Abstract
In the cyanogenic crop cassava (Manihot esculenta, Crantz), the final step in cyanide production is the conversion of acetone cyanohydrin, the deglycosylation product of linamarin, to cyanide plus acetone. This process occurs spontaneously at pH greater than 5.0 or enzymatically and is catalyzed by hydroxynitrile lyase (HNL). Recently, it has been demonstrated that acetone cyanohydrin is present in poorly processed cassava root food products. Since it has generally been assumed that HNL is present in all cassava tissues, we reinvestigated the enzymatic properties and tissue-specific distribution of HNL in cassava. We report the development of a rapid two-step purification protocol for cassava HNL, which yields an enzyme that is catalytically more efficient than previously reported (Hughes, J., Carvalho, F., and Hughes, M. [1994] Arch Biochem Biophys 311: 496–502). Analyses of the distribution of HNL activity and protein indicate that the accumulation of acetone cyanohydrin in roots is due to the absence of HNL, not to inhibition of the enzyme. Furthermore, the absence of HNL in roots and stems is associated with very low steady-state HNL transcript levels. It is proposed that the lack of HNL in cassava roots accounts for the high acetone cyanohydrin levels in poorly processed cassava food products.
The cyanogenic glycosides are a group of nitrile-containing plant secondary compounds that yield cyanide (cyanogenesis) following their enzymatic breakdown. The functions of cyanogenic glycosides remain to be determined in many plants; however, in some plants they have been implicated as herbivore deterrents and as transportable forms of reduced nitrogen (Belloti and Arias, 1993; Selmar, 1993; McMahon et al., 1995). It is estimated that between 3,000 and 12,000 plant species produce and sequester cyanogenic glycosides, including many important crop species (Kakes, 1990; Poulton, 1990) such as sorghum, almonds, lima beans (nondomesticated), and white clover. The most agronomically important of the cyanogenic crops, however, is the tropical root crop cassava (Manihot esculenta, Crantz). More than 153 million tons of cassava are produced annually, and it is the major source of calories for many people living in the tropics, particularly sub-Saharan Africa (Cock, 1985).
All cassava tissues, with the exception of seeds, contain the cyanogenic glycosides linamarin (>90% total cyanogen) and lotaustralin (<10% total cyanogen; for review, see McMahon et al., 1995). Leaves have the highest cyanogenic glycoside levels (5.0 g linamarin/kg fresh weight), whereas roots have approximately 20-fold lower linamarin levels. In addition to tissue-specific differences, there are cultivar-dependent differences in root cyanogen levels. Total root linamarin levels range between 100 and 500 mg linamarin/kg fresh weight for low- and high-cyanogenic cultivars, respectively. No cassava cultivars, however, lack cyanogenic glycosides.
Cyanogenesis is initiated in cassava when the plant tissue is damaged. Rupture of the vacuole releases linamarin, which is hydrolyzed by linamarase, a cell wall-associated β-glycosidase (McMahon et al., 1995). Hydrolysis of linamarin yields an unstable hydroxynitrile intermediate, acetone cyanohydrin, plus Glc. Acetone cyanohydrin spontaneously decomposes to acetone and HCN at pH >5.0 or temperatures >35°C and can be broken down enzymatically by HNL (Cutler and Conn, 1981; Yemm and Poulton, 1986; Wajant and Mundry, 1993; Wajant et al., 1994; White et al., 1994; White and Sayre, 1995; Zheng and Poulton, 1995; Hasslacher et al., 1996; Wajant and Pfizenmaier, 1996).
Various health disorders are associated with the consumption of cassava, which contains residual cyanogens. These disorders include hyperthyroidism, tropical ataxic neuropathy, and konzo (Osuntokun, 1981; Cock, 1985; Tylleskar et al., 1992; Rosling et al., 1993). Cyanide poisoning from high-cyanogenic cassava is typically associated with insufficient consumption of Cys and Met in the diet. Reduced sulfur-containing compounds are substrates for the detoxification of cyanide catalyzed by the enzymes rhodanese and/or β-cyanoalanine synthase (Castric et al., 1972; Kakes, 1990; Nambisan, 1993). Until recently, it had been assumed that all of the residual cyanogen present in cassava foods was in the form of linamarin. This assumption was based on the observation that acetone cyanohydrin is unstable and that the cyanide generated from acetone cyanohydrin is readily volatilized during food processing. Recently, however, it was demonstrated that the major cyanogen present in some poorly processed cassava roots was acetone cyanohydrin, not linamarin (Tylleskar et al., 1992). These results suggested that the spontaneous (high pH and/or temperature) and enzymatic breakdown of acetone cyanohydrin was reduced or inhibited in roots. In part, the high, residual acetone cyanohydrin levels could be attributed to the low-pH conditions established during the soaking (fermentation) of roots for food preparation. This hypothesis, however, does not address the contribution of HNL activity to root acetone cyanohydrin turnover and root cyanogenesis. To characterize the role of HNL in root cyanogenesis, we have determined the abundance, distribution, and kinetic properties of HNL in different cassava tissues. Our results indicate that differences in organ-specific patterns of HNL expression, and not inhibition of HNL activity, account for the absence of HNL activity in cassava roots.
