Abstract
People with lower extremity peripheral artery disease (PAD) have greater functional impairment and faster functional decline than those without PAD. We describe methods for the Group Oriented Arterial Leg Study (GOALS), an ongoing randomized controlled clinical trial designed to determine whether a Group-Mediated Cognitive Behavioral (GMCB) intervention improves functional performance in PAD participants, compared to a health education control condition. In GOALS, PAD participants are randomized to either an intervention or a health education control condition in a parallel design. Both conditions consist of weekly group sessions with other PAD participants. In the intervention, cognitive behavioral techniques are used to assist participants in setting and adhering to home-based walking exercise goals. Participants are encouraged to walk for exercise at home at least five days per week. In the control condition, participants receive lectures on health-related topics. After six months of on-site weekly sessions, participants are transitioned to telephone follow-up for another six months. Participants in the intervention are asked to continue home walking exercise. The primary outcome is change in six-minute walk performance between baseline and six-month follow-up. Secondary outcomes include change in six-minute walk performance at 12-month follow-up, and change in treadmill walking performance, the Walking Impairment Questionnaire, quality of life, and physical activity at six and 12-month follow-up. In conclusion, if our group-mediated cognitive behavioral intervention is associated with improved walking performance in individuals with PAD, results will have major public health implications for the large and growing number of people with PAD.
Keywords: Peripheral artery disease, intermittent claudication, clinical trial, exercise
Introduction
Eight million men and women in the United States and 10–15% of community dwelling men and women age 65 and older have lower extremity peripheral arterial disease (PAD) (1). Patients with PAD have greater functional impairment, increased rates of functional decline, and increased mobility loss compared to persons without PAD (2–6). Even asymptomatic PAD, or PAD that is not accompanied by exertional leg symptoms, is associated with greater functional impairment and faster functional decline compared to individuals without PAD (2,3,5). The functional impairment associated with PAD contributes to a loss of independence, higher rates of hospitalization, and poor quality of life (6,7).
Few therapies have been identified that improve lower extremity functioning or prevent functional decline in PAD. Supervised treadmill exercise programs significantly improve walking performance in patients with PAD, including among those with and without intermittent claudication symptoms (8,9). These supervised treadmill exercise programs require attending exercise sessions at an exercise facility at least three times per week. However, supervised exercise programs can be expensive and are not typically paid for by medical insurance, including Medicare. Transportation to an exercise facility three or more times weekly can be logistically challenging and costly. Because of these barriers, few PAD patients participate in supervised exercise programs (10,11).
Current clinical practice guidelines indicate that there are insufficient data to support the effectiveness of home-based walking exercise programs in patients with PAD (12). Prior studies have yielded mixed results regarding the ability of home-based exercise programs to improve walking performance in patients with PAD (13–17). However, in some prior studies, sample sizes were extremely small and did not structure the intervention to specifically target barriers to home-based walking exercise.
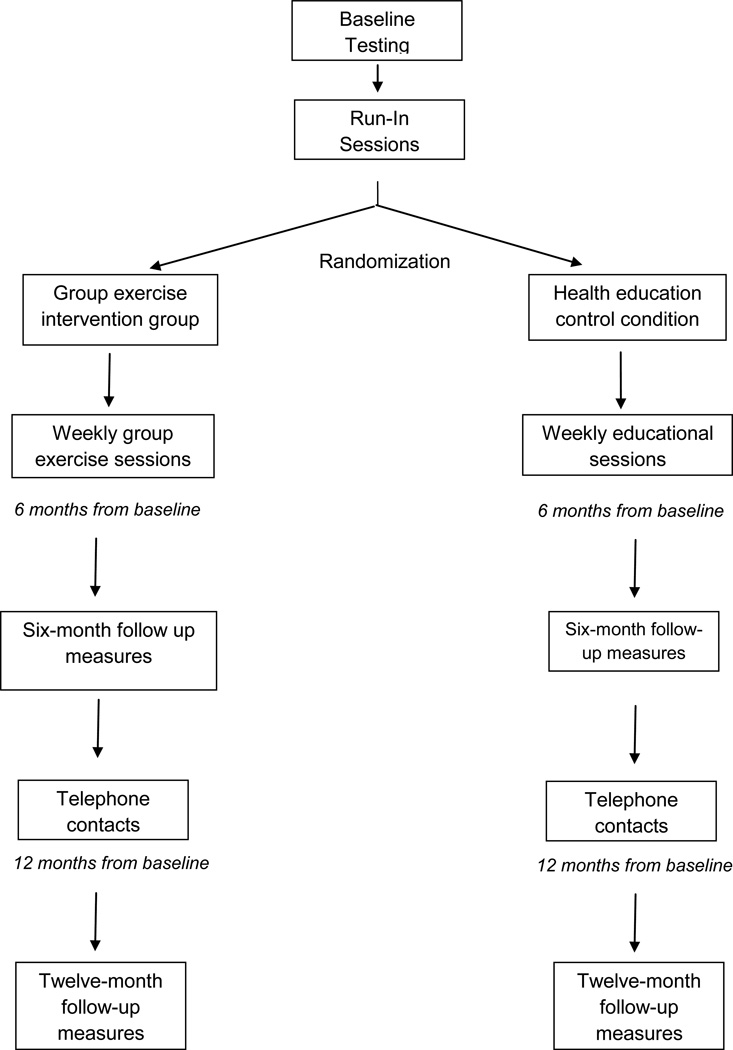
The Group Oriented Arterial Leg Study (GOALS) was designed to determine whether weekly group sessions with a trained facilitator can help PAD patients adhere to a home-based walking exercise intervention, resulting in improved six-minute walk performance at six-month follow-up in participants with PAD. If successful, the intervention will provide a novel therapeutic option to improve functional performance and prevent mobility loss in the large and growing number of patients disabled by PAD.
