Abstract
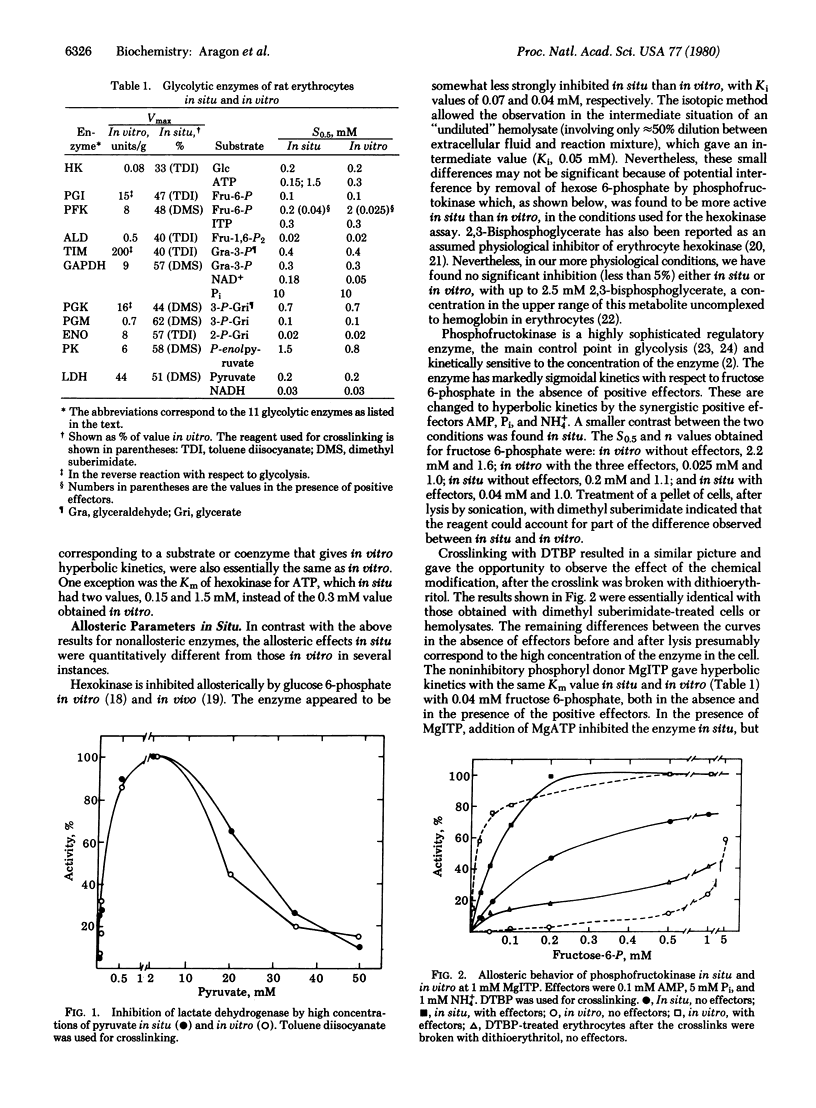
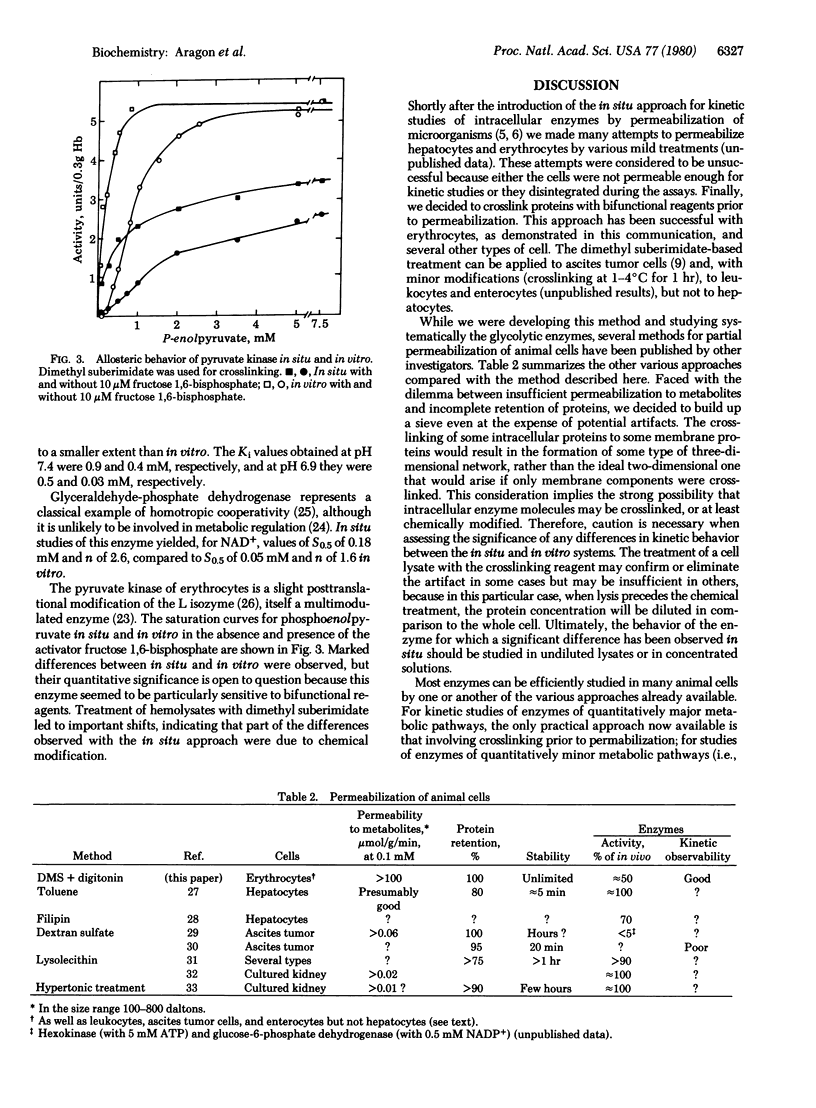
Intracellular enzymes in erythrocytes can be made accessible for in situ kinetic studies by treating the cells with bifunctional reagents to crosslink proteins, thus creating a network that allows subsequent permeabilization by delipidation without escape of intracellular proteins. Dimethyl suberimidate, dimethyl 3,3'-dithiobispropionimidate, and toluene-2,4-diisocyanate have been used successfully as crosslinking reagents, and digitonin has been used for delipidation. In a systematic study of the in situ behavior of the 11 glycolytic enzymes of rat erythrocytes, it was observed that Km and Vmax values for the majority of the enzymes are essentially the same in situ as in vitro. Lactate dehydrogenase (L-lactate:NAD+ oxidoreductase, EC 1.1.1.27) is inhibited by excess of pyruvate as much in situ as in vitro. Hexokinase (ATP:D-hexose 6-phosphotransferase, EC 2.7.1.1) was allosterically inhibited by glucose 6-phosphate nearly as much in situ as in vitro but was not affected by 2,3-biphosphoglycerate. The allosteric properties of 6-phosphofructokinase (ATP:D-fructose 6-phosphate 1-phosphotransferase, EC 2.7.1.11), glyceraldehyde-phosphate dehydrogenase [D-glyceraldehyde-3-phosphate:NAD+ oxidoreductase (phosphorylating), EC 1.2.1.12], and pyruvate kinase (ATP: pyruvate 2-O-phosphotransferase, EC 2.7.1.40) in situ were qualitatively similar to those observed in vitro, but some important quantitative differences were noticed. Particularly striking was the much greater activity of phosphofructokinase in situ compared to that in vitro at physiological concentrations of effector metabolites.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aragón J. J., Sols A. Glyceraldehyde-3-phosphate dehydrogenase activity studied under physiological conditions with a linear assay. Biochem Biophys Res Commun. 1978 Jun 29;82(4):1098–1103. doi: 10.1016/0006-291x(78)90300-5. [DOI] [PubMed] [Google Scholar]