MATERIALS AND METHODS
Cassava (Manihot esculenta, Crantz) was provided by the International Center for Tropical Agriculture (Cali, Colombia). Plants were maintained in growth chambers at 28°C with 12 h of light and dark. Sterile plants were grown on propagation medium for cassava containing Murashige-Skoog salts (Murashige and Skoog, 1962) or in liquid medium using the LIFERAFT support system (GIBCO-BRL) (Roca, 1984; Lin et al., 1995).
Protein Purification and HNL Enzyme Assays
Cassava leaves were homogenized for 1 min in a blender in the presence of a low-pH buffer (10 mL/g fresh weight) containing 100 mm sodium phosphate, pH 4.0, 500 mm NaCl, 3 mm DTT, and 1.0% PVP-40. The slurry was filtered through Miracloth (Calbiochem) and denatured proteins were removed by centrifugation at 25,000g for 10 min. The supernatant was brought to 40% ammonium sulfate saturation in the presence of 0.05% (v/v) Tween 20, and contaminating proteins (linamarase) were precipitated on ice for 3 h followed by pelleting at 25,000g for 30 min (Mkpong et al., 1990). The supernatant was extensively dialyzed against 10 mm sodium phosphate, pH 5.6, 50 mm NaCl, and 0.05% (v/v) Tween 20 for 24 h to yield the apparently purified protein. All steps were carried out at 4°C.
For determination of the native Mr, the protein was concentrated by ultrafiltration using a Centricon-10 filter (Amicon, Beverly, MA) and loaded onto a Sephracryl-200 column equilibrated in 100 mm sodium phosphate, pH 7.0, and 200 mm NaCl. The column was standardized with RNase A (13,700 D), chymotrypsin (25,000 D), ovalbumin (43,000 D), BSA (67,000 D), and blue dextran 2000 (Pharmacia), and the elution profile was monitored at 280 nm.
HNL enzyme assays were performed in 4 mL of 50 mm sodium phosphate, pH 5.0, using from 5 to 100 μg of protein in the presence of various concentrations of acetone cyanohydrin. Reactions were run for 15 min at room temperature. Control reactions had only buffer and acetone cyanohydrin (28 mm for assays of purification fractions). After 15 min, 10 μL of reaction was transferred to a tube containing 20 mL of 50 mm sodium phosphate, pH 4.0, and assayed for HCN using the Spectroquant cyanide detection kit (EM Science, Gibbstown, NJ). A standard cyanide response curve was generated using known amounts of KCN Spontaneous rates of cyanide production from acetone cyanohydrin (decomposition) were subtracted from enzyme-catalyzed reaction rates.
Immunoblots
Plant tissues were frozen in liquid nitrogen and ground in a mortar and pestle with 100 mm sodium phosphate, pH 7.0, and 500 mm NaCl for extraction of total soluble proteins. The extract was filtered through Miracloth and centrifuged at 100,000g for 1 h at 4°C. The protein concentration of the supernatant fraction was determined by the method of Bradford (1976) using BSA as a standard. SDS-PAGE, western transfers, and immunoblots were performed according to the method of Harlow and Lane (1988). Polyclonal antibodies were raised against purified HNL by the Ohio State University Antibody Center (Columbus). Immunodecorated bands were visualized using goat anti-mouse IgG alkaline phosphatase conjugate (Promega) and a colorimetric assay according to the procedure of Harlow and Lane (1988).
Localization of HNL by Immunofluorescence
Vibratome sections of cassava leaves were washed in 20 mm Tris, 500 mm NaCl, pH 7.5 (TBS) and blocked with 0.5% (w/v) BSA in TBS. After washing in TBS, sections were incubated in a 1:100 dilution of HNL polyclonal antibodies in 0.05% (v/v) Tween 20, plus 1% (w/v) gelatin in TBS for 1 h. The sections were washed twice with TBS and then incubated in the dark with a 1:30 dilution of goat anti-rabbit IgG fluorescein conjugate (Calbiochem) in TBS with 0.5% (w/v) BSA. Slides were stored at 4°C in the dark until photographed using an Axiovert 100 microscope (Zeiss).
