Abstract
We characterized two Lactobacillus plantarum virulent siphophages, ATCC 8014-B1 (B1) and ATCC 8014-B2 (B2), previously isolated from corn silage and anaerobic sewage sludge, respectively. Phage B2 infected two of the eight L. plantarum strains tested, while phage B1 infected three. Phage adsorption was highly variable depending on the strain used. Phage defense systems were found in at least two L. plantarum strains, LMG9211 and WCSF1. The linear double-stranded DNA genome of the pac-type phage B1 had 38,002 bp, a G+C content of 47.6%, and 60 open reading frames (ORFs). Surprisingly, the phage B1 genome has 97% identity with that of Pediococcus damnosus phage clP1 and 77% identity with that of L. plantarum phage JL-1; these phages were isolated from sewage and cucumber fermentation, respectively. The double-stranded DNA (dsDNA) genome of the cos-type phage B2 had 80,618 bp, a G+C content of 36.9%, and 127 ORFs with similarities to those of Bacillus and Lactobacillus strains as well as phages. Some phage B2 genes were similar to ORFs from L. plantarum phage LP65 of the Myoviridae family. Additionally, 6 tRNAs were found in the phage B2 genome. Protein analysis revealed 13 (phage B1) and 9 (phage B2) structural proteins. To our knowledge, this is the first report describing such high identity between phage genomes infecting different genera of lactic acid bacteria.
INTRODUCTION
Lactobacilli are widely used in a variety of food fermentation processes, where they contribute to the flavor and texture of final products. They also produce organic acids, and the resulting low pH protects fermented products from degradation by spoilage microorganisms (15). In recent years, the industrial relevance of lactobacilli has been significantly enhanced by their increasing use as probiotics (12) or as a biotechnological tool (32).
Lactobacillus plantarum is commonly found as part of the natural microflora of fermented foods (dairy, vegetables, and meats) (12, 53, 68). This lactic acid bacterium may also be added as a starter or adjunct culture, in both cases improving the organoleptic characteristics of the final products (2, 12, 14, 15, 48, 49). Additionally, many L. plantarum strains possess documented probiotic properties, and marketed functional foods contain these strains (12, 53). L. plantarum can be used as a probiotic starter culture in the production of functional foods, taking advantage of, among others, its ability to grow in milk. However, the increasing use of L. plantarum as a starter or adjunct culture can lead to phage infections in industrial environments, with adverse effects on the final product (25, 51).
Phage infection is still one of the persistent causes of substandard dairy fermentation processes (60). Virulent phages can lyse starter cultures, yielding low-quality products that lead to economic losses. Consequently, efficient control measures to minimize problems caused by phage attacks become essential. In order to carry out successful antiphage strategies, knowledge about phage population and biology is needed (27, 39).
To date, over 30 L. plantarum phages, isolated from several sources, have been reported (16, 70, 72). All belong to the Caudovirales order (tailed phages, double-stranded DNA genome) (1, 70), and members belonging to each of the three Caudovirales families have been isolated: Siphoviridae (19 phages), Myoviridae (5 phages), and Podoviridae (1 phage). Other L. plantarum phages have been reported but not classified (70). Therefore, L. plantarum phages are relatively diverse and found in a wide variety of niches.
To our knowledge, only four L. plantarum phage genomes have been sequenced. Phage g1e (Siphoviridae, temperate) was isolated from plant materials and has a 42,259-bp genome with a G+C content of 43.1% and 62 open reading frames (ORFs) (37). Phage Sha1 (Siphoviridae, temperate) was isolated from kimchi and has a 41,726-bp genome with a G+C content of 40.6% and 58 putative ORFs (72). Phage JL-1 (Siphoviridae, virulent) was isolated from fermented cucumbers (43) and possesses a 36,700-bp genome with a G+C content of 39.4% and 52 ORFs. Finally, phage LP65 (Myovidiae, virulent) was isolated from fermented meat and has a very large genome of 131,573 bp with a G+C content of 37.3% and 165 ORFs (10).
