Abstract
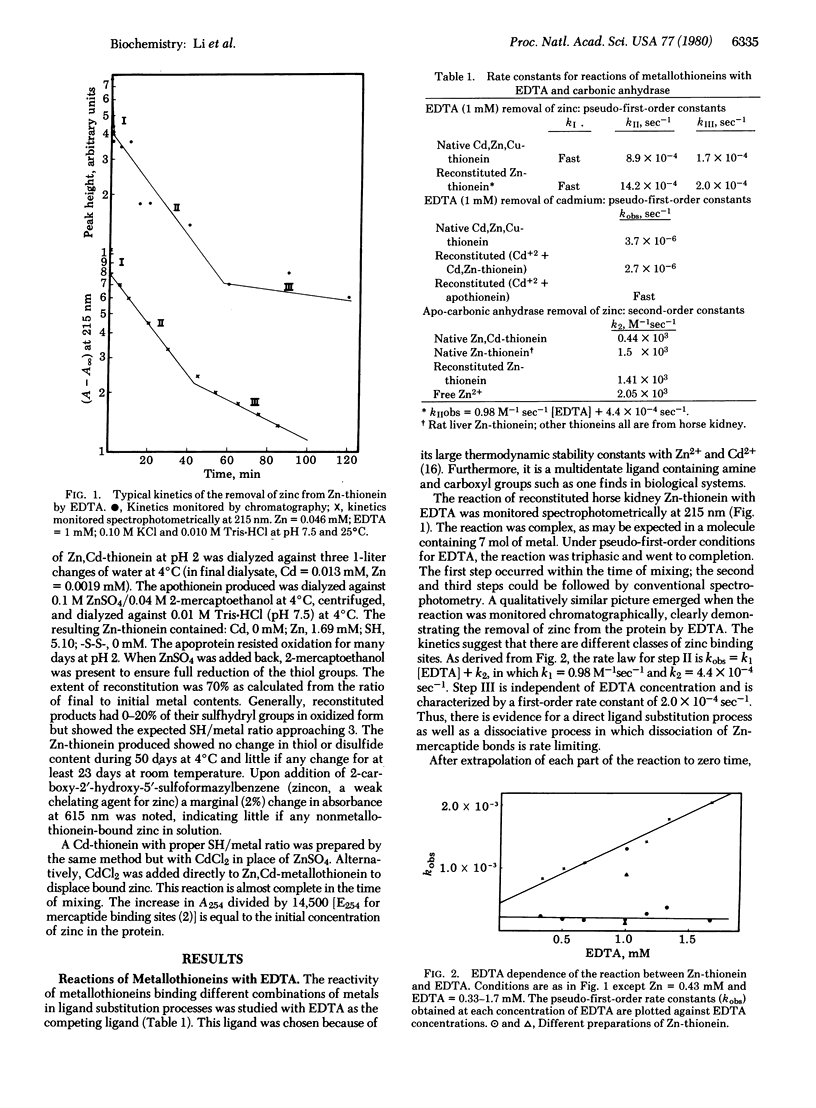
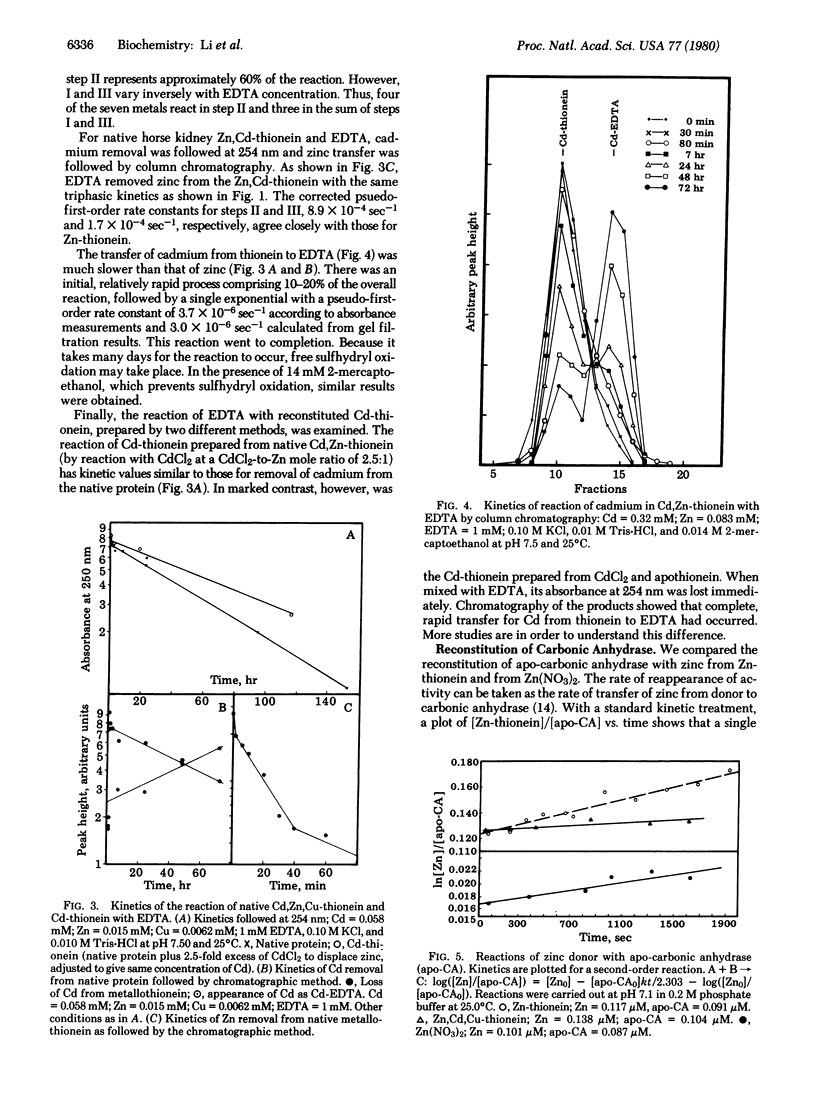
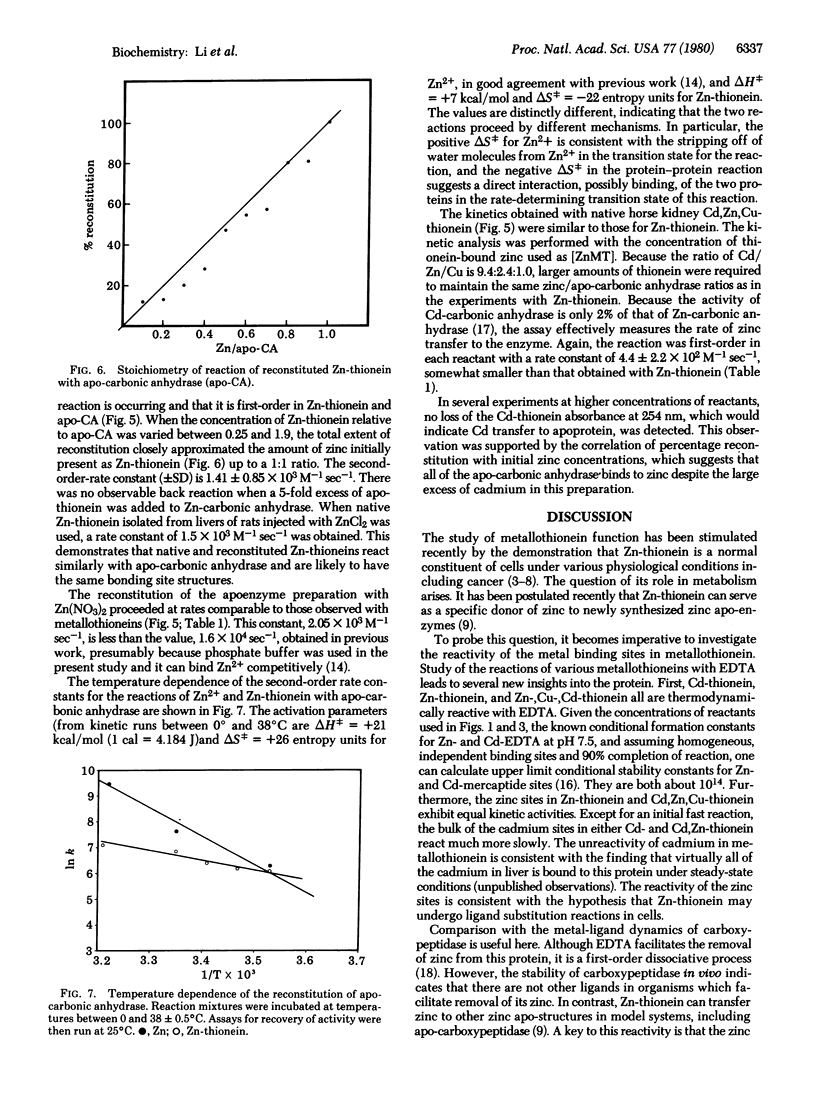
The reactions of Zn-, Zn,Cd-, and Cd-thioneins with EDTA and apo-carbonic anhydrase have been studied. The ligand substitution reaction of zinc with EDTA is multiphasic, having both associative and dissociative components in the rate expression. The cadmium sites are about 2 orders of magnitude less reactive. In contrast, apo-carbonic anhydrase abstracts zinc from Zn-thionein and Zn,Cd-thionein in second-order processes that are 2-3 orders of magnitude more rapid than those involving EDTA and approach the rate for unligated Zn2+ with the apo-protein. In comparison with other zinc proteins, Zn-thionein contains unusually reactive metal sites, suggesting that this protein may be a physiological zinc transfer protein, able to donate zinc to zinc-requiring apo macromolecules.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Billo E. J., Brito K. K., Wilkins R. G. Kinetics of formation and dissociation of metallocarboxypeptidases. Bioinorg Chem. 1978 Jun;8(6):461–475. doi: 10.1016/0006-3061(78)80001-5. [DOI] [PubMed] [Google Scholar]
- COLEMAN J. E., VALLEE B. L. Metallocarboxypeptidases. J Biol Chem. 1960 Feb;235:390–395. [PubMed] [Google Scholar]
- COLEMAN J. E., VALLEE B. L. Metallocarboxypeptidases: stability constants and enzymatic characteristics. J Biol Chem. 1961 Aug;236:2244–2249. [PubMed] [Google Scholar]
- Coleman J. E. Human carbonic anhydrase. Protein conformation and metal ion binding. Biochemistry. 1965 Dec;4(12):2644–2655. doi: 10.1021/bi00888a014. [DOI] [PubMed] [Google Scholar]