Isolation of Cassava HNL cDNA
Cassava leaf total RNA was isolated using the method of Baker et al. (1990) and supplied to Clontech Laboratories (Palo Alto, CA) for preparation of a λ ZAP cDNA library. The phage library was screened for an HNL cDNA clone using a 30-base oligonucleotide corresponding to the first 30 nucleotides of the coding sequence of a cassava HNL cDNA clone (Hughes et al., 1994), according to the procedures of Ausubel et al. (1994). Five independently isolated, positive phagemids were transformed into Escherichia coli XL1-Blue, according to the manufacturer's instructions (Clontech). DNA sequence analysis was carried out using the PRISM Ready Reaction DyeDeoxy Terminator Cycle Sequencing Kit (Perkin-Elmer). All regions of one of the clones were sequenced at least twice in both directions, and a second, independently isolated clone was sequenced once in each direction. The DNA sequence of the full-length cDNA was analyzed using the PC Gene program (Intelligenetics, Mountain View, CA) and the BLAST program (http://www.ncbi.nlm.nih.gov/) for protein sequence comparisons. The HNL cDNA sequence was identical to that deposited in GenBank (accession no. Z29091).
Northern Blots
Total RNA was extracted from leaves, stems, and roots of sterile cassava plants grown in liquid Murashige-Skoog medium supported by the LIFERAFT system (Lin et al., 1995) using TRIzol reagent (GIBCO-BRL). Because of low RNA yields using the regular TRIzol protocol, the manufacturer's modifications for RNA recovery in the presence of proteoglycans and polysaccharides were followed. Denaturing RNA (2.2 m formaldehyde) gel electrophoresis was performed according to the method of Farrell (1993). RNA was transferred to Duralon-UV membrane (Stratagene) according to the manufacturer's instructions. Radioactive probes for northern blots were prepared using the Radprime DNA-labeling system (GIBCO-BRL) and the cassava cDNA clone was used as a probe. Radioactively labeled bands were quantified using a phosphor imager and Imagequant software (Molecular Dynamics, Sunnyvale CA).
RESULTS
Purification and Characterization of Cassava HNL
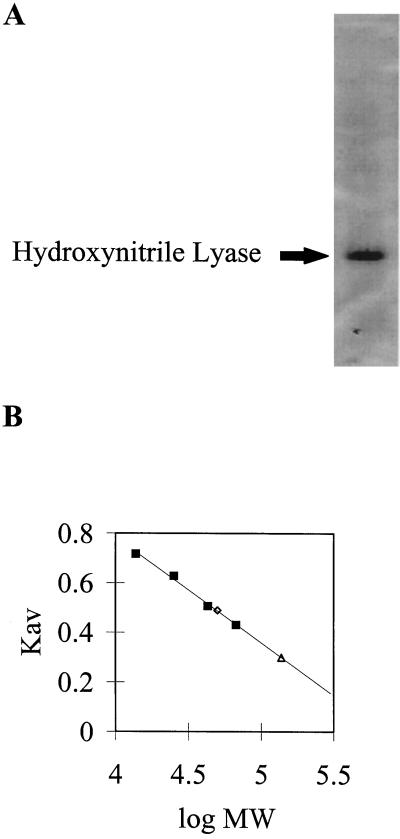
We have purified HNL to apparent homogeneity from whole leaves using a simplified two-step purification procedure (Table I; Fig. 1A). Previous HNL purification strategies required as many as 12 steps, which prolonged the period of isolation, possibly resulting in anomalous enzyme kinetics. Components of the isolation protocol that were essential for the recovery of the highly active enzyme included homogenization of the tissue in an extraction buffer having a high salt concentration (500 mm NaCl), the inclusion of PVP (1%, w/v), and dialysis against buffer containing Tween 20 (0.05%, v/v). In addition, the use of low-pH (4.0) sodium phosphate extraction buffer denatured most of the soluble proteins other than HNL and linamarase (Mkpong et al., 1990). Linamarase was subsequently removed by precipitation with 40% saturated ammonium sulfate. Spectroscopic analyses of the isolated protein indicated that it did not contain flavin, as is typical for class I HNLs (data not shown).
Table I.
Purification of cassava leaf HNL
| Fraction | Total Protein | Total Activity | Specific Activity | Purification Factor |
|---|---|---|---|---|
| mg | mmol cyanide h−1 | mmol cyanide mg−1 protein h−1 | ||
| Crude extract | 5.31 | 3.24 | 0.61 | |
| Ammonium sulfate | 0.95 | 10.9 | 11.5 | 18.9 |
Figure 1.
A, Coomassie blue-stained SDS-PAGE profile of isolated cassava leaf HNL obtained from the ammonium sulfate fraction (5 μg of total protein). The predicted molecular mass is 28.5 kD. The polypeptide profile of the crude, low-pH buffer extract yields essentially two proteins, linamarase and HNL (Mkpong et al., 1990). B, Native molecular weight (MW) of cassava HNL as determined by gel-permeation chromatography. Protein molecular mass standards were: RNase A, 13.7 kD; chymotrypsinogen A, 25 kD; ovalbumin, 43 kD; and BSA, 67 kD. HNL and linamarase eluted at 50.1 and 138 kD, respectively. ⋄, HNL; ▪, standards; and ▵, linamarase.