Other L. plantarum phages have been analyzed in some detail; studies mainly included thermal and chemical sensitivities, and there were some preliminary genetics studies (9, 16, 44, 54, 65, 74). Overall, research on Lactobacillus phages has progressed over the past decade, but our knowledge of their biology and genetic composition is still limited and lags somewhat behind that of other industrially relevant phages (70).
The aim of this work was to carry out the characterization of two available L. plantarum phages. Phages ATCC 8014-B1 and ATCC 8014-B2 (herein referred to as B1 and B2, respectively) were previously isolated from corn silage and anaerobic sewage sludge (21).
MATERIALS AND METHODS
Bacterial strains, phages, and culture conditions.
L. plantarum strains were grown at 37°C in MRS broth (Difco). L. plantarum ATCC 8014 was used as the host strain for phages B1 and B2. For phage amplification, MRS was supplemented with 10 mM CaCl2 (MRS-Ca). Phage stocks were prepared as described previously (56) and stored as lysates at 4°C. Phage counts, expressed as PFU per milliliter, were obtained using the double-layer plaque titration method (64). Bacterial strains are maintained at the INLAIN Collection (Argentina) and the Félix d'Hérelle Reference Center for Bacterial Viruses of the Université Laval (Canada; www.phage.ulaval.ca) as frozen stocks in MRS broth containing 15% (vol/vol) glycerol. Phages B1 and B2 as well as the host L. plantarum ATCC 8014 were purchased from the American Type Culture Collection (Manassas, VA; www.atcc.org).
Electron microscopy.
Ten microliters of 2% phosphotungstic acid (pH 7.0) was put in a clean sterile petri dish. A 200-mesh Formvar-carbon-coated copper grid (Pelco International) was deposited face down on the staining solution for 30 s. Then, 10 μl of a purified phage suspension (1010 PFU ml−1) was mixed with the stain by pipetting up and down. After 90 s, the grid was deposited face up on blotting paper. The grid was dried for 5 min and observed at 80 kV using a JEOL 1230 transmission electron microscope (62).
Microbiological assays.
The host range of L. plantarum phages B1 and B2 was assessed by spotting 5 microliters of 10−2 and 10−4 dilutions of a high-titer lysate (109 PFU ml−1) on top of agar containing one of the eight L. plantarum strains tested (see Table 1). To study the phage adsorption process, L. plantarum cultures were grown in MRS to an optical density at 600 nm of 0.6 to 0.8, after which they were in contact with phage B1 or B2 at a final concentration of 103 PFU ml−1. The phage-containing cultures were incubated at 37°C for 15 min, and then we proceeded as described elsewhere (22). To determine the presence of active natural defense mechanisms against phages B1 and B2, the efficiency of plaquing (EOP) was calculated by dividing the phage titer on the test L. plantarum strain by the titer of the phage on the phage-sensitive host strain L. plantarum ATCC 8014. For phage-host systems showing reduced EOP values, two phage plaques obtained on the restrictive strain were purified and propagated on the same strain. The lysate obtained (modified phage) was titrated on both strains (original sensitive host and the restrictive strain) to determine a second EOP value. Modified phages were then propagated again on L. plantarum ATCC 8014, and the resulting lysate (unmodified phage) was titrated on both strains (4).
Table 1.