- ELLMAN G. L. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959 May;82(1):70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- Hidalgo H. A., Koppa V., Bryan S. E. Induction of cadmium-thionein in mouse tumor cells. Toxicol Appl Pharmacol. 1978 Aug;45(2):521–530. doi: 10.1016/0041-008x(78)90114-x. [DOI] [PubMed] [Google Scholar]
- Hunt J. B., Rhee M. J., Storm C. B. A rapid and convenient preparation of apocarbonic anhydrase. Anal Biochem. 1977 May 1;79(1-2):614–617. doi: 10.1016/0003-2697(77)90444-4. [DOI] [PubMed] [Google Scholar]
- KAGI J. H., VALLEE B. L. Metallothionein: a cadmium and zinc-containign protein from equine renal cortex. II. Physico-chemical properties. J Biol Chem. 1961 Sep;236:2435–2442. [PubMed] [Google Scholar]
- Koch J., Wielgus S., Shankara B., Saryan L. A., Shaw C. F., Petering D. H. Zinc-, copper- and cadmium-binding protein in Ehrlich ascites tumour cells. Biochem J. 1980 Jul 1;189(1):95–104. doi: 10.1042/bj1890095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kägi J. H., Himmelhoch S. R., Whanger P. D., Bethune J. L., Vallee B. L. Equine hepatic and renal metallothioneins. Purification, molecular weight, amino acid composition, and metal content. J Biol Chem. 1974 Jun 10;249(11):3537–3542. [PubMed] [Google Scholar]
- Li T. Y., Chen J. F., Watters K. L., McFarland J. T. Identification of enzyme coupling sites with aromatic diazonium salts-a resonance raman study. Arch Biochem Biophys. 1979 Oct 15;197(2):477–486. doi: 10.1016/0003-9861(79)90270-4. [DOI] [PubMed] [Google Scholar]
- Oh S. H., Deagen J. T., Whanger P. D., Weswig P. H. Biological function of metallothionein. V. Its induction in rats by various stresses. Am J Physiol. 1978 Mar;234(3):E282–E285. doi: 10.1152/ajpendo.1978.234.3.E282. [DOI] [PubMed] [Google Scholar]