Methods
Overview
The institutional review board of Northwestern University approved the protocol. All participants provide written informed consent. Because our power calculations are based on 100 participants completing each arm, and because we anticipated a drop-out rate of 18%, the GOALS Trial aimed to recruit a total of 240 participants.
Eligibility
The inclusion criterion is an ankle brachial index (ABI) ≤ 0.90. Potential participants with a resting ABI ≥ 0.91 and ≤ 1.00 at baseline are eligible if their ABI drops by at least 20% following a heel-rise test (18,19). Prior study shows that an ABI decline > 20% after the heel-rise test is highly correlated with ABI declines after a treadmill exercise stress test (18,19). Potential participants with a resting ABI greater than 0.90 are also potentially eligible if they provide data from a certified vascular laboratory demonstrating prior lower extremity ischemia or if they have medical record documented evidence of prior lower extremity revascularization. We included participants both with and without classic symptoms of intermittent claudication, since prior studies show that even PAD patients without intermittent claudication symptoms have greater functional impairment and faster functional decline, compared to individuals without PAD (2–5). In addition, prior studies show that PAD participants both with and without intermittent claudication symptoms significantly improve their walking performance in response to a supervised treadmill exercise intervention (8). Exclusion criteria and justification for each criterion are listed in Table 1.
Table 1.
Exclusion Criteria and Justification for Exclusion Criteria in the Group Oriented Arterial Leg Study (GOALS) trial.
| Exclusion Category/Justification for Exclusion | Specific Exclusion criteria |
|---|---|
| Exclusion criteria selected because they may interfere with the participant’s ability to participate fully in the study intervention | Below or above-knee amputation |
| Wheelchair confinement | |
| Inability to walk at least 50 during the six-minute walk test without stopping | |
| Use of a walking aid (excluding canes) | |
| Unable/ unwilling to return to medical center weekly for six months | |
| Failure to complete study run-in phase within three weeks | |
| Walking impairment primarily limited by a cause other than PAD | |
| Stopping during treadmill/ six-minute walk for symptoms other than leg ischemia | |
| Current foot ulcer or critical limb ischemia | |
| Significant visual or hearing impairment | |
| Poor fit for study based on investigator judgment | |
| Exclusion criteria selected because they may influence study outcomes independently of study participation | Major surgery, lower extremity revascularization, or myocardial infarction during the previous three months |
| Major surgery or lower extremity revascularization planned within the next 12 months | |
| Major medical illness including treatment for cancer during previous 12 months | |
| Current participation in other clinical trial or participation in another exercise trial within the last year | |
| Completion of cardiac rehabilitation within the last three months | |
| Parkinson’s Disease | |
| Requires oxygen with activity or exercise | |
| Criteria selected because they indicate that study participation may be unsafe. | >Class II New York Heart Association heart failure or angina (at rest or with minimal exertion) |
| Increase in angina pectoris symptoms during the previous six months | |
| Baseline stress test consistent with coronary ischemia, indeterminate for presence of coronary ischemia, or left bundle branch block without a perfusion stress test demonstrating no reversible ischemia within previous three months1 | |
| Criteria selected because they may influence the ability to obtain accurate responses to study questionnaires | Mini-Mental Status Examination score <23 |
These participants may potentially become eligible at a later date if they undergo a stress test with imaging with their physician and the results show no active ischemia.
Recruitment and screening
Participants are identified from Chicago and surrounding regions using newspaper and radio advertising, targeted mailings to community-dwelling men and women over age 65, mailed letters to patients diagnosed with PAD in the non-invasive vascular laboratories at Northwestern Memorial Hospital, posted flyers, and outreach to vascular surgeons at Northwestern Memorial Hospital.
Potential participants are first screened for eligibility by telephone. Those who are eligible and interested after the telephone eligibility interview attend a baseline study visit. Participants who are still eligible after completing the first baseline visit are asked to return for a second visit for a treadmill exercise stress test.
Run-in period
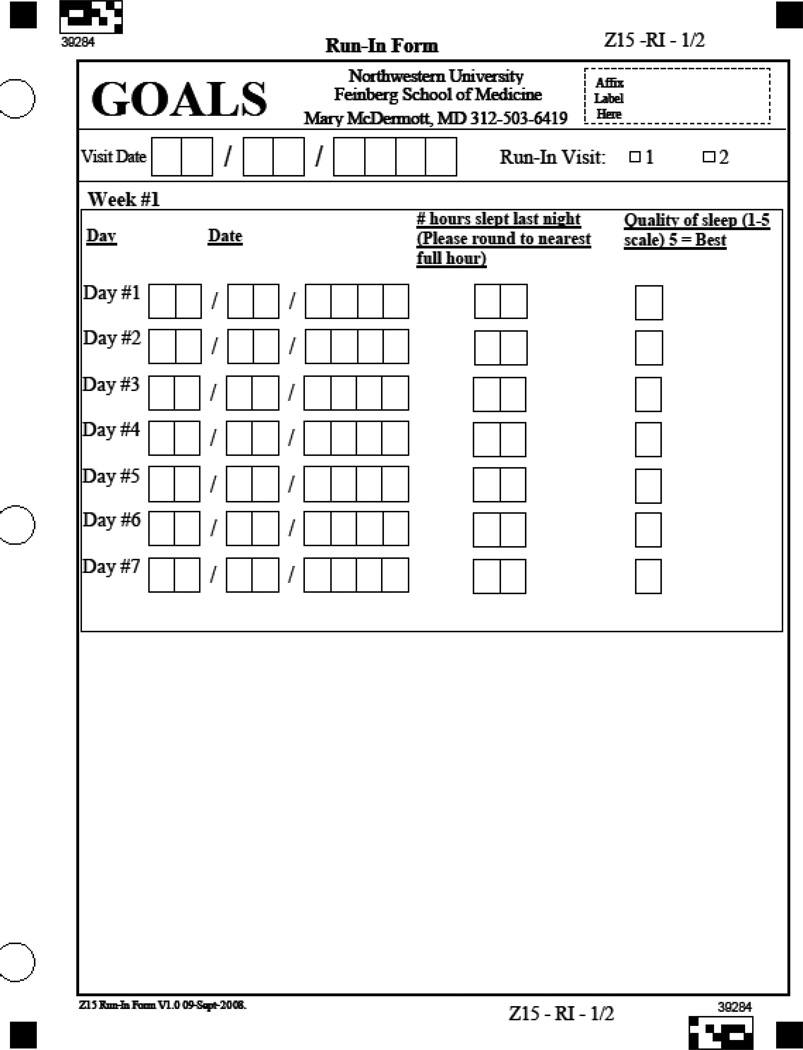
To identify potential participants who may not adhere to the weekly on-site sessions, participants are asked to attend two one-hour health education run-in sessions after completing baseline testing. These two sessions must be attended within three weeks in order for participants to remain eligible. To help ensure that participants randomized to the intervention will adhere to the requirement to record walking exercise performed each day, all potential participants are asked to record the number of hours slept each night and the quality of sleep using a 1 to 5 scale (Appendix A). Participants are asked to complete these sleep records during a two week period.