- Bañuelos M., Gancedo C. In situ study of the glycolytic pathway in Saccharomyces cerevisiae. Arch Microbiol. 1978 May 30;117(2):197–201. doi: 10.1007/BF00402308. [DOI] [PubMed] [Google Scholar]
- Blume K. G., Beutler E. Galactokinase from human erythrocytes. Methods Enzymol. 1975;42:47–53. doi: 10.1016/0076-6879(75)42091-2. [DOI] [PubMed] [Google Scholar]
- Bock P. E., Frieden C. Phosphofructokinase. I. Mechanism of the pH-dependent inactivation and reactivation of the rabbit muscle enzyme. J Biol Chem. 1976 Sep 25;251(18):5630–5636. [PubMed] [Google Scholar]
- CRANE R. K., SOLS A. The non-competitive inhibition of brain hexokinase by glucose-6-phosphate and related compounds. J Biol Chem. 1954 Oct;210(2):597–606. [PubMed] [Google Scholar]
- Cahn R. D., Zwilling E., Kaplan N. O., Levine L. Nature and Development of Lactic Dehydrogenases: The two major types of this enzyme form molecular hybrids which change in makeup during development. Science. 1962 Jun 15;136(3520):962–969. doi: 10.1126/science.136.3520.962. [DOI] [PubMed] [Google Scholar]
- Castellot J. J., Jr, Miller M. R., Lehtomaki D. M., Pardee A. B. Comparison of DNA replication and repair enzymology using permeabilized baby hamster kidney cells. J Biol Chem. 1979 Aug 10;254(15):6904–6908. [PubMed] [Google Scholar]
- Castellot J. J., Jr, Miller M. R., Pardee A. B. Animal cells reversibly permeable to small molecules. Proc Natl Acad Sci U S A. 1978 Jan;75(1):351–355. doi: 10.1073/pnas.75.1.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J. J., Jones M. E. Effect of 5-phosphoribosyl-a-pyrophosphate on de novo pyrimidine biosynthesis in cultured Ehrlich ascites cells made permeable with dextran sulfate 500. J Biol Chem. 1979 Apr 25;254(8):2697–2704. [PubMed] [Google Scholar]
- Chiba H., Sasaki R. Functions, of 2,3-bisphosphoglycerate and its metabolism. Curr Top Cell Regul. 1978;14:75–116. doi: 10.1016/b978-0-12-152814-0.50007-1. [DOI] [PubMed] [Google Scholar]
- Conway A., Koshland D. E., Jr Negative cooperativity in enzyme action. The binding of diphosphopyridine nucleotide to glyceraldehyde 3-phosphate dehydrogenase. Biochemistry. 1968 Nov;7(11):4011–4023. doi: 10.1021/bi00851a031. [DOI] [PubMed] [Google Scholar]
