Abstract
Background
Papillary thyroid cancer (PTC) recurrence risk is difficult to predict. No current risk classification system incorporates BRAF mutational status. Here, we assess the incremental value of BRAF mutational status in predicting PTC recurrence relative to existing recurrence risk algorithms.
Methods
Serial data were collected for a historical cohort having undergone total thyroidectomy for PTC over a five-year period. Corresponding BRAFV600E testing was performed and Cox proportional hazard regression modeling, with and without BRAF status, was used to evaluate existing recurrence risk algorithms.
Results
The five-year cumulative PTC recurrence incidence within our 356 patient cohort was 15%. 205 (81%) of associated archived specimens were successfully genotyped and 110 (54%) harbored the BRAFV600E mutation. The five-year cumulative recurrence incidence among BRAFV600E patients was 20%, versus 8% among BRAF wild type. BRAFV600E was significantly associated with time to recurrence when added to the following algorithms: AMES (HR 2.43 [1.08–5.49]), MACIS category (HR 2.46 [1.09–5.54]), AJCC-TNM (HR 2.51 [1.11, 5.66]), and ATA recurrence-risk category (HR 2.44 [1.08–5.50]), and model discrimination improved (incremental c-index range 0.046–0.109).
Conclusions
Addition of BRAF mutational status to established risk algorithms improves discrimination of recurrence risk in patients undergoing total thyroidectomy for PTC.
INTRODUCTION
Papillary thyroid carcinoma (PTC) is the most common type of thyroid malignancy, and the annual global incidence of this cancer subtype is increasing1–3. The majority of PTCs are effectively treated using a combination of surgical and radioiodine-based therapies, with associated 20-year cause-specific survival rates exceeding 90%4. Despite prompt and appropriate surgical and medical therapies, a subset of these cancers will behave aggressively, recurring locally and metastasizing early. These more virulent cancers are associated with higher rates of morbidity and mortality than are their less aggressive counterparts4. Therefore, early recognition of aggressive cancers is important, since more aggressive initial therapy may improve outcomes.
Numerous clinical, cellular and subcellular features of PTC are associated with cancer virulence. Clinical variables include gender, age at diagnosis, family history, and history of ionizing radiation exposure5–6. In addition, several cellular variants are linked to aggressive malignant behavior, including the tall-cell, hobnail and diffuse sclerosing subtypes7–8. At the molecular level, mutations in the BRAF protooncogene modulate thyroid cell growth and division, and appear to increase PTC aggressiveness9–14.
A number of formal scoring systems have been developed to estimate prognosis among patients diagnosed with PTC. These prognostic instruments include the AMES (Age at diagnosis, presence of Metastasis, Extent of disease and tumor Size), the MACIS (presence of Metastasis, Age at diagnosis, Completeness of surgical resection, presence of Invasion, tumor Size), the AJCC–TNM (American Joint Committee on Cancer - Tumor, lymph Node, distant Metastasis), and the ATA (American Thyroid Association) systems4, 15–16. Of these, the ATA algorithm was specifically designed to assess recurrence risk, while the remaining instruments were developed to estimate overall survival. Although these prognostic instruments are useful in risk-stratifying patients, we hypothesize that inclusion of BRAF mutational status could improve the discrimination of these scoring systems. Further, because the disease specific survival among PTC patients is generally excellent, we use recurrence, a more common and likely more relevant prognostic feature of PTC, as our clinical endpoint. We therefore assayed the additive value of BRAF gene mutation analysis to the predictive capacity of conventional risk-stratification algorithms, adapting each model specifically to assess recurrence risk.
MATERIALS AND METHODS
Patients
All patients having a confirmed diagnosis of PTC after undergoing at least a total thyroidectomy at Massachusetts General Hospital between 2000 and 2005 were included in our analysis (N = 356). Patients having distant metastatic disease (as demonstrated by perioperative imaging), recurrent PTC, or in whom other thyroid cancer variants (i.e. follicular thyroid cancer) were diagnosed were excluded. All patient data, including demographics, clinical history, operative details, and formal pathology were abstracted retrospectively from the electronic medical record and/or from the paper chart in compliance with the Internal Review Board of Massachusetts General Hospital. Papillary microcarcinoma (tumors < 1.0 cm in diameter) was included in our analysis if it was the fine needle aspiration (FNA) biopsy-proven preoperative index nodule, but was excluded when found incidentally on final pathology. For all cases, recurrence was defined as new, structural PTC discovered by cytology of an FNA biopsy or on formal surgical pathology in a previously treated patient, with a clinical disease-free interval (i.e. physical exam, neck ultrasound). Serum thyroglobulin levels were not, in and of themselves, considered an indication of persistence or recurrence.