Outcome measures
Study outcome measures are listed in Table 2 along with timing of follow-up testing.
Table 2.
Study outcome measures
| Measurement Time Point | |||
|---|---|---|---|
| Baseline | Six-month follow-up. |
Twelve-month follow-up | |
| Six-minute walk | X | X1 | X2 |
| Treadmill walking performance | X | X2 | |
| Walking Impairment Questionnaire | X | X2 | X2 |
| Short-Form 12 (Health-Related Quality of Life Measure) | X | X2 | X2 |
| Physical activity level | X | X2 | X2 |
| Measures of behavioral mediators4 | X | X3 | X3 |
1=Primary outcome measure
2=Secondary outcome measure
3=Exploratory outcome measure
4=These measures are detailed in Table 3.
Six-minute walk
PAD patients have limited walking endurance (3,4). The six-minute walk test is an objective measure of walking endurance that is well validated in PAD patients and has excellent test re-test reliability in PAD (8). Treadmill walking performance has been traditionally used to measure change in walking endurance in response to interventions in PAD participants and has advantages of being performed at controlled speed and grade. Although the six-minute walk is self-paced, the six-minute walk has some advantages over treadmill testing as a primary outcome (20–22). For example, treadmill walking is associated with balance problems and anxiety in older patient populations (21–22). In contrast, corridor walking, measured with the six-minute walk, is more familiar and acceptable to older study participants, such as those with PAD (21–22).
Following a standardized protocol, participants walk up and down a 100-foot hallway for up to six minutes with instruction to cover as much distance as possible (3,4,8). Distance completed after six minutes and distance at onset of leg pain is recorded.
Treadmill walking performance
Maximal treadmill walking distance and distance to onset of ischemic leg symptoms during treadmill walking are measured using the Gardner-Skinner protocol (23). In the Gardner protocol, speed is maintained at 2.0 miles per hour (mph) and the grade increases by 2% every two minutes. If participants are unable to walk at 2.0 mph, treadmill speed is started at 0.5 mph and increased by 0.5 mph every two minutes until the speed reaches 2.0 mph, after which the grade is increased by 2% every two minutes.
Quality of life and participant reported walking impairment
The Physical Functioning domain of the Medical Outcome Study Short Form-12 (SF-12) is used to assess functional status (24). The SF-12 is well-validated as compared to the original SF-36 questionnaire and minimizes participant burden (24,25). Participant self-assessment of walking performance is measured using the distance, speed, and stair-climbing scores from the Walking Impairment Questionnaire (WIQ). The WIQ is a PAD-specific measure of self-reported walking limitations including walking distance, walking speed, and stair climbing (26). Each domain is scored on a scale of 0–100, with 0 representing the most extreme limitation and 100 representing no difficulty.
Physical activity
Habitual physical activity is measured objectively over 7 days using a vertical accelerometer (Caltrac, Muscle Dynamics Fitness Network, Inc., Torrance, California) according to established methods (27–29). The vertical accelerometer is programmed with identical information for all participants to allow comparison of physical activity regardless of variation in participant age, sex, and body mass index.
Targeted behavioral mediators of our intervention
The study intervention is designed to target barriers to physical activity in individuals with PAD. A series of questionnaires, listed in Table 3, allows for measurement of change in potential behavioral barriers to exercise in response to the intervention
Table 3.
Measures of change in targeted behavioral mediators of the study intervention
| Proposed Mechanism | Measurement Plan |
|---|---|
| 1. Desire for physical competence | 1. Desire for Physical Competence Questionnaire |
| 2. Self-efficacy for functional performance | 2. Walking Efficacy Questionnaire |
| 3. Satisfaction with physical function | 3. Physical Function Satisfaction Questionnaire |
| 4. Efficacy for barriers to exercise | 4. Barriers Efficacy Questionnaire |
| 5. Acceptance of exertional leg pain | 5. Pain Perception Questionnaire |
| 6. Control for functional independence | 6. Beliefs about Physical Activity Questionnaire |
Desire for physical competence questionnaire and walking efficacy questionnaire
Desire for physical competence reflects older adults’ motivation to perform tasks requiring distinct physiological demands (30). In the GOALS trial, the intervention aims to increase PAD participants’ self-efficacy for their ability to walk varying distances at a brisk pace without stopping. The walking efficacy questionnaire is administered to assess patients’ confidence in their ability to walk various distances at a brisk pace without stopping. The desire for physical competence questionnaire is administered to assess participants’ desire to walk each corresponding distance at a brisk pace without stopping. The response uses a 5-point scale ranging from “No Desire Whatsoever” to “Very Strong Desire”. When administered to 62 participants with PAD on two occasions, these questionnaires had good test re-test reliability (r=0.77), respectively (31).