As shown in Figure 1A, purified cassava HNL had a subunit molecular mass of 28.5 kD, similar to that reported by Hughes et al. (1994) and Wajant and Pfizenmaier (1996). The native enzyme, however, had a molecular mass of 50.1 kD, suggesting that it was a dimer (Fig. 1B). These results are in contrast to those of Hughes et al. (1994), Chueskul and Chulavatnatol (1996), and Wajant and Pfizenmaier (1996). Hughes et al. (1994) reported that the native molecular mass of HNL was a 92-kD homotrimer, whereas the latter two groups reported that the native enzyme was a tetramer of 102 to 110 kD, respectively. In part this difference in native molecular mass may be attributed to differences in the ionic strength of the buffer used for size-exclusion chromatography. Hughes et al. (1994) reported that higher-ionic-strength buffers facilitate the formation of higher-ordered homomeric complexes. Chueskul and Chulavatnatol (1996), however, observed a homotetrameric native enzyme structure by gel filtration in the absence of salts.
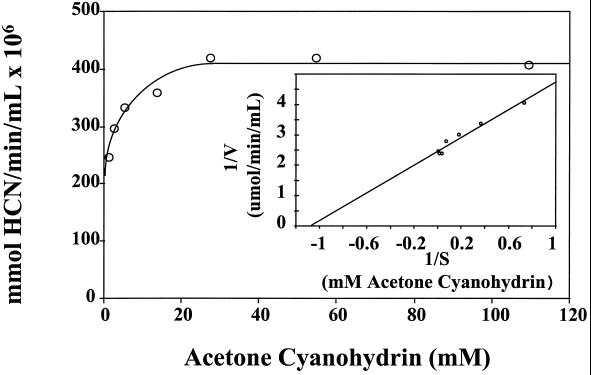
As shown in Table I and in Figure 2, the rapidly isolated enzyme had a higher Vmax (11.5 mmol cyanide mg−1 protein h−1) and a lower Km (0.93 mm) than those previously reported for HNL isolated from whole leaves (Kakes and Harvoort, 1992; Wajant and Pfizenmaier, 1996). Furthermore, the enzyme exhibited typical Michaelis-Menten kinetics in marked contrast to the results reported by Hughes et al. (1994). They reported that cassava HNL exhibited sigmoidal non-Michaelis-Menten kinetics and had a Km and Vmax of 119 mm and 2.2 mmol cyanide mg−1 protein h−1, respectively, indicative of a low catalytic efficiency (Kcat/Km). Similar to the results presented here, Chueskul and Chulavatnatol (1996) reported that the cassava petiolar HNL had simple Michaelis-Menten kinetics; however, the kinetic properties (Km and Vmax of 2.1 mm and 2.9 mmol HCN mg−1 protein h−1, respectively) of the HNL isolated by this group were indicative of a catalytically less efficient enzyme than that isolated by the two-step purification procedure.
Figure 2.
Substrate-dependent enzyme kinetics for the isolated cassava leaf HNL. Open circles indicate substrate concentration-dependent rate kinetics for the purified enzyme. Inset shows a double reciprocal plot of the data.
Tissue-Specific Localization of HNL in Cassava
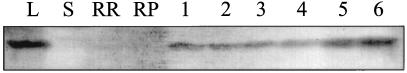
To determine the tissue- and organ-specific localization of HNL, total soluble proteins were extracted from leaves, stems, and roots for determination of HNL activity. In addition, the soluble proteins were separated by SDS-PAGE for western-blot analysis using HNL-specific antibodies. Unlike leaves, no detectable HNL activity was present in root and/or stem crude extracts (data not shown). To determine whether HNL protein was present in these extracts, but possibly inactive, equal quantities of leaf, root, and stem soluble protein extracts were co-electrophoresed with purified HNL and transferred to a membrane for western-blot analysis. As shown in Figure 3, HNL was detectable only in the leaf protein extract. Based on the relative cross-reactivity of the HNL present in leaves we estimate that HNL represents approximately 7% of the total leaf soluble protein. In contrast, the soluble extracts of stems, root parenchyma, and root rind did not contain detectable quantities of HNL (less than 0.3% of the total protein extracted).
Figure 3.
Western-blot analysis of the distribution and abundance of HNL in different cassava tissues. Each tissue lane contained 30 μg of total soluble protein. The primary antibody was generated against purified cassava leaf HNL. Lane L, Leaf; lane S, stem; lane RR, root rind; and lane RP, root parenchyma. Lanes 1 to 6, Purified HNL equivalent to 0.084, 0.168, 0.336, 0.672, 1.34, and 2.68 μg of HNL, respectively.
