Abstract
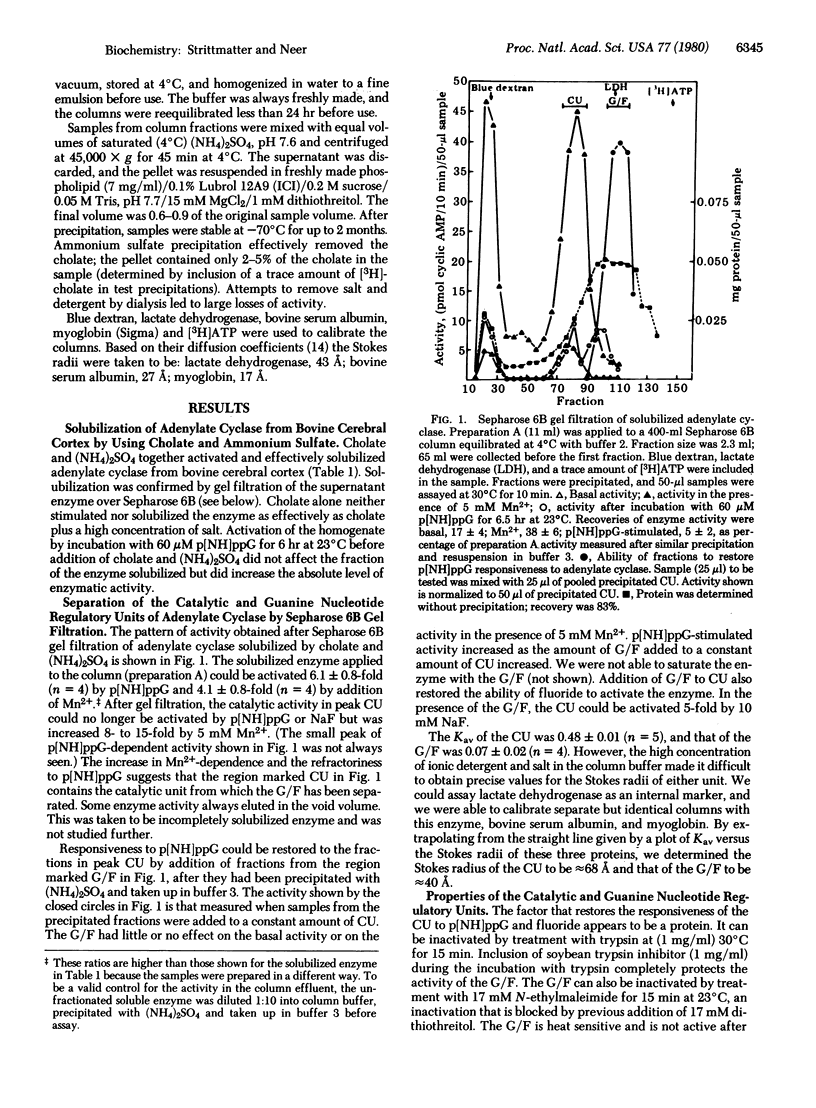
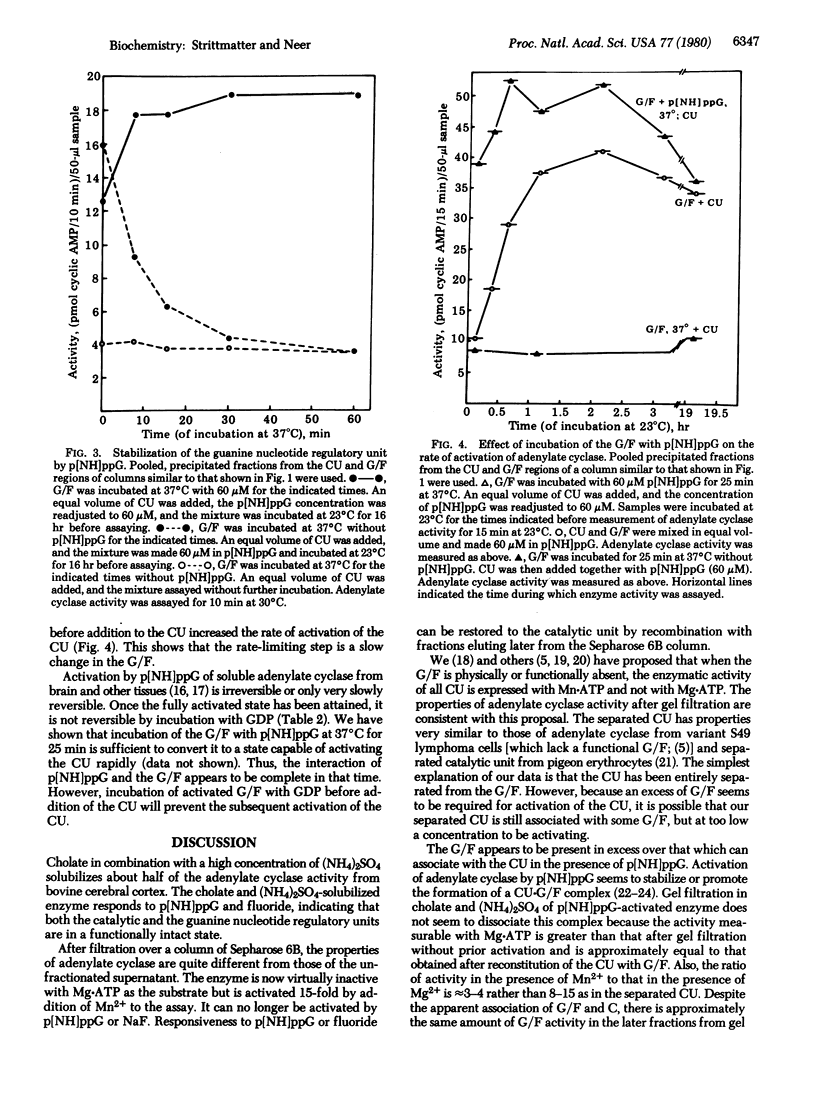
Adenylate cyclase from bovine brain cortex was solubilized with 14 mM cholate and 1 M (NH4)2SO4. Gel filtration over a column of Sepharose 6B separated the catalytic unit (CU) from a factor (G/F) that confers responsiveness to 5'-guanylyl imidophosphate (p[NH]ppG) or fluoride. The separated CU, which elutes with a Kav, of 0.48 +/- 0.01 (n=5), is not responsive to p[NH]ppG or fluoride and is relatively inactive when Mg . ATP is the substrate but activated 8-15-fold by Mn2+. The separated G/F elutes with a Kav of 0.70 +/- 0.02 (n=4). It restores the responsiveness of the CU to p[NH]ppG and fluoride. Activation of the enzyme by p[NH]ppG before solubilization does not decrease the amount of G/F eluting with a Kav of 0.7. Therefore, the G/F is probably present in brain cortex in excess over the CU. p[NH]ppG stabilizes the G/F but not the CU against thermal inactivation, suggesting that it interacts with G/F and not with CU. Incubation of the G/F with p[NH]ppG before addition of CU markedly increases the rate of activation of the reconstituted enzyme by p[NH]ppG. We propose, therefore, that the rate-limiting step in adenylate cyclase activation is a process in G/F alone and not a slow conformational change in CU or a slow association of G/F with CU. Binding of p[NH]ppG to the isolated G/F appears to be readily reversible; the ability of fully activated G/F to stimulate CU can be blocked if GDP is added before CU. In contrast, after the CU has been activated by interaction with G/F, GDP cannot reverse the activation. This suggests that association with the CU increases the affinity of G/F for p[NH]ppG.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cassel D., Selinger Z. Mechanism of adenylate cyclase activation through the beta-adrenergic receptor: catecholamine-induced displacement of bound GDP by GTP. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4155–4159. doi: 10.1073/pnas.75.9.4155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann F. M. Solubilization and reconstitution of dopamine-sensitive adenylate cyclase from bovine caudate nucleus. J Biol Chem. 1979 Jan 25;254(2):255–258. [PubMed] [Google Scholar]