Mutational testing
Of the unselected patient population meeting inclusion criteria, archived paraffin-embedded primary tumor specimens were available for 252 patients. The diagnosis of PTC in each case was verified and specific carcinoma-containing domain(s) in each primary tumor block were marked for DNA extraction by an endocrine pathologist (P.M.S). BRAF testing was performed for primary tumor samples only (recurrent disease was not assayed) and DNA extraction, processing and genotyping were performed as described previously17–18. Of the 252 paraffin samples available, tumor DNA was successfully extracted from 239 specimens and, of the resulting genomic templates, BRAFV600E genotyping was successful in 205 cases. Single nucleotide extension, a PCR-based genotyping modality affording highly sensitive detection of single nucleotide mutations, was performed to specifically assay the BRAF V600 position17–18. Each reaction was performed in duplicate and no disagreements in BRAF mutational status were encountered between duplicate reactions. Of note, given the known propensity of paraffin-preserved genetic material to spontaneously degrade over time, the quality of extracted genomic DNA was found to decline relative to the age of the paraffin embedded samples.
Statistical analysis
Predictors of PTC recurrence were selected to reflect known risk factors for recurrence and decreased survival among PTC patients. Variables were collapsed and/or categorized according to clinically relevant subgroups and/or convention. Age was assayed as both a binary (above and below 45 years) and a continuous variable. Tumor size was subcategorized according to the AJCC-TNM tumor staging system and PTC tumor subtypes were categorized relative to their respective associated recurrence risk: low-risk (follicular variant of PTC (FVPTC), warthin-like, cribiform-morular variant), normal risk (classical, oncocytic, solid types), and high-risk (tall-cell variant, diffuse-sclerosing). Pathological features, including extrathyroidal extension (ETE), lymphovascular invasion (LVI), lymph node positivity (central/level VI and lateral/levels II–V) and tumor multifocality, were analyzed as dichotomous variables and, in each case, were only considered present when specifically described in the final pathology report (as per convention). Missing demographic, clinical, and mutational variables were excluded from the final analysis. Crude hazard ratios correlating covariates to time to recurrence were assessed using Cox proportional hazards regression modeling. Person time was defined as the interval from surgery until the time of first confirmed recurrence or until the patient’s last documented endocrine/surgical follow-up. Isolated elevations in thyroglobulin levels that did not prompt biopsy or additional treatment(s) were not considered recurrences. The proportional hazard assumption was verified by assessing the significance of a multiplicative interaction term of each variable with time in the final model as well as based on Schoenfeld residuals. Composite variables were developed based on known risk-classification systems and adjusted survival analyses were performed after the addition of BRAF mutational status: AMES as low vs. high-risk, ATA-recurrence risk as low, intermediate, or high risk, and MACIS score and AJCC-TNM stage as linear variables. Kaplan-Meier curves and log-rank tests were used to compare disease-free survival between groups. Categories were collapsed for increased power where appropriate and for illustrative purposes. The area under the receiver-operator curve (c-index) of each classification system, with and without the addition of BRAF mutational status, was calculated. The c-index demonstrates the degree to which the model discriminates (0.5 - no predictive ability, 1 - perfect discrimination) between recurrent and nonrecurrent cases. A p-value < 0.05 was used to define statistical significance.
RESULTS
Cohort characteristics
All patients who underwent total thyroidectomy for PTC at Massachusetts General Hospital between 2000 and 2005 were included in the study cohort (Table 1). The mean age among the 356 patients meeting these criteria was 43.4 years (range 15 – 84), 87 were male (24.4%) and thirty had tumor diameters greater than 4 cm. Extrathyroidal extension was present in 66 cases: two with gross ETE (T4a) and 64 with minimal ETE (T3). AJCC-TNM staging distribution for the cohort was as follows: Stage I - 77.8%, Stage II - 3.9%, Stage III - 14.6%, and Stage IV - 3.7% (www.cancerstaging.org). 55 patients developed recurrence during the study period and the five-year disease-free survival was 84.8%. Of patients who recurred during the study period, mean time to recurrence was 1.94 (+/− 0.21) years. Overall five-year survival was 98.7%. Of the four deaths occurring within the study period, three were directly attributable to complications associated with distant PTC metastases.