Satisfaction with physical function
Satisfaction with physical function is assessed by the physical function satisfaction questionnaire, a measure validated by Reboussin et al (32). The physical function subscale has six items. Each item is rated on a 7-point scale ranging from −3 to +3. Numbers on the scale are anchored by the following phrases: very dissatisfied (−3), somewhat dissatisfied (−2), a little dissatisfied (−1), neither (0), a little satisfied (+1), somewhat satisfied (+2), very satisfied (+3). Scores are averaged to yield a total score ranging from −3 to +3. Previous study shows that this measure has an alpha reliability above 0.90 and changes in response to physical activity interventions (32,33).
Efficacy for barriers to exercise
A barriers efficacy questionnaire (fourteen items, score range = 0–140) is used to evaluate social problem solving necessary to address barriers to changing behavior (34). Previous study in 62 participants with PAD demonstrated a test re-test reliability of r=0.66.
Acceptance of exertional leg pain
Exertional leg symptoms are a significant barrier to walking activity in individuals with PAD. Thus, a successful intervention to increase walking activity in PAD should in part focus on management of walking-related leg discomfort. The pain perception questionnaire (score range = 0–98) is derived from the chronic pain acceptance questionnaire (CPAQ) (35–36) and measures the association of the intervention with changes in acceptance of exertional leg pain in the study population. Previous study in 62 participants with PAD demonstrated good test re-test reliability (r =0.74) (31).
Control for functional independence
The beliefs about physical activity questionnaire (score range = 0–18) evaluates patients’ control of their functional abilities. This questionnaire is used because the GOALS intervention is designed to enhance patients’ perceived control over their ability to perform daily activities despite the limitations imposed by their disease. Previous study in 62 participants with PAD demonstrated acceptable test re-test reliability (r =0.75) (31).
Additional measures
Leg symptoms
Leg symptoms are characterized using the San Diego claudication questionnaire (37). Intermittent claudication is defined as exertional calf pain that does not begin at rest, necessitates walking to be ceased, and resolves within 10 minutes of rest.
Ankle brachial index
The ABI is measured to determine eligibility of potential participants. Because prior randomized controlled trials of supervised treadmill exercise in participants with PAD did not demonstrate changes in the ABI after exercise, the ABI is not repeated after the six-minute walk or treadmill stress test [8,9]. After participants rest supine for five minutes, a hand-held Doppler probe (Nicolet Vascular Pocket Dop II, Golden, CO) is used to measure systolic pressures in this order: right brachial, dorsalis pedis, and posterior tibial arteries and left dorsalis pedis, posterior tibial, and brachial arteries. Pressures are repeated in reverse order, based on prior study (3,4,7). The ABI is calculated in each leg by dividing average pressures in each leg by the average of the four brachial pressures (38). Average brachial pressures in the arm with highest pressure were used when one brachial pressure was higher than the opposite brachial pressure in both measurement sets, and the two brachial pressures differed by 10 or more mm Hg in at least one measurement set, since in such cases subclavian stenosis was possible (39). Previous study demonstrates that this method of ABI measurement is associated meaningfully with the degree of functional impairment and rates of functional decline among older men and women (4,40). Participants with a resting ABI of 0.90 to 0.99 underwent a heel-rise test, in which the ABI was repeated after 50 heel-rises performed at a rate of one per second (41,42). Individuals with a resting ABI of 0.90 to 0.99 whose ABI dropped by ≥ 20% after the heel-rise test were eligible for study participation (41,42). Previous studies demonstrate that a drop in the ABI after heel-rise exercise is strongly correlated with the degree of drop in the ABI after an exercise treadmill stress test (41,42).
Randomization
Randomization is performed approximately every three months, once a minimum of 12 eligible participants have accrued. This method helps ensure that each study condition (intervention and health education control) has approximately six participants in each group. The GMCB intervention relies in part on the ability of group support to assist participants in achieving their walking goals. Therefore, a minimum of six participants in each intervention group was considered ideal. Subjects who were not randomized within three months of their baseline testing were required to repeat baseline testing before randomization. In addition, subjects who experienced major changes in their clinical status between baseline testing and randomization, such as a myocardial infarction, coronary revascularization, or lower extremity revascularization were required to repeat baseline testing and re-evaluate eligibility before randomization.
Eligible participants are randomly assigned by computer to either the GMCB intervention or the health education control condition using a randomly permuted block method. Randomization is stratified by whether the participant’s six-minute walk performance is above vs. below the baseline median value for the six-minute walk test in our previously randomized controlled clinical trial of supervised treadmill exercise (7).
Study interventions
During the first six months following randomization, participants attend weekly sessions at Northwestern medical center in their respective groups (GMCB intervention vs. health education control condition) (Phase I). After completing six-month follow-up testing, participants are transitioned to telephone contact with study staff (Phase II). In this phase of the study, staff members contact participants by telephone bi-weekly for the first three months of Phase II and monthly for the final three months of Phase II.