- Johnson G. L., Kaslow H. R., Bourne H. R. Genetic evidence that cholera toxin substrates are regulatory components of adenylate cyclase. J Biol Chem. 1978 Oct 25;253(20):7120–7123. [PubMed] [Google Scholar]
- Krishna G., Weiss B., Brodie B. B. A simple, sensitive method for the assay of adenyl cyclase. J Pharmacol Exp Ther. 1968 Oct;163(2):379–385. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lefkowitz R. J., Caron M. G. Characteristics of 5'-guanylyl imidodiphosphate-activated adenylate cyclase. J Biol Chem. 1975 Jun 25;250(12):4418–4422. [PubMed] [Google Scholar]
- Levinson S. L., Blume A. J. Altered guanine nucleotide hydrolysis as basis for increased adenylate cyclase activity after cholera toxin treatment. J Biol Chem. 1977 Jun 10;252(11):3766–3774. [PubMed] [Google Scholar]
- Londos C., Lad P. M., Nielsen T. B., Rodbell M. Solubilization and conversion of hepatic adenylate cyclase to a form requiring MnATP as substrate. J Supramol Struct. 1979;10(1):31–37. doi: 10.1002/jss.400100104. [DOI] [PubMed] [Google Scholar]
- Londos C., Salomon Y., Lin M. C., Harwood J. P., Schramm M., Wolff J., Rodbell M. 5'-Guanylylimidodiphosphate, a potent activator of adenylate cyclase systems in eukaryotic cells. Proc Natl Acad Sci U S A. 1974 Aug;71(8):3087–3090. doi: 10.1073/pnas.71.8.3087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naya-Vigne J., Johnson G. L., Bourne H. R., Coffino P. Complementation analysis of hormone-sensitive adenylate cyclase. Nature. 1978 Apr 20;272(5655):720–722. doi: 10.1038/272720a0. [DOI] [PubMed] [Google Scholar]
- Neer E. J., Echeverria D., Knox S. Increase in the size of soluble brain adenylate cyclase with activation by guanosine 5'-(beta, gamma-imino)triphosphate. J Biol Chem. 1980 Oct 25;255(20):9782–9789. [PubMed] [Google Scholar]
- Neer E. J. Physical and functional properties of adenylate cyclase from mature rat testis. J Biol Chem. 1978 Aug 25;253(16):5808–5812. [PubMed] [Google Scholar]
- Neer E. J. Size and detergent binding of adenylate cyclase from bovine cerebral cortex. J Biol Chem. 1978 Mar 10;253(5):1498–1502. [PubMed] [Google Scholar]