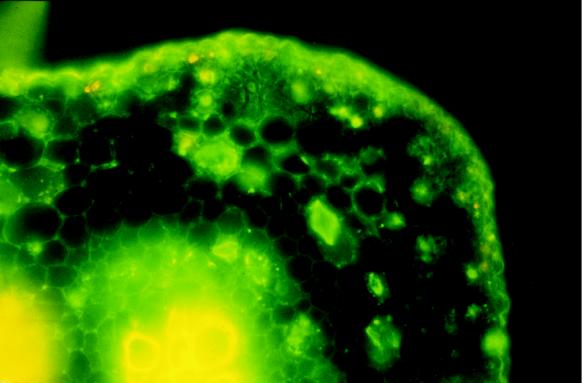
Previously, we have demonstrated that leaf apoplast extracts were enriched (8-fold) in HNL activity relative to whole-leaf extracts, suggesting that HNL was probably localized in the cell walls (White et al., 1994; White and Sayre, 1995). To test this hypothesis, we carried out in situ immunolocalization of HNL in cassava leaves. Vibratome sections of cassava leaves were incubated with polyclonal antibodies raised against HNL purified from total leaf extracts. The antibodies labeled the cell walls of parenchyma, mesophyll, and epidermal cells (Fig. 4). Preimmune serum did not label the leaf material. These results demonstrate that HNL is present in cell walls, similar to the pattern reported for linamarase (Mkpong et al., 1990).
Figure 4.
Immunofluorescent localization of HNL in cassava leaf tissue. The primary antibody was generated against cassava leaf HNL and the secondary antibody was labeled with fluorescein. Preimmune sera did not cross-react with the cassava tissue (data not shown). The yellow fluorescence associated with the central vascular bundle and the epidermal cells is the result of autofluorescence. In some cells a yellow-orange or red punctate fluorescence is due to the combined fluorescence emission of chloroplasts (red) and fluorescein (green).
Tissue-Specific Abundance of HNL mRNA
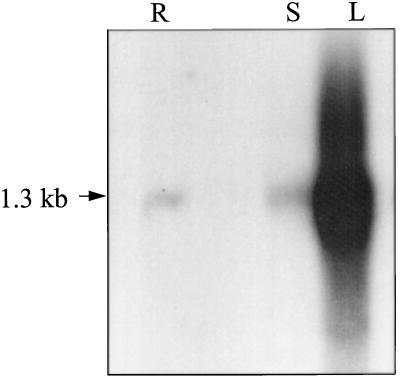
To characterize the molecular basis for the differential expression of HNL we measured the steady-state HNL mRNA levels in cassava leaves, stems, and roots. Total RNA was isolated from leaves, stems, and roots of plants grown under sterile conditions. Equal loadings (based on readings at A260 and densitometry of ethidium bromide-stained rRNA bands [nondenaturing gels]) of total RNA extracts were electrophoretically separated and probed on northern blots using a 32P-labeled cassava HNL cDNA. A single hybridizing band of approximately 1.3 kb was detected in the leaf total RNA but was present in much reduced amounts in the stem and root RNA populations (Fig. 5). Based on phosphor-imager analyses, the steady-state HNL mRNA levels present in stems and roots were equivalent to 2 and 6%, respectively, of that present in leaves.
Figure 5.
Northern-blot analysis of HNL transcript abundance in different cassava tissues. Lane R, Root; lane S, stem; and lane L, leaf tissue. Equal loadings (based on A280 readings and ethidium bromide staining intensity of rRNAs) of total RNA are present in each lane.
DISCUSSION
We have developed a simple two-step procedure for the purification of HNL from cassava leaves. A key step in the isolation protocol was the use of a low-pH (4.0) extraction buffer, which denatures most of the cassava leaf proteins with the exception of HNL and linamarase (Mkpong et al., 1990). The high ionic strength of the extraction buffer and presence of detergent during the subsequent dialysis were necessary to prevent protein aggregation and inactivation of the enzyme. HNL isolated by this procedure had a 5- and 2-fold higher specific activity than that previously reported by Hughes et al. (1994) and Wajant and Pfizenmaier (1996).
In contrast to the results reported by Hughes et al. (1994), we observed typical Michaelis-Menten kinetics for the cassava HNL isolated by the two-step protocol. The Km for acetone cyanohydrin was 0.9 mm, 100-fold lower than that reported by Hughes et al. (1994). Furthermore, cyanide production was saturated by 30 mm acetone cyanohydrin, much less than that (300 mM) previously reported (Hughes et al., 1994). Significantly, the kinetic properties reported here for cassava HNL are similar to those previously reported for linamarase (Mkpong et al., 1990). Since the relative abundance of HNL and linamarase in cassava leaves is similar, it is unlikely that HNL is rate limiting (kinetically) for cyanogenesis in leaves (Mkpong et al., 1990; White and Sayre, 1995).