- Gupta R. K., Benovic J. L., Rose Z. B. The determination of the free magnesium level in the human red blood cell by 31P NMR. J Biol Chem. 1978 Sep 10;253(17):6172–6176. [PubMed] [Google Scholar]
- Hilderman R. H., Goldblatt P. J., Deutscher M. P. Preparation and characterization of liver cells made permeable to macromolecules by treatment with toluene. J Biol Chem. 1975 Jun 25;250(12):4796–4801. [PubMed] [Google Scholar]
- Hofer H. W., Krystek E. Determination of the kinetic parameters of phosphofructokinase dissociation. FEBS Lett. 1975 May 1;53(2):217–220. doi: 10.1016/0014-5793(75)80023-8. [DOI] [PubMed] [Google Scholar]
- Hulme E. C., Tipton K. F. The dependence of phosphofructokinase kinetics upon protein concentration. FEBS Lett. 1971 Jan 25;12(4):197–200. doi: 10.1016/0014-5793(71)80019-4. [DOI] [PubMed] [Google Scholar]
- Karadsheh N. S., Uyeda K., Oliver R. M. Studies on structure of human erythrocyte phosphofructokinase. J Biol Chem. 1977 May 25;252(10):3515–3524. [PubMed] [Google Scholar]
- Kasahara M. A permeability change in Ehrlich ascites tumor cells caused by dextran sulfate and its repair by ascites fluid or Ca2+ ions. Arch Biochem Biophys. 1977 Dec;184(2):400–407. doi: 10.1016/0003-9861(77)90367-8. [DOI] [PubMed] [Google Scholar]
- Kitajima M., Sekiguchi W., Kondo A. A modification of red blood cells by isocyanates. Bull Chem Soc Jpn. 1971 Jan;44(1):139–143. doi: 10.1246/bcsj.44.139. [DOI] [PubMed] [Google Scholar]
- Lad P. M., Hammes G. G. Physical and chemical properties of rabbit muscle phosphofructokinase cross-linked with dimethyl suberimidate. Biochemistry. 1974 Oct 22;13(22):4530–4537. doi: 10.1021/bi00719a009. [DOI] [PubMed] [Google Scholar]
- Marie J., Garreau H., Kahn A. Evidence for a postsynthetic proteolytic transformation of human erythrocyte pyruvate kinase into L-type enzyme. FEBS Lett. 1977;78(1):91–94. doi: 10.1016/0014-5793(77)80280-9. [DOI] [PubMed] [Google Scholar]