Host range and adsorption rates of phages B1 and B2 on L. plantarum strains
| Strain | Sourcea | Phage B1 |
Phage B2 |
||
|---|---|---|---|---|---|
| EOP | Adsorption (%) | EOP | Adsorption (%) | ||
| ATCC 8014 | ATCC | 1.0 | 99.6 ± 4.8 | 1.0 | 90.8 ± 3.0 |
| WCSF1 | Human saliva (NCBIM collection) | 1.5 × 10−2 | 19.0 ± 1.4 | —b | 0 |
| LMG9211 | Human saliva (BCCM collection) | 4.0 × 10−3 | 2.3 ± 0.4 | — | 92.4 ± 1.8 |
| PLN | NSLAB (INLAIN collection) | — | 0 | 1.0 | 98.5 ± 1.5 |
| SMQ-1113 | Industrial strain | -– | 1.1 ± 0.7 | -– | 14.4 ± 3.9 |
| SMQ-1114 | Industrial strain | -– | 8.2 ± 2.3 | -– | 3.2 ± 1.0 |
| SMQ-1115 | Industrial strain | -– | 9.5 ± 2.5 | -– | 12.3 ± 1.6 |
| SMQ-1116 | Industrial strain | -– | 13.7 ± 3.6 | -– | 10.4 ± 6.4 |
ATCC, American Type Culture Collection; BCCM, Belgian Coordinated Collections of Microorganisms; NCBIM, National Collection of Industrial and Marine Bacteria; NSLAB, nonstarter lactic acid bacteria; INLAIN, Instituto de Lactología Industrial.
—, not determined, as the phage does not infect the strain.
Phage DNA preparation and sequencing.
Genomic DNA of phages B1 and B2 was isolated using a Maxi lambda DNA purification kit (Qiagen) with modifications (19). The restriction profiles of phage B1 and B2 DNA were compared to confirm differences. Restriction endonucleases (Roche Diagnostics) were used as recommended by the manufacturer. The DNA fragments were separated in a 0.8% agarose gel, stained with ethidium bromide, and photographed under UV illumination. Genome sequencing was performed at the Plateforme d'ADN génomique de l'Université Laval (Université Laval, Québec, Canada) using a GS-FLX Titanium apparatus (Roche) and the 454 pyrosequencing technique. For phage B1, 39,144 reads were generated and assembled into a single contig with a coverage of 430-fold. For phage B2, 4,670 reads were generated and assembled into a single contig with a coverage of 18-fold. The extremities of the genomes were determined by sequencing ligated phage DNA preparations using converging PCR primers at the genomic platform of the Centre Hospitalier de l'Université Laval with an ABI Prism 3100 apparatus.
Bioinformatics analysis.
Sequence analyses were performed using BioEdit (30). Open reading frames (ORFs) were first identified using the GenMark program (46) and were further confirmed with ORFinder (http://www.ncbi.nlm.nih.gov/gorf/gorf.html). An ORF was considered valid if it had AUG, UUG, or GUG as the starting codon, encoded at least 29 amino acids (aa), and was preceded by an L. plantarum Shine-Dalgarno sequence (AGAAAGGAGGTGATC) (5). Function was attributed to an ORF using Blast2go (http://blast2go.bioinfo.cipf.es/start_blast2go) and BLASTp (NCBI [http://blast.ncbi.nlm.nih.gov/Blast.cgi]). The annotations were supported by searching for protein functional domains using the NCBI Conserved Domain Database (http://www.ncbi.nlm.nih.gov/Structure/cdd/wrpsb.cgi) and EMBL InterProScan (http://www.ebi.ac.uk/Tools/InterProScan/). The tRNAs were identified using the tRNAscan-SE server (http://lowelab.ucsc.edu/tRNAscan-SE) and the ARAGORN program (41). Codon usage was determined through the DNA 2.0 Web server (Menlo Park, CA) and the Count-codon program available on the Kazusa DNA Research Institute Web page (http://www.kazusa.or.jp/codon/). The bacterial codon usage for the L. plantarum host strains was obtained from the Kazusa DNA Research Institute database.
Analyses of phage B1 and B2 structural proteins.