Table 1.
Clinicopathologic characteristics of the patient cohort studied (N=356).
| Variable | Distribution (N, %) (n=356) | BRAF tested patients (n = 205) | No BRAF data (n = 151) | |
|---|---|---|---|---|
| Male | 87 (24.4) | 53 (25.9) | 34 (22.5) | 0.53 |
| Age at surgery (mean, SD) | 43.4 (±14.4) | 42.1 (±13.7) | 45.1 (±15.2) | 0.17 |
| AJCC TNM – Tumor stage | ||||
| < 2cm | 188 (53.0) | 102 (49.8) | 86 (57.3) | |
| 2–4 cm | 79 (22.3) | 48 (23.4) | 31 (20.7) | |
| > 4 cm or extrathyroidal extension | 88 (24.8) | 55 (26.8) | 33 (22.0) | 0.37 |
| Tumor subtype* | ||||
| Normal risk | 292 (81.8) | 163 (79.5) | 129 (85.4) | |
| Low risk | 56 (15.7) | 36 (17.6) | 19 (12.6) | |
| High risk | 9 (2.5) | 6 (2.9) | 3 (2.0) | 0.39 |
| Central neck LN positivity | 107 (30.1) | 58 (28.6) | 49 (32.5) | 0.49 |
| Lateral neck LN positivity | 47 (13.2) | 32 (15.6) | 15 (9.9) | 0.15 |
| Lymphovascular invasion | 26 (7.3) | 20 (9.8) | 6 (4.0) | 0.04 |
| Tumor multifocality | 172 (48.3) | 90 (44.1) | 82 (54.3) | 0.07 |
| Recurrences | 55 (15.5) | 30 (14.6) | 25 (16.6) | 0.10 |
| BRAFV600E+ | - | 110/205 (53.6) | - | |
Low risk – Follicular variant of PTC, warthin-like, cribiform-morular variant. Normal risk – classical PTC, mixed FVPTC/classical PTC, solid, oncocytic. High risk – tall cell variant, diffuse-sclerosing.
Within the study cohort, 252 archived paraffin-embedded surgical specimens were available for genotyping. Tumor DNA extraction was accomplished for 239 of these samples and BRAF genotyping was subsequently successful in 205 cases (81%). The BRAFV600E mutation was present in 110 (54%) of genotyped samples. No significant differences in tumor size, extrathyroidal extension, and proportion of patients who recurred existed between those patients with and without BRAF testing (Table 1). Moreover, five-year disease-free survival among the 205 patients for whom successful BRAF tumor genotyping was achieved was 85.9%, similar to the entire cohort (N = 356).
Crude hazard ratios associating each covariate with time to PTC recurrence are shown in Table 2. None of the patients with FVPTC (53/356) recurred, while recurrence did develop in 18.5% of classical PTC cases and in 25.0% of tumors featuring tall-cell variant histology. Variables associated with time to recurrence included male gender, T3 tumor stage, central or lateral neck lymph node involvement, lymphovascular invasion, tumor multifocality, and BRAFV600E mutation. 302 patients (85%) received adjuvant RAI following initial thyroidectomy for PTC, with the mean dose among these patients being 94 mCi. Adjuvant RAI therapy was not associated with recurrence risk on univariate analysis (p = 0.08) or when adjusted for stage (p = 0.13).
Table 2.
