Abstract
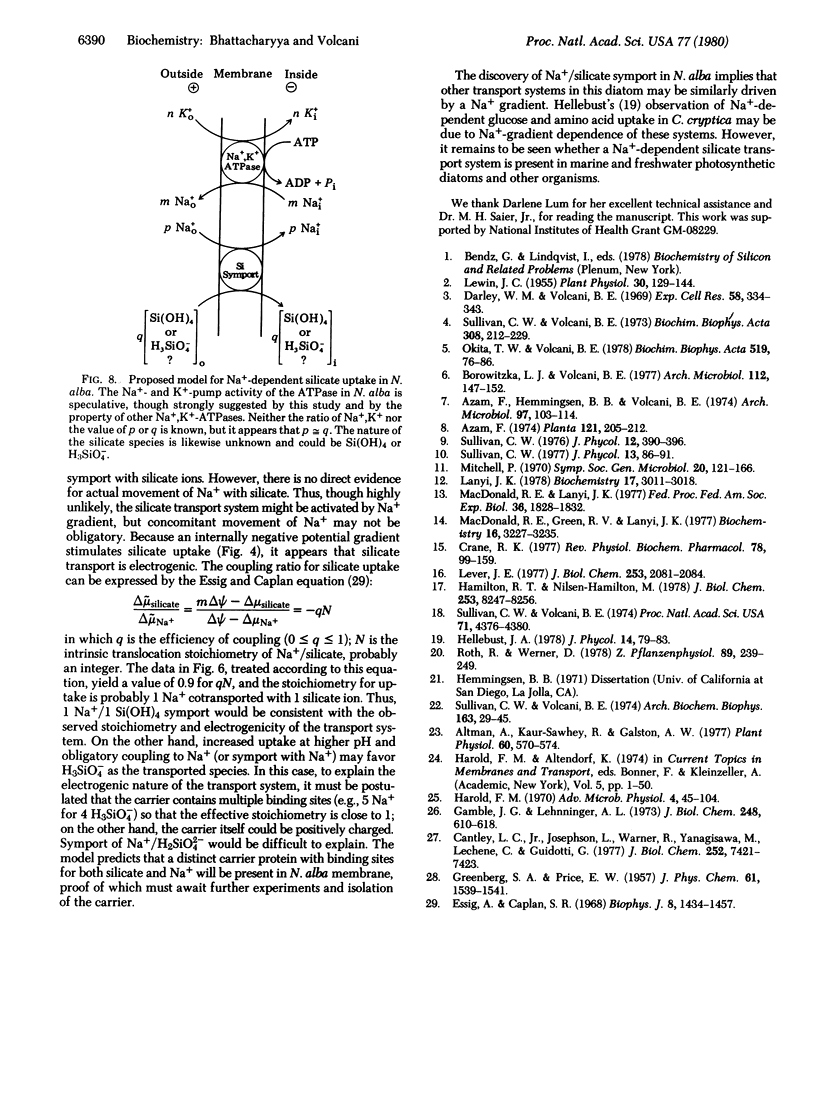
Silicate uptake by Nitzschia alba cells is higher in medium containing Na+ than in media lacking Na+ but containing K+, Rb+, NH4+, Li+, or choline+. The initial rate is inhibited by monensin and gramicidin but not by valinomycin or nigericin and is less sensitive to inhibition by carbonyl cyanide m-chlorophenylhydrazone (CCCP). In isolated membrane vesicles, silicate is taken up when a Na+ gradient is imposed across the membrane or is generated by cytoplasmic Na+,K+-ATPase. H+ or K+ gradients in either direction do not stimulate uptake. Na+-gradient-dependent uptake is inhibited by monensin but not by CCCP, valinomycin, or vanadate, which inhibits the cytoplasmic Na+,K+-ATPase. Uptake increases if an internally negative potential is imposed across the membrane. The vesicular uptake shows saturation kinetics with a Km of 62 μM and a Vmax of 4.1 nmol/mg of protein per min. In intact cells, the initial rate of silicate uptake increases with pH up to 9.5. Thus, in N. alba, silicate is symported with Na+, and the transport system is driven by the Na+ gradient that is generated and maintained across the membrane by the activity of Na+,K+-ATPase.