GMCB intervention (phase I)
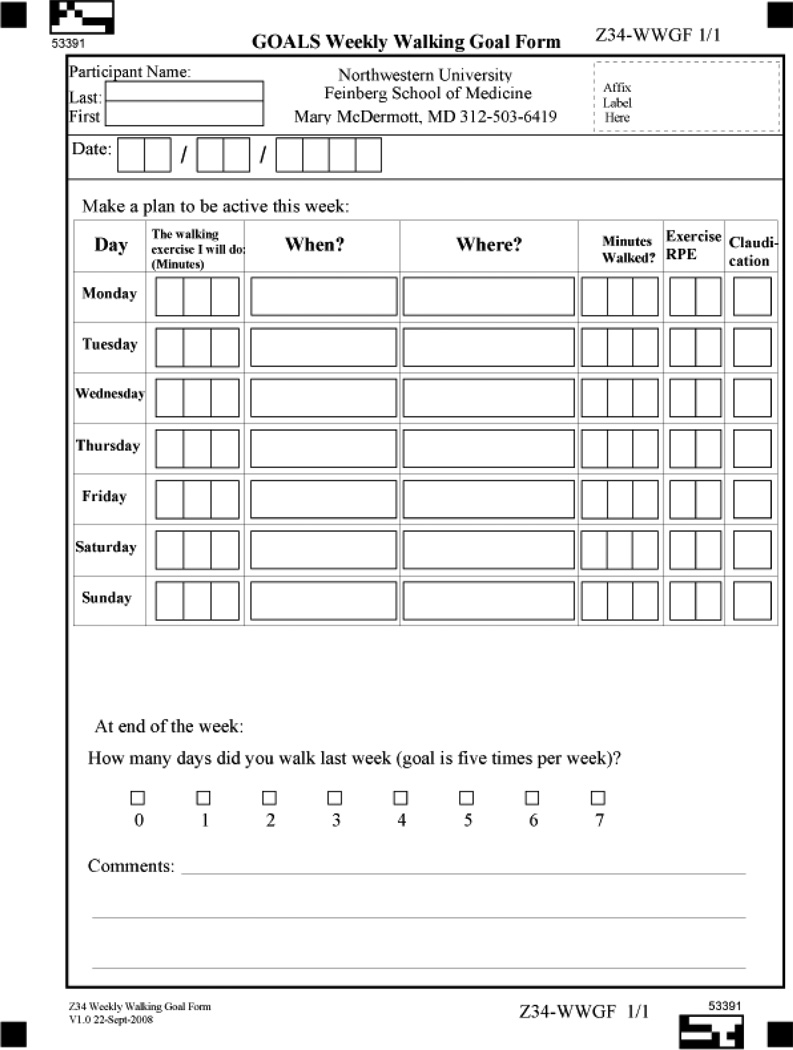
The GMCB intervention is designed to help participants set and adhere to daily walking exercise goals. During the weekly sessions, participants meet in a group with other PAD participants, led by a trained facilitator. The sessions last for approximately 90 minutes, with 45 minutes devoted to facilitator-led discussions of adhering to home-based walking exercise and 45 minutes devoted to walking exercise around an indoor track at the exercise facility. Each group discussion has a specific topic, led by the facilitator, with a handout for study participants. Session topics include an overview of PAD, benefits of walking exercise for PAD, goal-setting, self-monitoring, and managing pain and fatigue during exercise. A list of all topics is shown in Table 4. Group discussion is encouraged and participants are asked to share personal challenges and triumphs. At the end of each group session, participants complete a walking goal form (see Appendix B), on which they are asked to list specific walking goals for at least five days during the coming week. Participants are encouraged to engage in over-ground walking (rather than treadmill walking), since over-ground walking more closely simulates walking activity in daily life. Participants are encouraged to walk until they experience severe leg discomfort (i.e. a severity of 4 or 5 on a scale of 0–5). They are then advised to rest until the leg discomfort subsides sufficiently that they can resume walking. Participants who do not experience exertional leg symptoms are asked to walk to an intensity of 12–14 on the Borg Rating of Perceived Exertion (RPE) scale (40). During the week they are asked to record their actual walking activity each day, the severity of their leg pain on a 0–5 scale during exercise, and their rating of perceived exertion (RPE) during exercise using the Borg scale (40). Participants return their completed forms to the facilitator at each weekly session. The facilitator reviews each completed form and provides brief individual feedback to participants. Individuals are asked to increase their walking activity over time with the goal of achieving at least 50 minutes of walking per session at least five days per week. The rate of increase in walking duration is individualized according to participants’ ability.
Table 4.
Group Mediated Cognitive Behavioral Topics Covered During Phase I of the Intervention
|
GMCB intervention (phase II)
After completing six-month follow-up testing, participants are transitioned to an exclusively home-based exercise program for the final six months of the study (Phase II). In this phase of the study, participants are assisted with continued adherence to their home-based exercise program by telephone contact. The group facilitator telephones participants weekly for the first three months and monthly for the final three months. The telephone calls last approximately 10 minutes. During these telephone contacts, the facilitator reviews the participant’s walking progress and assists them with overcoming barriers to exercise. Participants are asked to continue to complete weekly walking trackers and mail them back to the facilitator.
Health education control condition (phase I)
The health education control condition is designed to provide weekly group sessions with other PAD participants, without imparting information about exercise or behavior change. In the weekly group sessions, physicians and other healthcare professionals provide educational information on a topic of interest to the study participants. Topics include management of hypertension, cancer screening, preventing falls, and vaccinations. A list of representative topics is shown in Table 5. The sessions last for approximately 60 minutes.
Table 5.
Health Education Control Presentation Topics
|
Health education control condition (phase II)
After completing six-month follow-up testing, participants in the health education control condition are contacted by telephone at the same frequency as participants in Phase II of the GMCB intervention. Health education materials, similar to those discussed in Phase I, are mailed to participants and reviewed by telephone. The telephone call duration was designed to last a similar length as compared to the intervention telephone calls. The purpose of this phase of the health education control condition is to provide participants in this arm of the study with similar telephone contact time to those in the intervention, without providing information about exercise or behavior changes.
Statistical analysis
Characteristics of participants randomized to the intervention and the health education control condition will be compared to ensure balanced randomization. For our primary outcome, we will use a two-sample two-sided t test to compare change in six-minute walk performance between the intervention and health education control conditions at six-month follow-up. Analyses will be performed according to the intention to treat principle. Therefore, a minimum adherence rate is not required for participants to be included in analyses. We will also conduct longitudinal analysis using the generalized estimating equation (GEE) approach with six-minute walking distance measured over time as the response variable to compare change in six-minute walking distance between the intervention and health education control conditions. For the secondary specific aims, similar two-sample t and rank tests, multivariate regression analyses and GEE will be performed for each outcome of interest, respectively. After completing our intention-to-treat analyses, we will repeat analyses using a pattern-mixture model to impute missing data.