Assessment of associations between individual clinicopathologic tumor variables and time to PTC recurrence (events = 55).
| Variable | Hazard ratio | Confidence interval | p-value |
|---|---|---|---|
| Male | 2.08 | 1.21–3.59 | 0.01 |
| Age at surgery (per 10 yrs) | 1.02 | 0.85–1.23 | 0.83 |
| AJCC TNM - Tumor stage | |||
| T1 (< 2cm (reference)) | |||
| T2 (2–4 cm) | 1.06 | 0.50–2.24 | 0.88 |
| T3 (≥ 4 cm or minimal extrathyroidal extension) | 2.34 | 1.30–4.23 | 0.005 |
| T4 (gross ETE) | 4.30 | 0.58–31.9 | 0.15 |
| Tumor histological subtype | |||
| Normal risk (reference) | |||
| Low risk* | 0.00 | - | - |
| High risk | 1.20 | 0.29–4.94 | 0.80 |
| Central neck LN positivity | 1.87 | 1.10–3.19 | 0.02 |
| Lateral neck LN positivity | 2.69 | 1.48–4.86 | 0.001 |
| Lymphovascular invasion | 2.89 | 1.45–5.73 | 0.002 |
| Tumor multifocality | 2.11 | 1.21–3.67 | 0.01 |
| BRAFV600E+ | 2.62 | 1.17–5.88 | 0.02 |
No patients with low risk histological subtypes recurred leading to model non-convergence.
Applying the AMES, MACIS, AJCC-TNM, and ATA algorithms to our cohort afforded moderate recurrence discrimination in each case, with c-index range varying between 0.554 and 0.627 (Table 3). Of the tested risk classification systems, most were associated with 5-year recurrence on univariate analysis; AMES (HR 3.98 [1.77–8.96]), MACIS score (HR 1.37 [1.04–5.54]), AJCC-TNM stage (HR 1.35 [0.96, 1.90]), and ATA recurrence-risk category (HR 2.26 [1.16–4.40]). BRAFV600E was independently associated with risk of recurrence when added to each model; AMES (HR 2.43 [1.08–5.49]), MACIS score (HR 2.46 [1.09–5.54]), AJCC-TNM stage (HR 2.51 [1.11, 5.66]), and ATA recurrence-risk category (HR 2.44 [1.08–5.50]) and improved model discrimination (incremental c-index range 0.046–0.109) (Table 3). Kaplan-Meier curves stratified by collapsed risk-algorithm categories and BRAF status illustrate this relationship (Figure 1). The log-rank estimate is significant in all cases.
Table 3.
Discrimination of conventional classification systems and additive predictive value of BRAF status.
| Model | c-index (95% CI) | c-index with BRAF (95% CI) |
|---|---|---|
| AMES (low versus high risk) | 0.597 (0.519, 0.675) | 0.676 (0.590, 0.761) |
| MACIS categories (< 6, 6–6.99, 7–7.99, ≥ 8) | 0.556 (0.486, 0.626) | 0.665 (0.584, 0.746) |
| AJCC-TNM stage | 0.555 (0.469, 0.641) | 0.638 (0.550, 0.726) |
| ATA recurrence risk (low versus intermediate/high) | 0.628 (0.539, 0.716) | 0.674 (0.584, 0.765) |
Fig 1.
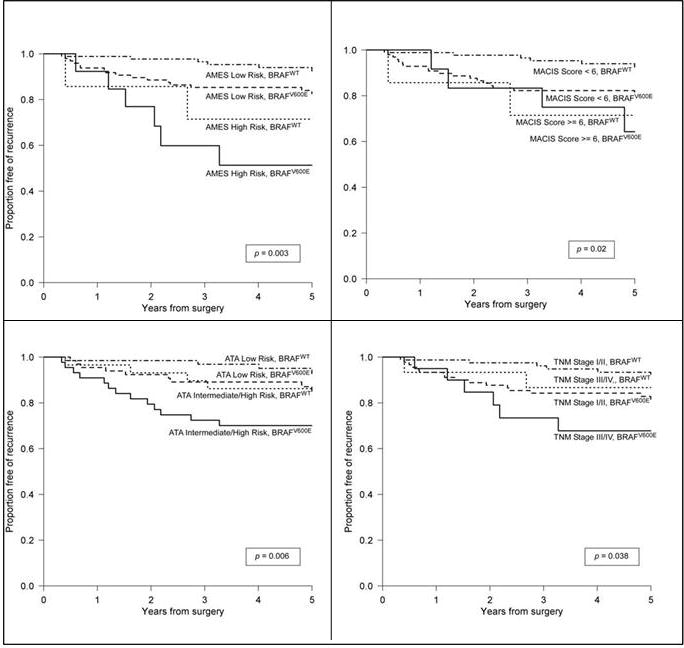
Disease-free survival within the study cohort, organized by specific PTC prognosis algorithm, with and without inclusion of BRAF mutational status.