In all cyanogenic plants surveyed to date it is apparent that the cyanogenic glycoside and its corresponding cyanogenic enzymes are localized in different cellular compartments or tissues. This compartmentalization prevents cyanogenesis until the tissue is disrupted. In some cyanogenic plants the separation of substrate and cyanogenic enzymes is at a subcellular level. In rubber tree, endosperm cells contain linamarin and HNL in the cytoplasm, whereas linamarase is located in the apoplast (Poulton, 1990; Selmar, 1993). In some cyanogenic plants the separation of substrate and cyanogenic enzyme(s) is at a tissue level. In sorghum (Sorghum bicolor) leaves, the cyanogenic glycoside dhurrin is located in vacuoles of leaf epidermal cells, whereas the β-glucosidase and HNL are localized in the cytoplasm and plastids of mesophyll cells (Poulton, 1990; Wajant et al., 1994). In cassava leaves linamarin has been localized to the vacuoles, whereas linamarase is localized to cell walls and laticifers (Mkpong et al., 1990; Pancoro and Hughes, 1992; Hughes et al., 1994; McMahon et al., 1995) Recently, we demonstrated that both linamarase and HNL were enriched (8-fold relative to whole leaves) in cassava leaf apoplast extracts (White et al., 1994; White and Sayre, 1995). These results suggested that HNL may be present in the cell walls. The HNL immunolocalization experiments reported here indicate that HNL is indeed localized in leaf cell walls similar to linamarase, thus precluding cyanogenesis without tissue disruption (Mkpong et al., 1990). Since both linamarase and HNL have relatively low affinities for their substrates (Km = 1.0 mm), compartmentalization of both cyanogenic enzymes in the cell wall would be expected to facilitate cyanogen turnover relative to compartmentalization of the two enzymes at different sites.
Previous studies have demonstrated that linamarin and its β-glucosidase, linamarase, are present in all cassava organs except seeds (McMahon et al., 1995). Since acetone cyanohydrin has been detected in processed cassava roots, it was necessary to determine whether roots contained HNL. Using the improved enzyme solubilization and assay procedures we were unable to detect HNL activity in cassava roots and stems. Furthermore, immunoblot analyses indicated that HNL was present in leaves but absent from roots and stems. The molecular basis for the absence of HNL from roots and stems could be attributed to very low steady-state HNL transcript levels (relative to leaves), suggesting that HNL expression is regulated at a pretranslational level. Organ-specific localization of HNL is not unique among cyanogenic plants. Similar to cassava, sorghum and flax (Linum usitatissimum) contain HNL only in leaves and not in stems or roots (Wajant et al., 1994). In sorghum, however, the roots also lack dhurrin (cyanogenic glycoside) and its corresponding β-glycosidase. In contrast, cyanogenic varieties of white clover lack HNL activity in all tissues (for review, see Poulton, 1990). Cyanogenesis in white clover is apparently dependent on the instability of the hydroxynitrile. In cassava roots, however, the absence of HNL results in high acetone cyanohydrin levels and reduced cyanogenesis, particularly at low pH.
The absence of HNL in roots raises the question as to whether there is any apparent biological relevance for its absence. Two possible scenarios are considered. First, HNL activity may be toxic to the root. Recently, it has been demonstrated that cassava roots are capable of synthesizing linamarin (Du et al., 1995; McMahon and Sayre, 1995; McMahon et al., 1995; White and Sayre, 1995). It is not known, however, whether linamarin is also transported (apoplastically) between shoots and roots or between root cells. One mechanism for apoplastically transporting cyanogenic glycosides is as its glycoside, linustatin (Lykkesfeldt and Moller, 1994). In rubber tree seedlings, linustatin is transported from the seed to the growing hypocotyl (Selmar, 1993). Since linustatin cannot be hydrolyzed by cell wall-bound linamarase, its apoplastic transport is not cyanogenic. At the sink site, linustatin is deglycosylated by one of two mechanisms to produce acetone cyanohydrin. The acetone cyanohydrin is then either reglycosylated to produce linamarin or is broken down to cyanide, which is then refixed by β-cyanoalanine synthase (ultimately to synthesize Asn). If a similar apoplastic linustatin transport and deglycosylation pathway were to operate in cassava roots, then cyanide production from acetone cyanohydrin could be accelerated by root HNL and potentially poison the root.
An alternative explanation for the apparent absence of HNL in roots and stems is that HNL may be transiently expressed only during pathogen attack. The basis for this hypothesis is the high degree of similarity (33–55%) between the predicted protein sequence of the rice (Oryza sativa L.) pir gene(s) and that of cassava HNL (Reimann et al., 1995). Expression of the rice pir gene is induced during fungal infection. Although it is apparent that the molecular basis for pathogen resistance encoded by the rice pir gene (lipase activity) differs from that of cassava HNL, it is conceivable that the highly similar N-terminal domain of the pir proteins and cassava HNL may have similar functions, possibly related to targeting fungal pathogens. It is noted, however, that some fungi are capable of metabolizing cyanide and are more pathogenic on plant cultivars that are more cyanogenic (Birk et al., 1996). Both of these hypotheses, however, are testable.