Phage lysates were concentrated with polyethylene glycol (PEG) and purified using two CsCl gradients (61). Purified phages were recovered by ultracentrifugation using a Beckman SW41 Ti rotor at 35,000 rpm (210,053 × g) for 3 h, followed by a second ultracentrifugation using a Beckman NVT65 rotor at 60,000 rpm (342,317 × g) for 18 h. The phage preparations were then dialyzed against phage buffer (0.05 M Tris-HCl [pH 7.5], 0.1 M NaCl, 8 mM MgSO4). Purified phages (4 × 1011 PFU ml−1) were treated as described elsewhere (62). Briefly, phages were mixed with 4× loading buffer and boiled for 5 min. The samples were sonicated for 5 s with an ultrasonic Sonifier W-350 cell disrupter. Proteins were then separated by migration on a 12% SDS-polyacrylamide gel (1.5 mm thick). The Coomassie-stained protein bands of interest were excised from the gel and identified by liquid chromatography-tandem mass spectrometry (LC-MS/MS) at the Centre Protéomique de l'Est du Québec (Université Laval, Quebec, Canada). These results were analyzed using the Scaffold Proteome software (13, 33, 55). Purified phage lysates were also directly analyzed by LC-MS/MS.
Nucleotide sequence accession numbers.
The complete genome sequences of phages B1 and B2 have been deposited in GenBank under accession numbers JX486087 and JX486088, respectively.
RESULTS AND DISCUSSION
Electron microscopy.
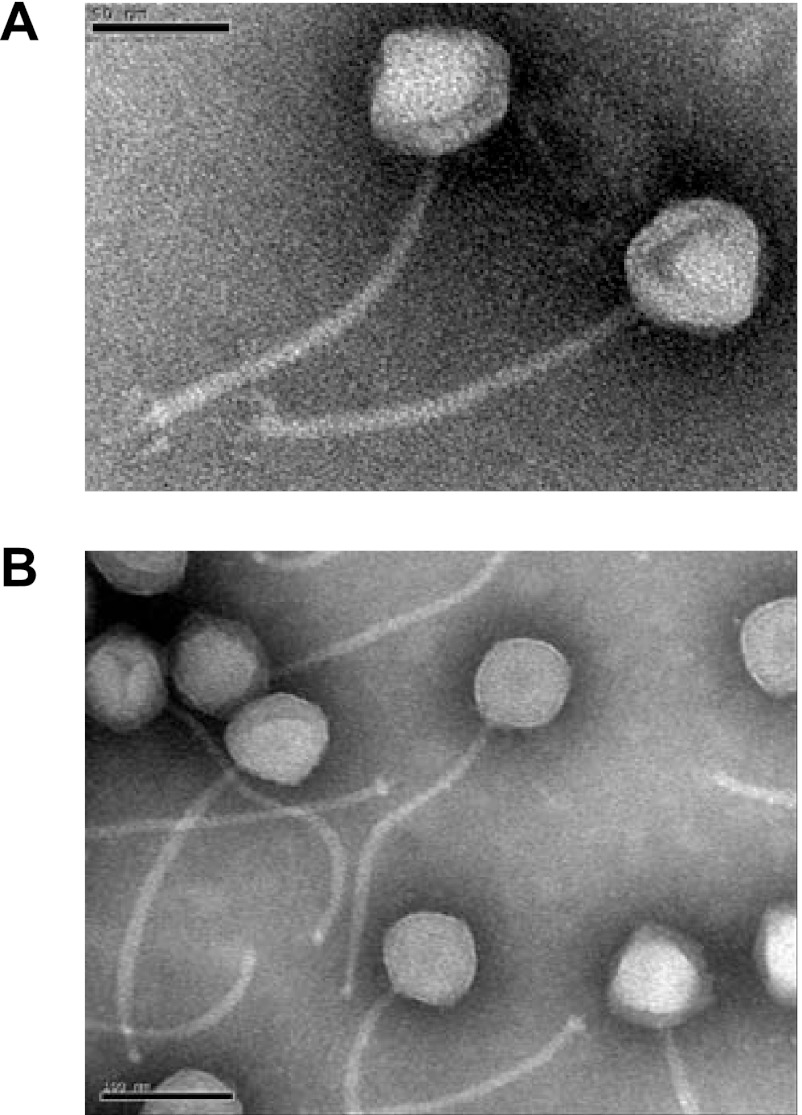
Both B1 and B2 phages have long noncontractile tails (Fig. 1) and belong to the Siphoviridae family, as do most characterized L. plantarum phages (70). Phage B1 has an icosahedral capsid with an estimated diameter of 54 ± 3 nm and a tail of 157 ± 10 nm in length and 8 ± 1 nm in width. The baseplate appears somewhat complex, with spikes or fibers (Fig. 1A). Phage B2 has an icosahedral capsid with a diameter of 74 ± 2 nm and a tail of 240 ± 3 nm in length and 10 ± 1 nm in width (Fig. 1B). Other investigators previously reported a larger capsid diameter (110 nm) and a much longer tail (500 nm) for L. plantarum phage B2 (54). Although dimensions may vary due to the use of different electron microscopes and methodologies (59), this cannot explain such large differences. At this time, it is unclear why such a discrepancy exists.