Power calculations
One hundred patients in each group who complete follow-up testing provides 80% power to detect a minimum detectable difference (MDD) of 0.40 pooled standard deviations (SD) of the change in six-minute walk performance between the two conditions at the significance level of 0.05. Based on the fact that 194 participants have been randomized into the GOALS trial and that the current six-month drop-out rate is 6.5%, the MDD will be 0.42 SD = 36.83 meters, using a two-tailed test. A previously completed clinical trial of supervised exercise in participants with PAD demonstrated that a 0.40 pooled SD was equivalent to 35.08 meters (8). Results from prior randomized clinical trials involving home-based walking exercise in patients with PAD suggest that a difference of about 0.40 SD or greater can be expected in study outcomes between the two study arms (15–17).
Discussion
Previous randomized controlled clinical trials demonstrate that supervised treadmill exercise interventions significantly improve treadmill walking performance as compared to a control group (8,9). Based on these prior studies, current clinical practice guidelines recommend supervised treadmill walking exercise for individuals with PAD (Class I recommendation, Level of Evidence-A) (12). However, many patients with PAD do not have access to supervised treadmill exercise, in part because medical insurance typically does not cover the cost of supervised treadmill exercise. In addition, supervised treadmill exercise requires transportation to an exercise center three times weekly, which may be impractical or burdensome. For these reasons, many physicians do not recommend supervised treadmill exercise for patients with PAD (41). Most patients with PAD do not participate in supervised treadmill exercise programs (42).
Older randomized trials analyzing home-based exercise programs in participants with PAD have included very small sample sizes and have yielded mixed results (13–17). In 2011, two randomized controlled clinical trials of home-base exercise programs for patients with PAD were published with sample sizes exceeding 100 participants (13,14). Gardner et al randomized 119 participants with PAD and exertional leg symptoms to one of three study conditions: a) home-based walking exercise, b) supervised treadmill exercise, or c) control (14). Participants in the home-based walking exercise condition were instructed to walk three times per week at a self selected pace, beginning at 20 minutes per session and working up to 45 minutes per session by the end of the intervention. Participants in the home-based exercise arm were provided with a pedometer to track their progress and also maintained an exercise log-book. They met with an exercise physiologist approximately every two weeks to receive feedback about their walking exercise progress. At twelve-week follow-up, participants in the home-based walking exercise arm increased their peak treadmill walking time from 402 to 526 seconds (P<0.01) and those in the supervised treadmill exercise arm increased their peak treadmill walking time from 325 to 540 seconds (P<0.001). Each exercise condition significantly increased their peak treadmill walking time as compared to the control condition. There were no significant differences in the improvement in treadmill walking performance between the home-based and supervised treadmill exercise groups (14). This study by Gardner et al suggests that a home-based walking exercise program can significantly improve treadmill walking performance in individuals with PAD. However, a trial by Collins et al of 145 participants with diabetes and PAD concluded that home-based walking interventions do not improve walking distance, compared to a control condition (13). The home-based walking exercise intervention in the study by Collins et al consisted of once weekly walking group sessions with a study facilitator and other PAD participants. PAD participants were also encouraged to walk at home at least three days per week for 50 minutes each session. Participants were provided with pedometers and encouraged to increase the number of steps by 50 at each walking session. Participants were telephoned bi-weekly and strategies for adhering to a home-based walking program were discussed. At six-month follow-up, there was no difference in change in maximum treadmill walking distance between the home-based walking exercise group vs. the control group (+24.5 meters vs. +39.2 meters). To our knowledge, the studies completed by Gardner et al and Collins et al are the largest completed randomized controlled clinical trials that tested the ability of a home-based walking exercise program to improve treadmill walking performance in participants with PAD. However, these two trials yielded substantially different results. Reasons for these differences may relate to distinct inclusion criteria (the trial by Collins et al included only PAD participants with diabetes mellitus), the duration of the interventions, and the specific nature of the interventions. As compared to these prior two trials, the GOALS trial is expected to have a larger sample size and includes a group-based intervention that is specifically focused on overcoming challenges to home-based exercise. Similar to the study by Gardner et al, the GOALS trial includes PAD participants both with and without diabetes mellitus. In addition, the six-minute walk primary outcome in GOALS may be better suited to the over-ground walking exercise that is encouraged in the GOALS intervention.
The GOALS trial is also unique because it includes PAD participants who are asymptomatic or who have exertional leg symptoms other than intermittent claudication. Most PAD patients do not have classical symptoms of intermittent claudication (1–3), thus findings of the GOALS trial will be generalizable to the large proportion of PAD participants without classic symptoms of intermittent claudication.
Promoting home-based walking exercise in patients with PAD is particularly challenging due to ischemic leg symptoms typically experienced during walking exercise in patients with PAD and the high prevalence of comorbid diseases in patients with PAD. If effective, a home-based walking exercise program that employs a group-mediated cognitive behavioral intervention could have far-reaching implications for the large and growing number of patients with PAD who do not have access to a supervised treadmill exercise programs.
Figure 1.
Study Overview
Acknowledgments
Funding
This work was supported by the National Heart Lung and Blood Institute [grant number R01-HL088589] and by the National Institute on Aging.