DISCUSSION
The natural history of PTC and, possibly, the responsiveness of this malignancy to therapy, may be estimated on the basis of associated clinical, histologic and genetic variables. A number of algorithms incorporating these variables have been designed to estimate disease prognosis following initial PTC treatment. These algorithms include the AMES, MACIS, AJCC-TNM, and ATA algorithms. In each case, these models have been shown to provide moderate discrimination in predicting disease recurrence among patients treated for PTC.
B-Raf is a serine/threonine protein kinase member of the MAP kinase signal transduction cascade. Disregulation of this mitogenic pathway is associated with malignant transformation and somatic mutation of the BRAF gene, encoding the B-Raf protein, may be directly oncogenic in multiple cell types19. The BRAFV600E mutation, in particular, is the most common oncogene found in PTC and is associated with enhanced tumor aggressiveness10. Prior work demonstrates increased risk of extrathyroidal tumor extension, lymph node metastasis and advanced TNM stage among PTC patients harboring the BRAFV600E mutation, which may also modulate the responsiveness of PTC to medical therapies9–11, 13, 19–21. In addition, Niemeier and colleagues provided initial evidence of an association between BRAF mutational status, clinicopathologic tumor features and recurrence risk using data from a case controlled study of papillary thyroid microcarcinomas, and these authors were able to develop an associated scoring system for assessment of recurrence risk in microPTC22. Most of these studies, however, do not assay a consecutive series of unselected patients and use cohorts enriched with tall cell PTC variants, tumors known to harbor BRAF mutations, and/or particularly aggressive tumors.
In this study, we assess the additive utility of BRAF mutational status in refining existing PTC risk stratification systems. Our data confirm previously identified variables associated with time to disease recurrence, including advanced tumor stage, cervical lymph node involvement, lymphovascular invasion and tumor multifocality. Interestingly, our data demonstrate an increased risk of disease recurrence among patients with lateral cervical lymph node involvement (N1b) compared to that associated with central cervical nodal metastases. In addition, BRAF mutational status was found to be an independent predictor of time to PTC recurrence. Application of BRAF mutational status to the AMES, MACIS, AJCC-TNM, and ATA prognostic algorithms was independently associated with disease recurrence in each case and model discrimination, although moderate, improved. Of note, recurrence risk discrimination, without inclusion of BRAF mutational status, was best for the ATA algorithm (Table 3). This finding is not surprising, given that the ATA instrument is the only system of the four tested that was originally designed to assess recurrence risk.
There are a number of potential limitations to our study. Of the 356 patients in our unselected cohort, archived tumor tissue was available in 239 cases. Of this subgroup, BRAF genotyping was successful for 205 specimens, with the age of archived, paraffin-embedded tissues correlating inversely with the probability of successful BRAF mutational status determination. Thus, assessment of the incremental value of BRAF genotyping to the predictive capacity of each algorithm was possible for 205 members of our 356 patient cohort (57.6%). Despite this limitation, our study proved to be of sufficient power to identify a statistically significant improvement in recurrence discrimination when BRAF mutational status was included in each model. Possible misclassification of data is expected to be nondifferential and therefore bias towards the null hypothesis. Finally, it is important to note that although each cohort member studied did undergo total thyroidectomy for PTC, the extent of additional surgical exploration, in particular with regard to prophylactic lymph node sampling, was not uniform and was difficult to verify retrospectively (i.e. prophylactic versus therapeutic central lymph node dissection). While our data do demonstrate a statistically significant association between the presence of cervical lymph node metastasis and disease recurrence, we found no significant relationship between the absolute number of level VI lymph nodes resected and recurrence risk. Whether the performance of prophylactic lymph node dissection impacts PTC recurrence risk is a matter of debate23–25. It is still possible that variations in the extent of nodal dissection performed between patients may have exerted an unquantifiable effect on the recurrence rate within the cohort population.