Keywords: Na+ gradient; Na+,K+-ATPase; membrane vesicles
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altman A., Kaur-Sawhney R., Galston A. W. Stabilization of Oat Leaf Protoplasts through Polyamine-mediated Inhibition of Senescence. Plant Physiol. 1977 Oct;60(4):570–574. doi: 10.1104/pp.60.4.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azam F., Hemmingsen B. B., Volcani B. E. Role of silicon in diatom metabolism. V. Silicic acid transport and metabolism in the heterotrophic diatom Nitzschia alba. Arch Microbiol. 1974 Apr 19;97(2):103–114. doi: 10.1007/BF00403050. [DOI] [PubMed] [Google Scholar]
- Borowitzka L. J., Volcani B. E. Role of silicon in diatom metabolism. VIII. Cyclic AMP and cyclic GMP in synchronized cultures of Cylindrotheca fusiformis. Arch Microbiol. 1977 Mar 1;112(2):147–152. doi: 10.1007/BF00429327. [DOI] [PubMed] [Google Scholar]
- Cantley L. C., Jr, Josephson L., Warner R., Yanagisawa M., Lechene C., Guidotti G. Vanadate is a potent (Na,K)-ATPase inhibitor found in ATP derived from muscle. J Biol Chem. 1977 Nov 10;252(21):7421–7423. [PubMed] [Google Scholar]
- Crane R. K. The gradient hypothesis and other models of carrier-mediated active transport. Rev Physiol Biochem Pharmacol. 1977;78:99–159. doi: 10.1007/BFb0027722. [DOI] [PubMed] [Google Scholar]
- Darley W. M., Volcani B. E. Role of silicon in diatom metabolism. A silicon requirement for deoxyribonucleic acid synthesis in the diatom Cylindrotheca fusiformis Reimann and Lewin. Exp Cell Res. 1969 Dec;58(2):334–342. doi: 10.1016/0014-4827(69)90514-x. [DOI] [PubMed] [Google Scholar]
- Essig A., Caplan S. R. Energetics of active transport processes. Biophys J. 1968 Dec;8(12):1434–1457. doi: 10.1016/S0006-3495(68)86565-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamble J. G., Lehninger A. L. Transport of ornithine and citrulline across the mitochondrial membrane. J Biol Chem. 1973 Jan 25;248(2):610–618. [PubMed] [Google Scholar]
- Hamilton R. T., Nilsen-Hamilton M. Transport of phosphate in membrane vesicles from mouse fibroblasts transformed by simian virus 40. J Biol Chem. 1978 Nov 25;253(22):8247–8256. [PubMed] [Google Scholar]
- Lanyi J. K. Coupling of aspartate and serine transport to the transmembrane electrochemical gradient for sodium ions in Halobacterium halobium. Translocation stoichiometries and apparent cooperativity. Biochemistry. 1978 Jul 25;17(15):3011–3018. doi: 10.1021/bi00608a012. [DOI] [PubMed] [Google Scholar]
- Lever J. E. Active phosphate ion transport in plasma membrane vesicles isolated from mouse fibroblasts. J Biol Chem. 1978 Apr 10;253(7):2081–2084. [PubMed] [Google Scholar]
- Lewin J. C. Silicon Metabolism in Diatoms. II. Sources of Silicon for Growth of Navicula Pelliculosa. Plant Physiol. 1955 Mar;30(2):129–134. doi: 10.1104/pp.30.2.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald R. E., Greene R. V., Lanyi J. K. Light-activated amino acid transport systems in Halobacterium halobium envelope vesicles: role of chemical and electrical gradients. Biochemistry. 1977 Jul 12;16(14):3227–3235. doi: 10.1021/bi00633a029. [DOI] [PubMed] [Google Scholar]
- MacDonald R. E., Lanyi J. K. Light-activated amino acid transport in Halobacterium halobium envelope vesicles. Fed Proc. 1977 May;36(6):1828–1832. [PubMed] [Google Scholar]
- Okita T. W., Volcani B. E. Role of silicon on diatom metabolism. IX. Differential synthesis of DNA polymerases and DNA-binding proteins during silicate starvation and recovery in Cylindrotheca fusiformis. Biochim Biophys Acta. 1978 Jun 22;519(1):76–86. doi: 10.1016/0005-2787(78)90063-1. [DOI] [PubMed] [Google Scholar]
- Sullivan C. W., Volcani B. E. Isolation and characterization of plasma and smooth membranes of the marine diatom Nitzschia alba. Arch Biochem Biophys. 1974 Jul;163(1):29–45. doi: 10.1016/0003-9861(74)90451-2. [DOI] [PubMed] [Google Scholar]
- Sullivan C. W., Volcani B. E. Role of silicon in diatom metabolism. 3. The effects of silicic acid on DNA polymerase, TMP kinase and DNA synthesis in Cylindrotheca fusiformis. Biochim Biophys Acta. 1973 May 10;308(2):212–229. doi: 10.1016/0005-2787(73)90151-2. [DOI] [PubMed] [Google Scholar]
- Sullivan C. W., Volcani B. E. Synergistically stimulated (Na+,K+)-adenosine triphosphatase from plasma membrane of a marine diatom. Proc Natl Acad Sci U S A. 1974 Nov;71(11):4376–4380. doi: 10.1073/pnas.71.11.4376. [DOI] [PMC free article] [PubMed] [Google Scholar]