Overall, it is apparent that the presence of acetone cyanohydrin in ruptured or processed cassava roots can in part be attributed to the absence of HNL. It is suggested that supplementary addition of HNL during cassava root processing or the expression of HNL in roots of transgenic cassava plants may facilitate the reduction of cyanogen content, resulting in a healthier food product.
ACKNOWLEDGMENTS
We would like to thank Yakang Lin, Matthew Geisler, Greg Bell, Dr. Zhenbiao Yang, Dr. Fred Sack, and Dr. Miller McDonald for the use of equipment. We would also like to thank Dr. Terrence Graham and Dr. Michael Evans for useful discussions.
Abbreviation:
- HNL
hydroxynitrile lyase
Footnotes
This work was supported in part by the Rockefeller Foundation and the Consortium for Plant Biotechnology Research (R.T.S.).
LITERATURE CITED
- Ausubel F, Brent R, Kingston R, Moore D, Seidman J, Smith J, Struhl K, Albright L, Coen D, Varki A and others. Current Protocols in Molecular Biology, Vol 1. New York: John Wiley & Sons; 1994. [Google Scholar]
- Baker SS, Rugh CL, Kamalay JC. RNA and DNA isolation from recalcitrant plant tissues. Biotechniques. 1990;9:268. [PubMed] [Google Scholar]
- Birk R, Bravdo B, Shoseyov O. Detoxification of cassava by Aspergillus niger B-1. Appl Microbiol Biotechnol. 1996;45:411–414. [Google Scholar]
- Belloti A, Arias B (1993) The possible role of HCN on the biology and feeding behavior of the cassava burrowing bug (Cyrtomenus bergi Froeschner). In WM Roca, AM Thro, eds, Proceedings of the First International Scientific Meeting of the Cassava Biotechnology Network, Centro Internacional Agricultura Tropical, Cali, Colombia, pp 406–409
- Bradford M. A rapid and sensitive method for quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Castric PA, Farnden KF, Conn EE. Cyanide metabolism in higher plants. 5. The formation of asparagine from β-cyanoalanine. Arch Biochem Biophys. 1972;152:62–69. doi: 10.1016/0003-9861(72)90193-2. [DOI] [PubMed] [Google Scholar]
- Chueskul S, Chulavatnatol M. Properties of alpha-hydroxynitrile lyase from the petiole of cassava (Manihot esculenta, Crantz) Arch Biochem Biophys. 1996;334:401–405. doi: 10.1006/abbi.1996.0471. [DOI] [PubMed] [Google Scholar]
- Cock JH (1985) Cassava: New Potential for a Neglected Crop. Westfield Press, London
- Cutler A, Conn E. The biosynthesis of cyanogenic glycosides in Linum usitatissumum (Linen flax) in vitro. Arch Biochem Biophys. 1981;212:468–474. doi: 10.1016/0003-9861(81)90389-1. [DOI] [PubMed] [Google Scholar]
- Du L, Bokanga M, Moller BL, Halker BA. The biosynthesis of cyanogenic glucosides in roots of cassava. Phytochemistry. 1995;39:323–326. [Google Scholar]
- Farrell R. RNA Methodologies: A Laboratory Guide for Isolation and Characterization. San Diego, CA: Academic Press; 1993. [Google Scholar]
- Harlow E, Lane D. Antibodies: A Laboratory Manual. Cold Spring Harbor, NY: Cold Spring Harbor Press; 1988. [Google Scholar]
- Hasslacher M, Schall M, Hayn M, Griengl H, Kohlwein S, Schwab H. Molecular cloning of the full-length cDNA of (S)-hydroxynitrile lyase from Hevea brasiliensis. J Biol Chem. 1996;271:5884–5891. doi: 10.1074/jbc.271.10.5884. [DOI] [PubMed] [Google Scholar]
- Hughes J, Carvalho F, Hughes M. Purification, characterization, and cloning of α-hydroxynitrile lyase from cassava (Manihot esculenta Crantz) Arch Biochem Biophys. 1994;311:496–502. doi: 10.1006/abbi.1994.1267. [DOI] [PubMed] [Google Scholar]
- Kakes P. Properties and functions of the cyanogenic system in higher plants. Euphytica. 1990;48:25–43. [Google Scholar]
- Kakes P, Harvoort H. Is there rhodanese in plants? Phytochemistry. 1992;31:1501–1505. [Google Scholar]
- Lin J, Fike R, Assad-Garcia N. Improved regeneration of plant tissues: a novel medium format and membrane-based liquid culture system. Focus. 1995;17:95–97. [Google Scholar]
- Lykkesfeldt, Moller BL. Cyanogenic glycosides in cassava, Manihot esculenta Crantz. Acta Chem Scand. 1994;48:178–180. [Google Scholar]