Appendix A
Appendix B
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Roger VL, Go AS, Lloyd-Jones DM, et al. Executive summary: Heart disease and stroke statistics-2012 update: A report from the American Heart Association. Circulation. 2012;125:188–197. doi: 10.1161/CIR.0b013e3182456d46. [DOI] [PubMed] [Google Scholar]
- 2.McDermott MM, Greenland P, Liu K, et al. Leg symptoms in peripheral arterial disease: associated clinical characteristics and functional impairment. JAMA. 2001;286:1599–1606. doi: 10.1001/jama.286.13.1599. [DOI] [PubMed] [Google Scholar]
- 3.McDermott MM, Liu K, Greenland P, Guralnik JM, Criqui MH, Chan C, Pearce WH, Schneider JR, Ferrucci L, Celic L, Taylor LM, Vonesh E, Martin GJ, Clark E. Functional decline in peripheral arterial disease: associations with the ankle brachial index and leg symptoms. JAMA. 2004;292:453–461. doi: 10.1001/jama.292.4.453. [DOI] [PubMed] [Google Scholar]
- 4.McDermott MM, Greenland P, Liu K, et al. The ankle brachial index is associated with leg function and physical activity: the Walking and Leg Circulation Study. Ann Intern Med. 2002;136:873–883. doi: 10.7326/0003-4819-136-12-200206180-00008. [DOI] [PubMed] [Google Scholar]
- 5.McDermott MM, Fried L, Simonsick E, Ling S, Guralnik JM. Asymptomatic peripheral arterial disease is independently associated with impaire lower extremity functioning: the Women’s Health and Aging Study. Circulation. 2000;101:1007–1012. doi: 10.1161/01.cir.101.9.1007. [DOI] [PubMed] [Google Scholar]
- 6.McDermott MM, Guralnik JM, Tian L, et al. Baseline functional performance predicts the rate of mobility loss in persons with peripheral arterial disease. J Am Coll Cardiol. 2007;50:974–982. doi: 10.1016/j.jacc.2007.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fried LP, Guralnik JM. Disability in older adults: evidence regarding significance, etiology, and risk. J Am Geriatr Soc. 1997;45:92–100. doi: 10.1111/j.1532-5415.1997.tb00986.x. [DOI] [PubMed] [Google Scholar]
- 8.McDermott MM, Ades P, Guralnik JM, et al. Treadmill exercise and resistance training in patients with peripheral arterial disease with and without intermittent claudication: a randomized controlled trial. JAMA. 2009;301:165–174. doi: 10.1001/jama.2008.962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Watson L, Ellis B, Leng GC. Exercise for intermittent claudication. Cochrane Database Syst Rev. 2008;4 doi: 10.1002/14651858.CD000990.pub2. CD000990. [DOI] [PubMed] [Google Scholar]
- 10.Falcone RA, Hirsch AT, Regensteiner JG, Treat-Jacobson D, Williams MA, Hiatt WR, Stewart KJ. Peripheral arterial disease rehabilitation: A review. J Cardiopulm Rehabil. 2003;23:170–175. doi: 10.1097/00008483-200305000-00002. [DOI] [PubMed] [Google Scholar]
- 11.Regensteiner JG. Exercise rehabilitation for the patient with intermittent claudication: a highly effective yet underutilized treatment. Curr Drug Targets Cardiovasc Haematol Disord. 2004;4:233–239. doi: 10.2174/1568006043336195. [DOI] [PubMed] [Google Scholar]
- 12.Hirsch AT, Haskal ZJ, Hertzer NR, et al. ACC/AHA Guidelines for the Management of Patients with Peripheral Arterial Disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report. J Vasc Interv Radiol. 2006;17:1383–1397. doi: 10.1097/01.RVI.0000240426.53079.46. [DOI] [PubMed] [Google Scholar]
- 13.Collins TC, Lunos S, Carlson T, et al. Effects of a home-based walking intervention on mobility and quality of life in people with diabetes and peripheral arterial disease. Diabetes Care. 2011;34:2174–2179. doi: 10.2337/dc10-2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gardner AW, Parker DE, Montgomery PS, et al. Efficacy of quantified home-based exercise and supervised exercise in patients with intermittent claudication. A randomized controlled trial. Circulation. 2011;123:491–498. doi: 10.1161/CIRCULATIONAHA.110.963066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Regensteiner JG, Meyer TJ, Krupski WC, et al. Hospital vs. home-based exercise rehabilitation for patients with peripheral arterial occlusive disease. Angiology. 1997;48:291–300. doi: 10.1177/000331979704800402. [DOI] [PubMed] [Google Scholar]
- 16.Savage P, Ricci MA, Lynn M, et al. Effects of home versus supervised exercise for patients with intermittent claudication. J Cardiopulm Rehabil. 2001;21:152–157. doi: 10.1097/00008483-200105000-00006. [DOI] [PubMed] [Google Scholar]
- 17.Patterson RB, Pinto B, Marcus B, et al. Value of a supervised exercise program for therapy of arterial claudication. J Vasc Surg. 1997;25:312–318. doi: 10.1016/s0741-5214(97)70352-5. [DOI] [PubMed] [Google Scholar]
- 18.Amirhamzeh MM, Chant JH, Rees JL, et al. A comparative study of treadmill tests and heel raising exercise for peripheral arterial disease. Eur J Vasc Endovasc Surg. 1997;13:301–305. doi: 10.1016/s1078-5884(97)80102-5. [DOI] [PubMed] [Google Scholar]
- 19.McPhail IR, Spittell PC, Weston SA, et al. Intermittent claudication: an objective office-based assessment. J Am Coll Cardiol. 2001;37:1381–1385. doi: 10.1016/s0735-1097(01)01120-2. [DOI] [PubMed] [Google Scholar]