- Nielsen T. B., Lad P. M., Preston M. S., Rodbell M. Characteristics of the guanine nucleotide regulatory component of adenylate cyclase in human erythrocyte membranes. Biochim Biophys Acta. 1980 Apr 17;629(1):143–155. doi: 10.1016/0304-4165(80)90273-1. [DOI] [PubMed] [Google Scholar]
- Pfeuffer T. Guanine nucleotide-controlled interactions between components of adenylate cyclase. FEBS Lett. 1979 May 1;101(1):85–89. [PubMed] [Google Scholar]
- Pfeuffer T., Helmreich E. J. Activation of pigeon erythrocyte membrane adenylate cyclase by guanylnucleotide analogues and separation of a nucleotide binding protein. J Biol Chem. 1975 Feb 10;250(3):867–876. [PubMed] [Google Scholar]
- Ross E. M., Gilman A. G. Biochemical properties of hormone-sensitive adenylate cyclase. Annu Rev Biochem. 1980;49:533–564. doi: 10.1146/annurev.bi.49.070180.002533. [DOI] [PubMed] [Google Scholar]
- Ross E. M., Gilman A. G. Resolution of some components of adenylate cyclase necessary for catalytic activity. J Biol Chem. 1977 Oct 25;252(20):6966–6969. [PubMed] [Google Scholar]
- Ross E. M., Howlett A. C., Ferguson K. M., Gilman A. G. Reconstitution of hormone-sensitive adenylate cyclase activity with resolved components of the enzyme. J Biol Chem. 1978 Sep 25;253(18):6401–6412. [PubMed] [Google Scholar]
- Sahyoun N., Schmitges C. J., Le Vine H., 3rd, Cuatrecasas P. Molecular resolution and reconstitution of the GPP (NH) P and NAF sensitive adenylate cyclase system. Life Sci. 1977 Dec 15;21(12):1857–1863. doi: 10.1016/0024-3205(77)90169-2. [DOI] [PubMed] [Google Scholar]
- Schramm M., Rodbell M. A persistent active state of the adenylate cyclase system produced by the combined actions of isoproterenol and guanylyl imidodiphosphate in frog erythrocyte membranes. J Biol Chem. 1975 Mar 25;250(6):2232–2237. [PubMed] [Google Scholar]
- Spiegel A. M., Downs R. W., Jr, Aurbach G. D. Separation of a guanine nucleotide regulatory unit from the adenylate cyclase complex with GTP affinity chromatography. J Cyclic Nucleotide Res. 1979;5(1):3–17. [PubMed] [Google Scholar]
- Toscano W. A., Jr, Westcott K. R., LaPorte D. C., Storm D. R. Evidence for a dissociable protein subunit required for calmodulin stimulation of brain adenylate cyclase. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5582–5586. doi: 10.1073/pnas.76.11.5582. [DOI] [PMC free article] [PubMed] [Google Scholar]