Fig 1.
Electron micrographs of the phages B1 (A) and B2 (B). Bars, 50 nm (A) and 100 nm (B).
Microbiological assays.
The results of the host range and adsorption tests are presented in Table 1. Each phage exhibited a distinctive host range but shared a common host (L. plantarum ATCC 8014). Phage B1 also replicated on L. plantarum strains WCSF1 and LMG9211, but the EOP was reduced. Surprisingly, under the conditions tested, the adsorption of phage B1 on strains LMG9211 and WCSF1 was very low, although clear plaques were formed. This low adsorption could be due to a limited number of phage receptors (in comparison with L. plantarum ATCC 8014) or their availability on the cell surface. Similar results were reported for Lactobacillus paracasei phages (8). Conversely, phage B2 was amplified on L. plantarum strain PLN and on its host ATCC 8014 (Table 1). Interestingly, phage B2 adsorbed well to strain LMG9211 without forming plaques (Table 1), suggesting the presence of phage resistance mechanisms in this strain (39). In general, phages were not able to adsorb on the other L. plantarum strains tested, suggesting the absence of receptors or perhaps adsorption blocking mechanisms (39).
Restriction/modification systems.
As indicated above, L. plantarum LMG9211 and WCSF1 seemed to carry a natural defense system, as the EOP of phage B1 was reduced (Table 1). Phage plaques were recovered from these two hosts (LMG9211 and WCSF1), purified, and amplified on each strain. These amplified phages had an EOP of 1.0 on L. plantarum ATCC 8014. When these phages were propagated again in their original host, L. plantarum ATCC 8014, the EOP values were reduced and similar to those shown in Table 1. This temporary host-specific immunity suggests the presence of a classical restriction/modification (R/M) system in both strains (52). Besides, the same specificity might be involved in both systems, since an EOP value of 1 was obtained when LMG9211-amplified phage was tested on L. plantarum WCSF1 and when WCSF1-amplified phage was tested on L. plantarum LMG9211. A type I restriction/modification system was previously identified in the genome of L. plantarum WCSF1, though its functionality was not demonstrated (36, 63).
Genome analysis.
Phages B1 and B2 have linear double-stranded DNA genomes comprising 38,002 bp and 80,618 bp, respectively. Nes et al. (54) reported a relatively similar genome size for phage B2 (73 kbp), which was calculated from the addition of the molecular sizes of DNA restriction fragments. Phage B1 has the highest GC content (47.6%) reported to date for an L. plantarum phage. The GC content of phage B2 was much lower, at 37.0%, but is similar to the GC content of the L. plantarum myophage LP65 (10). The GC content of the host strain L. plantarum ATCC 8014 was previously estimated at 45.1% (50), whereas genome sequencing of strain WCSF1 revealed a GC content of 44.5% (36, 63). The genomes of two other L. plantarum strains also have GC contents of 44.5 to 44.7% (71, 75). The GC contents were similar throughout the genomic sequences of both phages, although some noncoding regions in phage B2 were AT rich. The lower GC content of phage B2 may suggest that some genetic elements were derived from phages infecting other hosts (23, 31).