- McMahon J, White W, Sayre RT. Cyanogenesis in cassava (Manihot esculenta Crantz) J Exp Bot. 1995;46:731–741. [Google Scholar]
- McMahon JM, Sayre RT (1995) Cyanogenic glycosides: physiology and regulation of synthesis. In DL Gustine, HE Flores, eds, Phytochemicals and Health, Current Topics in Plant Physiology, Vol 15. American Society of Plant Physiologists, Rockville, MD, pp 112–121
- Mkpong O, Yan H, Chism G, Sayre RT. Purification, characterization and localization of linamarase in cassava. Plant Physiol. 1990;93:176–181. doi: 10.1104/pp.93.1.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murashige T, Skoog F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Plant Physiol. 1962;15:473–497. [Google Scholar]
- Nambisan B (1993) Cyanogenesis in cassava. In WM Roca, AM Thro, eds, Proceedings of the First International Scientific Meeting of the Cassava Biotechnology Network, Centro Internacional Agricultura Tropical, Cali, Colombia, pp 424–427
- Osuntokun BO. Cassava diet, chronic cyanide intoxification and neuropathy in Nigerian Africans. World Rev Nutr Diet. 1981;36:141–173. doi: 10.1159/000393156. [DOI] [PubMed] [Google Scholar]
- Pancoro A, Hughes MA. In situ localization of cyanogenic glucosidase (linamarase) gene expression in leaves of cassava (Manihot esculenta, Crantz) using non-isotopic riboprobes. Plant J. 1992;2:821–827. [Google Scholar]
- Poulton J. Cyanogenesis in plants. Plant Physiol. 1990;94:401–405. doi: 10.1104/pp.94.2.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reimann C, Hofmann C, Mauch F, Dudler R. Characterization of a rice gene induced by Pseudomonas syringe pv. syringe: requirement for a bacterial lemA gene function. Physiol Mol Plant Pathol. 1995;46:71–81. [Google Scholar]
- Roca WM (1984) Cassava. In WR Sharp, DA Evans, PV Ammirato, Y Yamada, eds, Handbook of Plant Cell Culture. McMillan Publishing, New York, pp 269–301
- Rosling H, Mlingi N, Tylleskar T, Banea M (1993) Causal mechanisms behind human diseases induced by cyanide exposure from cassava. In W Roca, A Thro, eds, Proceedings of the First International Scientific Meeting of the Cassava Biotechnology Network, Centro Internacional de Agricultura Tropical, Cali, Colombia, pp 366–375
- Selmar D. Transport of cyanogenic glycosides: uptake of linustatin by Hevea cotyledons. Planta. 1993;191:191–199. [Google Scholar]
- Tylleskar T, Cooke R, Banea M, Poulter N, Bikangi N, Rosling H. Cassava cyanogens and konzo, an upper motoneuron disease found in Africa. Lancet. 1992;339:208–211. doi: 10.1016/0140-6736(92)90006-o. [DOI] [PubMed] [Google Scholar]
- Wajant H, Mundry K-W. Hydroxynitrile lyase from Sorghum bicolor: a glycoprotein heterodimer. Plant Sci. 1993;89:127–133. [Google Scholar]
- Wajant H, Pfizenmaier K. Identification of potential active site residues in the hydroxynitrile lyase from Manihot esculenta by site-directed mutagenesis. J Biol Chem. 1996;271:25830–25834. doi: 10.1074/jbc.271.42.25830. [DOI] [PubMed] [Google Scholar]
- Wajant H, Riedel D, Bent S, Mundry K-W. Immunocytological localization of hydroxynitrile lyases from Sorghum bicolor L. and Linum usitatissimum L. Plant Sci. 1994;103:145–154. [Google Scholar]
- White W, McMahon J, Sayre RT. Regulation of cyanogenesis in cassava. Acta Hortic. 1994;375:69–78. [Google Scholar]
- White W, Sayre RT (1995) The characterization of hydroxynitrile lyase for the production of safe food products from cassava (Manihot esculenta, Crantz) In DL Gustine, HE Flores, eds, Phytochemicals and Health, Current Topics in Plant Physiology, Vol 15. American Society of Plant Physiologists, Rockville, MD, pp 303–304
- Yemm R, Poulton J. Isolation and characterization of mandelonitrile lyase from mature black cherry (Prunus serotina) seeds. Arch Biochem Biophys. 1986;247:440–445. doi: 10.1016/0003-9861(86)90604-1. [DOI] [PubMed] [Google Scholar]
- Zheng L, Poulton JE. Temporal and spatial expression of amygdalin hydrolase and (R)-(+)-mandelonitrile lyase in black cherry seeds. Plant Physiol. 1995;109:31–39. doi: 10.1104/pp.109.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]