Taken together, the data presented in this report demonstrate that the addition of BRAF gene mutational status adds incrementally to the predictive capacity of established PTC risk stratification systems. For each scoring system, assessment of BRAF mutational status resulted in a statistically significant increase in discriminatory accuracy. We also confirm that BRAF mutational status is, in itself, an independent predictor of time to PTC recurrence. Given our findings, the development of novel prediction model that includes mutational status of BRAF and potentially other genes (e.g. KRAS), is warranted. The addition of tumor subtypes, as suggested in the ATA guideline risk-system, may also add incrementally to recurrence prediction. A larger sample size with corresponding mutational status will allow for a more refined prediction model.
Acknowledgments
We would like to thank the following persons for their contributions to this work: Julie Miller, MS, Professional Research Assistant; Nancy R. Cook, ScD, Associate Professor of Medicine (Biostatistics), Harvard Medical School; Elkan F. Halpern, PhD, Director of Statistics, Instructor in radiology/statistics, Harvard medical school.
This work was funded in part by a grant award from the Ellison Foundation (Daniels) and by the Program in Cancer Outcomes Research Training Grant (NCI R25CA092203) (Lubitz)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Chen AY, Jemal A, Ward EM. Increasing incidence of differentiated thyroid cancer in the United States, 1988–2005. Cancer. 2009;115:3801–7. doi: 10.1002/cncr.24416. [DOI] [PubMed] [Google Scholar]
- 2.Davies L, Welch HG. Increasing incidence of thyroid cancer in the United States, 1973–2002. JAMA. 2006;295:2164–7. doi: 10.1001/jama.295.18.2164. [DOI] [PubMed] [Google Scholar]
- 3.Enewold L, Zhu K, Ron E, et al. Rising thyroid cancer incidence in the United States by demographic and tumor characteristics, 1980–2005. Cancer Epidemiol Biomarkers Prev. 2009;18:784–91. doi: 10.1158/1055-9965.EPI-08-0960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hay ID, Bergstralh EJ, Goellner JR, Ebersold JR, Grant CS. Predicting outcome in papillary thyroid carcinoma: development of a reliable prognostic scoring system in a cohort of 1779 patients surgically treated at one institution during 1940 through 1989. Surgery. 1993;114:1050–7. discussion 7-8. [PubMed] [Google Scholar]
- 5.Seaberg RM, Eski S, Freeman JL. Influence of previous radiation exposure on pathologic features and clinical outcome in patients with thyroid cancer. Arch Otolaryngol Head Neck Surg. 2009;135:355–9. doi: 10.1001/archoto.2009.13. [DOI] [PubMed] [Google Scholar]
- 6.Mazeh H, Benavidez J, Poehls JL, Youngwirth L, Chen H, Sippel RS. In patients with thyroid cancer of follicular cell origin, a family history of nonmedullary thyroid cancer in one first-degree relative is associated with more aggressive disease. Thyroid. 2012;22:3–8. doi: 10.1089/thy.2011.0192. [DOI] [PubMed] [Google Scholar]
- 7.Kazaure HS, Roman SA, Sosa JA. Aggressive Variants of Papillary Thyroid Cancer: Incidence, Characteristics and Predictors of Survival among 43,738 Patients. Ann Surg Oncol. 2011 doi: 10.1245/s10434-011-2129-x. [DOI] [PubMed] [Google Scholar]
- 8.Asioli S, Erickson LA, Sebo TJ, et al. Papillary thyroid carcinoma with prominent hobnail features: a new aggressive variant of moderately differentiated papillary carcinoma. A clinicopathologic, immunohistochemical, and molecular study of eight cases. Am J Surg Pathol. 2010;34:44–52. doi: 10.1097/PAS.0b013e3181c46677. [DOI] [PubMed] [Google Scholar]
- 9.Xing M, Westra WH, Tufano RP, et al. BRAF mutation predicts a poorer clinical prognosis for papillary thyroid cancer. J Clin Endocrinol Metab. 2005;90:6373–9. doi: 10.1210/jc.2005-0987. [DOI] [PubMed] [Google Scholar]