The phage genomic DNA was also digested with various restriction enzymes (EcoRV, HindIII, MluI, and SalI), and the profiles obtained were similar to the theoretical profiles obtained from the genomic data (NEBcutter), suggesting the absence of modified nucleotides (data not shown). The profile obtained for phage B2 was similar to that reported elsewhere (54). Analysis of the genome extremities indicated that phage B1 is a pac-type phage, like L. plantarum phages fri, JL-1, and LP65 (10, 43, 65), whereas phage B2 was classified as a cos-type phage, similar to SC921 phage (74). The cos site is 11 nucleotides long (5′-TGAGCGCCCTA-3′) (data not shown).
Sixty ORFs were identified for phage B1 and 127 ORFs for phage B2 (Tables 2 and 3; Fig. 2 and 3). They covered 93% (B1) and 87% (B2) of the genome length. A total of 56 ORFs (93%) for phage B1 and 65 ORFs (51%) for phage B2 had homology to previously characterized genes in public databases. However, a protein function could be attributed to products of only 25 ORFs (42%) for phage B1 and 37 ORFs (29%) for phage B2. The predominant starting codon was ATG for both phages (90% for B1, 86% for B2). Interestingly, four B1 ORFs share some identity with B2 ORFs, namely, B1 ORF15 and B2 ORF33, B1 ORF18 and B2 ORF36, B1 ORF22 and B2 ORF40, and B1 ORF35 and B2 ORF99. Of interest, ORF18 of phage B1 is likely involved in host recognition, and its identity with B2 ORF36 agrees with the observation that both phages infect the same host strain.
Table 2.
Open reading frames deduced from the genome of L. plantarum phage B1 and their predicted functions
| ORFa | Start (bp) | Stop (bp) | Predicted protein |
Putative RBS and start codonb | Predicted function | Best match(es) (extentc; % amino acid identity) | E value | Aligned protein |
|||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Size (aa) | Molecular mass (kDa) | pI | Size (aa) | GenBank accession no. | |||||||
| 1 | 242 | 553 | 103 | 11.4 | 6.3 | ATAAAGGAGATAACGgaATG | Terminase small subunit | clP1_010 (P. damnosus phage clP1) (99/103; 96) | 3.0E−64 | 139 | YP_004934175 |
| 2 | 550 | 1845 | 431 | 48.9 | 8.2 | AACATCGGGTTTCCCgaATG | Terminase large subunit | clP1_009 (P. damnosus phage clP1) (430/431; 99) | 0 | 431 | YP_004934174 |
| ORF440 (L. plantarum phage phiJL-1) (293/431; 68) | 0 | 440 | YP_223885 | ||||||||
| 3 | 1861 | 3402 | 513 | 58.4 | 4.8 | ATAACGGAGGAGTTAaaacATG | Portal protein | clP1_008 (P. damnosus phage clP1) (508/513; 99) | 0 | 513 | YP_004934173 |
| ORF506 (L. plantarum phage phiJL-1) (284/506; 56) | 0 | 506 | YP_223886 | ||||||||
| 4 | 3320 | 4132 | 270 | 30.6 | 8.8 | CGAAAGGCGGATTGAttatcaATG | Capsid protein | clP1_007 (P. damnosus phage clP1) (266/270; 99) | 0 | 270 | YP_004934172 |