- 20.McDermott MM, Ades PA, Dyer A, et al. Corridor-based functional performance measures correlate better with physical activity during daily life than treadmill measures in persons with peripheral arterial disease. J Vasc Surg. 2008;48:1231–1237. doi: 10.1016/j.jvs.2008.06.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Greig C, Butler F, Skelton D, et al. Treadmill walking in old age may not reproduce the real life situation. J Am Geriatr Soc. 1993;41:15–18. doi: 10.1111/j.1532-5415.1993.tb05941.x. [DOI] [PubMed] [Google Scholar]
- 22.Swerts PMJ, Mostert R, Wouters EFM. Comparison of corridor and treadmill walking in patients with severe chronic obstructive pulmonary disease. Phys Ther. 1990;70:439–442. doi: 10.1093/ptj/70.7.439. [DOI] [PubMed] [Google Scholar]
- 23.Gardner AW, Skinner JS, Cantwell BW, et al. Progressive vs. single-stage treadmill tests for evaluation of claudication. Med Sci Sports Exerc. 1991;23:402–408. [PubMed] [Google Scholar]
- 24.Ware J, Kosinski M, Keller SD. A 12-item Short-Form Health Survey: Construction of scales and preliminary tests of reliability and validity. Medical Care. 1996;34:220–233. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 25.Jenkinson C, Layte R. Development and Testing of the UK SF-12 (short form) J Health Services Research Policy. 1997;2:14–18. doi: 10.1177/135581969700200105. [DOI] [PubMed] [Google Scholar]
- 26.Regensteiner JG, Steiner JF, Panzer RJ, et al. Evaluation of walking impairment by questionnaire in patients with peripheral arterial disease. J Vasc Med Biol. 1990;2:142–152. [Google Scholar]
- 27.Richardson MT, Leon AS, Jacobs DR, et al. Ability of the Caltrac accelerometer to assess daily physical activity levels. J Cardiopulmonary Rehabil. 1995;15:107–113. doi: 10.1097/00008483-199503000-00003. [DOI] [PubMed] [Google Scholar]
- 28.McDermott MM, Ohlmiller SM, Liu K, et al. Gait alterations associated with walking impairment in people with peripheral arterial disease with and without intermittent claudication. J Am Geriatr Soc. 2001;49:747–754. doi: 10.1046/j.1532-5415.2001.49151.x. [DOI] [PubMed] [Google Scholar]
- 29.Garg PK, Liu K, Tian L, et al. Physical activity during daily life and functional decline in peripheral arterial disease. Circulation. 2009;119:251–260. doi: 10.1161/CIRCULATIONAHA.108.791491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rejeski W, Ip E, Katula J, White L. Older adults' desire for physical competence. Med Sci Sports Exercise. 2006;38(1):100–105. doi: 10.1249/01.mss.0000183231.61022.18. [DOI] [PubMed] [Google Scholar]
- 31.Rejeski WJ, Tian L, Liao Y, et al. Social cognitive constructs and the promotion of physical activity in patients with peripheral artery disease. J Cardiopulm Rehabil Prev. 2008;28:65–72. doi: 10.1097/01.HCR.0000311512.61967.6e. [DOI] [PubMed] [Google Scholar]
- 32.Reboussin BA, Rejeski WJ, Martin KA, et al. Correlates of satisfaction with body function and body appearance in middle- and older aged adults: The Activity Counseling Trial (ACT) Psychol Health. 2000;15(2):239–254. [Google Scholar]
- 33.Rejeski WJ, Shelton B, Miller ME, et al. Mediators of increased physical activity and change in subjective well-being: results from the Activity Counseling Trial (ACT) J Health Psychol. 2001;6:159–168. doi: 10.1177/135910530100600206. [DOI] [PubMed] [Google Scholar]
- 34.Garcia AW, King AC. Predicting long-term adherence to aerobic exercise: A comparison of two models. J Sport Exerc Psychol. 1991;13:394–410. [Google Scholar]
- 35.Geiser DS. Unpublished doctoral dissertation. USA: University of Nevada-Reno; 1992. A comparison of acceptance-focused and control-focused psychological treatments in a chronic pain treatment center. [Google Scholar]
- 36.Hayes SC, Wilson KG, Gifford EV, et al. Experimental avoidance and behavioral disorders: A functional dimensional approach to diagnosis and treatment. J Consult Clin Psychol. 1996;64:1152–1168. doi: 10.1037//0022-006x.64.6.1152. [DOI] [PubMed] [Google Scholar]
- 37.Criqui MH, Denenberg JO, Bird CE, et al. The correlation between symptoms and non-invasive test results in patients referred for peripheral arterial disease testing. Vasc Med. 1996;1:65–71. doi: 10.1177/1358863X9600100112. [DOI] [PubMed] [Google Scholar]
- 38.McDermott MM, Criqui MH, Liu K, et al. Lower ankle/brachial index, as calculated by averaging the dorsalis pedis and posterior tibial arterial pressures, and association with leg functioning in peripheral arterial disease. J Vasc Surg. 2000;32:1164–1171. doi: 10.1067/mva.2000.108640. [DOI] [PubMed] [Google Scholar]
- 39.Shadman R, Criqui MH, Bundens WP, et al. Subclavian artery stenosis: prevalence, risk factors, and association with cardiovascular diseases. J Am Coll Cardiol. 2004;44:618–623. doi: 10.1016/j.jacc.2004.04.044. [DOI] [PubMed] [Google Scholar]
- 40.Borg GV. Psychophysical bases of perceived exertion. Med Sci Sports Exerc. 1982;14:377–381. [PubMed] [Google Scholar]
- 41.McDermott MM, Hahn EA, Greenland P, et al. Atherosclerotic risk factor reduction in peripheral arterial disease. Results of a national survey. J Gen Intern Med. 2002;17:895–904. doi: 10.1046/j.1525-1497.2002.20307.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shalhoub J, Hamish M, Davies AH. Supervised exercise for intermittent claudication-an under-utlized tool. Ann R Coll Surg Engl. 2009;91:473–476. doi: 10.1308/003588409X432149. [DOI] [PMC free article] [PubMed] [Google Scholar]