- 10.Xing M. BRAF mutation in papillary thyroid cancer: pathogenic role, molecular bases, and clinical implications. Endocr Rev. 2007;28:742–62. doi: 10.1210/er.2007-0007. [DOI] [PubMed] [Google Scholar]
- 11.Elisei R, Ugolini C, Viola D, et al. BRAF(V600E) mutation and outcome of patients with papillary thyroid carcinoma: a 15-year median follow-up study. J Clin Endocrinol Metab. 2008;93:3943–9. doi: 10.1210/jc.2008-0607. [DOI] [PubMed] [Google Scholar]
- 12.Kim TY, Kim WB, Rhee YS, et al. The BRAF mutation is useful for prediction of clinical recurrence in low-risk patients with conventional papillary thyroid carcinoma. Clin Endocrinol (Oxf) 2006;65:364–8. doi: 10.1111/j.1365-2265.2006.02605.x. [DOI] [PubMed] [Google Scholar]
- 13.Nucera C, Porrello A, Antonello ZA, et al. B-Raf(V600E) and thrombospondin-1 promote thyroid cancer progression. Proc Natl Acad Sci U S A. 2010;107:10649–54. doi: 10.1073/pnas.1004934107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nucera C, Lawler J, Parangi S. BRAF(V600E) and microenvironment in thyroid cancer: a functional link to drive cancer progression. Cancer Res. 2011;71:2417–22. doi: 10.1158/0008-5472.CAN-10-3844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cooper DS, Doherty GM, Haugen BR, et al. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009;19:1167–214. doi: 10.1089/thy.2009.0110. [DOI] [PubMed] [Google Scholar]
- 16.Sanders LE, Cady B. Differentiated thyroid cancer: reexamination of risk groups and outcome of treatment. Arch Surg. 1998;133:419–25. doi: 10.1001/archsurg.133.4.419. [DOI] [PubMed] [Google Scholar]
- 17.Dias-Santagata D, Lam Q, Bergethon K, et al. A potential role for targeted therapy in a subset of metastasizing adnexal carcinomas. Mod Pathol. 2011;24:974–82. doi: 10.1038/modpathol.2011.48. [DOI] [PubMed] [Google Scholar]
- 18.Phadke PA, Rakheja D, Le LP, et al. Proliferative nodules arising within congenital melanocytic nevi: a histologic, immunohistochemical, and molecular analyses of 43 cases. Am J Surg Pathol. 2011;35:656–69. doi: 10.1097/PAS.0b013e31821375ea. [DOI] [PubMed] [Google Scholar]
- 19.Davies H, Bignell GR, Cox C, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949–54. doi: 10.1038/nature00766. [DOI] [PubMed] [Google Scholar]
- 20.Lee JH, Lee ES, Kim YS. Clinicopathologic significance of BRAF V600E mutation in papillary carcinomas of the thyroid: a meta-analysis. Cancer. 2007;110:38–46. doi: 10.1002/cncr.22754. [DOI] [PubMed] [Google Scholar]
- 21.Nehs MA, Nucera C, Nagarkatti SS, et al. Late intervention with anti-BRAF(V600E) therapy induces tumor regression in an orthotopic mouse model of human anaplastic thyroid cancer. Endocrinology. 2012;153:985–94. doi: 10.1210/en.2011-1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Niemeier LA, Akatsu HK, Song C, et al. A combined molecular-pathologic score improves risk stratification of thyroid papillary microcarcinoma. Cancer. 2011;118(8):2069–77. doi: 10.1002/cncr.26425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Costa S, Giugliano G, Santoro L, et al. Role of prophylactic central neck dissection in cN0 papillary thyroid cancer. Acta Otorhinolaryngol Ital. 2009;29:61–9. [PMC free article] [PubMed] [Google Scholar]
- 24.Sywak M, Cornford L, Roach P, Stalberg P, Sidhu S, Delbridge L. Routine ipsilateral level VI lymphadenectomy reduces postoperative thyroglobulin levels in papillary thyroid cancer. Surgery. 2006;140:1000–5. doi: 10.1016/j.surg.2006.08.001. discussion 5-7. [DOI] [PubMed] [Google Scholar]
- 25.Zuniga S, Sanabria A. Prophylactic central neck dissection in stage N0 papillary thyroid carcinoma. Arch Otolaryngol Head Neck Surg. 2009;135:1087–91. doi: 10.1001/archoto.2009.163. [DOI] [PubMed] [Google Scholar]


