a similar correlation was reported between obesity and biochemical failure in 939 patients treated with external beam radiotherapy [19]. On the basis of this study, we examined the correlation between obesity and the survival rate of patients with CRPC who received docetaxel treatment.
Many studies have been reported on the correlation between BMI and prostate cancer in Korean men. Lee et al. [20] reported that a higher BMI was significantly associated with extracapsular extension of tumors. However, most studies have focused on the effects of BMI on the prognosis of localized prostate cancer. To our knowledge, there have been no studies of the relation between BMI and CRPC in Korean men. This is a strength of the current study relative to the other reported studies.
In contrast with previously published studies on men with an earlier stage of disease, our results revealed a protective effect of an elevated BMI in patients with CRPC. A hypothesis that thin people are vulnerable to cancer cachexia, thus resulting in a poor prognosis, has been suggested. Cancer cachexia is considered a poor response to rapid progression and treatment of cancer [18]. In the present study, normal-weight patients showed lower hemoglobin levels and ECOG performance status and higher PSA levels than did overweight patients, even though the difference was not significant. In comparison, high-BMI patients are considered to accumulate more protein and calories than thin patients and, accordingly, are more efficient in enduring the cancer-cachetic-producing effect. Some studies have suggested that energy restriction appears to decrease cellular proliferation by ceasing progression through the cell cycle and enhancing apoptosis of cancer cells [21].
In previous studies, various hypotheses have been presented to explain the correlation between prostate cancer and obesity [22-24]. First, obesity, especially abdominal obesity, is associated with insulin resistance and hyperinsulinemia, which contributes to a high serum insulin level and insulin-like growth factor-I level facilitating the development of cancer. Moreover, obesity-associated high leptin levels adversely affect the survival of prostate cancer patients. Leptin is secreted from adipose cells and promotes angiogenesis. Recently, adiponectin, a major adipose cytokine that decreases in circulation in obesity and ameliorates obesity, was identified as an inhibitor of prostate cancer cell growth [25].
The results of the present study showed that the survival rate was significantly high in the CRPC group having a BMI≥23 kg/m2. As a retrospective study, a limitation of the present study is the sample size of 55, which is small compared with other studies. Despite this limitation, the present study is the first such study in this field in Korea. In the future, prospective and large-scale multi-institutional studies on the pathology of the correlation are expected to be conducted.
CONCLUSIONS
The findings from the current study suggest that BMI as well as other prognostic factors are independent prognostic factors in patients with CRPC who receive docetaxel treatment. With higher BMI, the cancer-specific survival rate was observed to improve more, in contrast with the correlation in earlier stages of prostate cancer. In addition, logPSA, Gleason score, hemoglobin level, alkaline phosphatase level, and metastasis to internal organs were significant prognostic factors. A large-scale, prospective study will be needed to elucidate the exact association between BMI and CRPC. Institutional Review Board (IRB) approval number: B-1207-164-114.
Footnotes
The authors have nothing to disclose.
References
- 1.Oh SW. Obesity and metabolic syndrome in Korea. Diabetes Metab J. 2011;35:561–566. doi: 10.4093/dmj.2011.35.6.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rodriguez C, Patel AV, Calle EE, Jacobs EJ, Chao A, Thun MJ. Body mass index, height, and prostate cancer mortality in two large cohorts of adult men in the United States. Cancer Epidemiol Biomarkers Prev. 2001;10:345–353. [PubMed] [Google Scholar]
- 3.Chi BH, Chang IH. Prostate cancer: recent trends in Korea. Urol Int. 2010;85:88–93. doi: 10.1159/000318677. [DOI] [PubMed] [Google Scholar]
- 4.Giovannucci E, Rimm EB, Liu Y, Leitzmann M, Wu K, Stampfer MJ, et al. Body mass index and risk of prostate cancer in U.S. health professionals. J Natl Cancer Inst. 2003;95:1240–1244. doi: 10.1093/jnci/djg009. [DOI] [PubMed] [Google Scholar]
- 5.Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348:1625–1638. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- 6.Snowdon DA, Phillips RL, Choi W. Diet, obesity, and risk of fatal prostate cancer. Am J Epidemiol. 1984;120:244–250. doi: 10.1093/oxfordjournals.aje.a113886. [DOI] [PubMed] [Google Scholar]