- Miller M. R., Castellot J. J., Jr, Pardee A. B. A general method for permeabilizing monolayer and suspension cultured animal cells. Exp Cell Res. 1979 May;120(2):421–425. doi: 10.1016/0014-4827(79)90404-x. [DOI] [PubMed] [Google Scholar]
- Otto M., Heinrich R., Jacobasch G., Rapoport S. A mathematical model for the influence of anionic effectors on the phosphofructokinase from rat erythrocytes. Eur J Biochem. 1977 Apr 1;74(2):413–420. doi: 10.1111/j.1432-1033.1977.tb11406.x. [DOI] [PubMed] [Google Scholar]
- ROSE I. A., O'CONNELL E. L. THE ROLE OF GLUCOSE 6-PHOSPHATE IN THE REGULATION OF GLUCOSE METABOLISM IN HUMAN ERYTHROCYTES. J Biol Chem. 1964 Jan;239:12–17. [PubMed] [Google Scholar]
- Rapoport T. A., Heinrich R., Rapoport S. M. The regulatory principles of glycolysis in erythrocytes in vivo and in vitro. A minimal comprehensive model describing steady states, quasi-steady states and time-dependent processes. Biochem J. 1976 Feb 15;154(2):449–469. doi: 10.1042/bj1540449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves R. E., Sols A. Regulation of Escherichia coli phosphofructokinase in situ. Biochem Biophys Res Commun. 1973 Jan 23;50(2):459–466. doi: 10.1016/0006-291x(73)90862-0. [DOI] [PubMed] [Google Scholar]
- Srere P. A., Matsuoka Y., Mukherjee A. Inhibition studies of rat citrate synthase. J Biol Chem. 1973 Dec 10;248(23):8031–8035. [PubMed] [Google Scholar]
- Wang K., Richards F. M. Reaction of dimethyl-3,3'-dithiobispropionimidate with intact human erythrocytes. Cross-linking of membrane proteins and hemoglobin. J Biol Chem. 1975 Aug 25;250(16):6622–6626. [PubMed] [Google Scholar]
- Wenzel K. W., Kurganov B. I., Zimmermann G., Yakovlev V. A., Schellenberger W., Hofmann E. Self-association of human erythrocyte phosphofructokinase. Kinetic behaviour in dependence on enzyme concentration and mode of association. Eur J Biochem. 1976 Jan 2;61(1):181–190. doi: 10.1111/j.1432-1033.1976.tb10010.x. [DOI] [PubMed] [Google Scholar]
- Wuntch T., Chen R. F., Vesell E. S. Lactate dehydrogenase isozymes: further kinetic studies at high enzyme concentration. Science. 1970 Jul 31;169(3944):480–481. doi: 10.1126/science.169.3944.480. [DOI] [PubMed] [Google Scholar]