- Ohtake H., Hasegawa K., Koga M. Zinc-binding protein in the livers of neonatal, normal and partially hepatectomized rats. Biochem J. 1978 Sep 15;174(3):999–1005. doi: 10.1042/bj1740999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petering D. H., Palmer G. Properties of spinach ferredoxin in anaerobic urea solution: a comparison with the native protein. Arch Biochem Biophys. 1970 Dec;141(2):456–464. doi: 10.1016/0003-9861(70)90162-1. [DOI] [PubMed] [Google Scholar]
- Pocker Y., Stone J. T. The catalytic versatility of erythrocyte carbonic anhydrase. 3. Kinetic studies of the enzyme-catalyzed hydrolysis of p-nitrophenyl acetate. Biochemistry. 1967 Mar;6(3):668–678. doi: 10.1021/bi00855a005. [DOI] [PubMed] [Google Scholar]
- Richards M. P., Cousins R. J. Metallothionein and its relationship to the metabolism of dietary zinc in rats. J Nutr. 1976 Nov;106(11):1591–1599. doi: 10.1093/jn/106.11.1591. [DOI] [PubMed] [Google Scholar]
- Romans A. Y., Graichen M. E., Lochmüller C. H., Henkens R. W. Kinetics and mechanism of dissociation of zinc ion from carbonic anhydrase. Bioinorg Chem. 1978 Sep;9(3):217–229. doi: 10.1016/s0006-3061(78)80007-6. [DOI] [PubMed] [Google Scholar]
- Sobocinski P. Z., Canterbury W. J., Jr, Mapes C. A., Dinterman R. E. Involvement of hepatic metallothioneins in hypozincemia associated with bacterial infection. Am J Physiol. 1978 Apr;234(4):E399–E406. doi: 10.1152/ajpendo.1978.234.4.E399. [DOI] [PubMed] [Google Scholar]
- Udom A. O., Brady F. O. Reactivation in vitro of zinc-requiring apo-enzymes by rat liver zinc-thionein. Biochem J. 1980 May 1;187(2):329–335. doi: 10.1042/bj1870329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yazgan A., Henkens R. W. Role of zinc (II) in the refolding of guanidine hydrochloride denatured bovine carbonic anhydrase. Biochemistry. 1972 Mar 28;11(7):1314–1318. doi: 10.1021/bi00757a031. [DOI] [PubMed] [Google Scholar]