- 7.Amling CL, Blute ML, Lerner SE, Bergstralh EJ, Bostwick DG, Zincke H. Influence of prostate-specific antigen testing on the spectrum of patients with prostate cancer undergoing radical prostatectomy at a large referral practice. Mayo Clin Proc. 1998;73:401–406. doi: 10.1016/S0025-6196(11)63720-8. [DOI] [PubMed] [Google Scholar]
- 8.Mottet N, Bellmunt J, Bolla M, Joniau S, Mason M, Matveev V, et al. EAU guidelines on prostate cancer. Part II: Treatment of advanced, relapsing, and castration-resistant prostate cancer. Eur Urol. 2011;59:572–583. doi: 10.1016/j.eururo.2011.01.025. [DOI] [PubMed] [Google Scholar]
- 9.Jee SH, Yun JE, Park EJ, Cho ER, Park IS, Sull JW, et al. Body mass index and cancer risk in Korean men and women. Int J Cancer. 2008;123:1892–1896. doi: 10.1002/ijc.23719. [DOI] [PubMed] [Google Scholar]
- 10.Wang YC, McPherson K, Marsh T, Gortmaker SL, Brown M. Health and economic burden of the projected obesity trends in the USA and the UK. Lancet. 2011;378:815–825. doi: 10.1016/S0140-6736(11)60814-3. [DOI] [PubMed] [Google Scholar]
- 11.Lopez-Jimenez F, Cortes-Bergoderi M. Update: systemic diseases and the cardiovascular system (i): obesity and the heart. Rev Esp Cardiol. 2011;64:140–149. doi: 10.1016/j.recesp.2010.10.010. [DOI] [PubMed] [Google Scholar]
- 12.Cosin Aguilar J, Hernandiz Martinez A, Masramon Morell X, Aristegui Urrestarazu R, Aguilar Llopis A, Zamorano Gomez JL, et al. Overweight and obesity in hypertensive Spanish patients. The CORONARIA study. Med Clin (Barc) 2007;129:641–645. doi: 10.1157/13112092. [DOI] [PubMed] [Google Scholar]
- 13.Zalesin KC, Franklin BA, Miller WM, Peterson ED, McCullough PA. Impact of obesity on cardiovascular disease. Endocrinol Metab Clin North Am. 2008;37:663–684. doi: 10.1016/j.ecl.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 14.Magheli A, Rais-Bahrami S, Trock BJ, Humphreys EB, Partin AW, Han M, et al. Impact of body mass index on biochemical recurrence rates after radical prostatectomy: an analysis utilizing propensity score matching. Urology. 2008;72:1246–1251. doi: 10.1016/j.urology.2008.01.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Irani J, Lefebvre O, Murat F, Dahmani L, Dore B. Obesity in relation to prostate cancer risk: comparison with a population having benign prostatic hyperplasia. BJU Int. 2003;91:482–484. doi: 10.1046/j.1464-410x.2003.04133.x. [DOI] [PubMed] [Google Scholar]
- 16.Adams KF, Schatzkin A, Harris TB, Kipnis V, Mouw T, Ballard-Barbash R, et al. Overweight, obesity, and mortality in a large prospective cohort of persons 50 to 71 years old. N Engl J Med. 2006;355:763–778. doi: 10.1056/NEJMoa055643. [DOI] [PubMed] [Google Scholar]
- 17.Freedland SJ, Aronson WJ, Kane CJ, Presti JC, Jr, Amling CL, Elashoff D, et al. Impact of obesity on biochemical control after radical prostatectomy for clinically localized prostate cancer: a report by the Shared Equal Access Regional Cancer Hospital database study group. J Clin Oncol. 2004;22:446–453. doi: 10.1200/JCO.2004.04.181. [DOI] [PubMed] [Google Scholar]
- 18.Halabi S, Ou SS, Vogelzang NJ, Small EJ. Inverse correlation between body mass index and clinical outcomes in men with advanced castration-recurrent prostate cancer. Cancer. 2007;110:1478–1484. doi: 10.1002/cncr.22932. [DOI] [PubMed] [Google Scholar]
- 19.Strom SS, Kamat AM, Gruschkus SK, Gu Y, Wen S, Cheung MR, et al. Influence of obesity on biochemical and clinical failure after external-beam radiotherapy for localized prostate cancer. Cancer. 2006;107:631–639. doi: 10.1002/cncr.22025. [DOI] [PubMed] [Google Scholar]
- 20.Lee SE, Lee WK, Jeong MS, Abdullajanov M, Kim DS, Park HZ, et al. Is body mass index associated with pathological outcomes after radical prostatectomy in Korean men? BJU Int. 2011;107:1250–1255. doi: 10.1111/j.1464-410X.2010.09592.x. [DOI] [PubMed] [Google Scholar]
- 21.Platz EA, Leitzmann MF, Michaud DS, Willett WC, Giovannucci E. Interrelation of energy intake, body size, and physical activity with prostate cancer in a large prospective cohort study. Cancer Res. 2003;63:8542–8548. [PubMed] [Google Scholar]
- 22.Freedland SJ, Aronson WJ. Obesity and prostate cancer. Urology. 2005;65:433–439. doi: 10.1016/j.urology.2004.08.035. [DOI] [PubMed] [Google Scholar]
- 23.Frystyk J, Vestbo E, Skjaerbaek C, Mogensen CE, Orskov H. Free insulin-like growth factors in human obesity. Metabolism. 1995;44(10 Suppl 4):37–44. doi: 10.1016/0026-0495(95)90219-8. [DOI] [PubMed] [Google Scholar]
- 24.Chan JM, Stampfer MJ, Giovannucci E, Gann PH, Ma J, Wilkinson P, et al. Plasma insulin-like growth factor-I and prostate cancer risk: a prospective study. Science. 1998;279:563–566. doi: 10.1126/science.279.5350.563. [DOI] [PubMed] [Google Scholar]
- 25.Bub JD, Miyazaki T, Iwamoto Y. Adiponectin as a growth inhibitor in prostate cancer cells. Biochem Biophys Res Commun. 2006;340:1158–1166. doi: 10.1016/j.bbrc.2005.12.103. [DOI] [PubMed] [Google Scholar]