| ORF273 (L. plantarum phage phiJL-1) (121/270; 45) | 2.0E−72 | 273 | YP_223887 | ||||||||
| 5 | 4232 | 4762 | 176 | 19.1 | 4.7 | ATTGAGGAGGAGAAAccatcATG | Scaffold protein | clP1_006 (P. damnosus phage clP1) (174/176; 99) | 1.0E−118 | 176 | YP_004934171 |
| ORF184 (L. plantarum phage phiJL-1) (70/161; 43) | 1.0E−27 | 184 | YP_223888 | ||||||||
| 6 | 4780 | 5640 | 286 | 30.9 | 4.8 | CGGAGGAACTTAAACaATG | Major capsid protein | clP1_005 (P. damnosus phage clP1 (284/286; 99) | 0 | 286 | YP_004934170 |
| ORF286 (L. plantarum phage phiJL-1) (198/286; 69) | 7.0E−138 | 286 | YP_223889 | ||||||||
| 7 | 5688 | 5915 | 75 | 7.2 | 4.4 | CAAAAGACCGCTAGCATG | Minor capsid protein | clP1_004 (P. damnosus phage clP1 (74/75; 99) | 9.0E−37 | 75 | YP_004934169 |
| ORF64b (L. plantarum phage phiJL-1) (33/49; 67) | 6.0E−11 | 64 | YP_223890 | ||||||||
| 8 | 5943 | 6314 | 123 | 14.1 | 5.0 | ATAATTAACGTACCCgtatggGTG | DNA packaging | clP1_003 (P. damnosus phage clP1) (113/115; 98) | 6.0E−74 | 115 | YP_004934168 |
| ORF113 (L. plantarum phage phiJL-1) (69/113; 61) | 2.0E−42 | 113 | YP_223891 | ||||||||
| 9 | 6311 | 6589 | 92 | 10.2 | 9.5 | GGGGTTCAGGTTCTTATG | clP1_002 (P. damnosus phage clP1) (90/92; 98) | 5.0E−57 | 92 | YP_004934167 | |
| ORF94 (L. plantarum phage phiJL-1) (49/93; 53) | 3.0E−27 | 94 | YP_223892 | ||||||||
| 10 | 6546 | 6959 | 137 | 15.1 | 9.3 | GAACGTGCCGTTATCaATG | Head to tail joining | clP1_001 (P. damnosus phage clP1) (123/125; 98) | 5.0E−85 | 125 | YP_004934166 |
| ORF125 (L. plantarum phage phiJL-1) (77/121; 64) | 3.0E−46 | 125 | YP_223893 | ||||||||
| 11 | 7035 | 7388 | 117 | 13.2 | 8.0 | CGCTAGGGGGTGTCAcaagATG | ORF117 (L. plantarum phage phiJL-1) (64/117; 55) | 2.0E−40 | 117 | YP_223894 | |
| 12 | 7407 | 8033 | 208 | 22.7 | 4.3 | AATGAGGAGTGTAaaaaaatATG | Major tail protein | clP1_057 (P. damnosus phage clP1) (208/208; 100) | 5.0E−150 | 208 | YP_004934222 |
| ORF199 (L. plantarum phage phiJL-1) (131/195; 67) | 4.0E−92 | 199 | ZP_03964227 | ||||||||
| 13 | 8064 | 8468 | 134 | 15.2 | 5.0 | AAAAAGGACGGTACCaacaaaATG | clP1_056 (P. damnosus phage clP1) (134/134; 100) | 1.0E−93 | 134 | YP_004934221 | |
| ORF139 (L. plantarum phage phiJL-1) (71/138; 51) | 7.0E−37 | 139 | YP_223896 | ||||||||
| 14 | 8546 | 8785 | 79 | 8.7 | 9.9 | GAAGCCGAGGCCGTCATG | clP1_055 (P. damnosus phage clP1) (79/79; 100) | 9.0E−48 | 79 | YP_004934220 | |
| ORF140 (L. plantarum phage phiJL-1) (23/79; 29) | 5.1E−2 | 140 | YP_223897 | ||||||||
| 15 | 8789 | 12043 | 1084 | 110.9 | 9.6 | TCGGAGGAGGTTAACgaATG | Tape measure protein | clP1_054 (P. damnosus phage clP1) (1067/1084; 98) | 0 | 1084 | YP_004934219 |
| ORF1133 (L. plantarum phage phiJL-1) (97/172; 56) | 5.0E−51 | 1133 | YP_223898 | ||||||||
| 16 | 12347 | 12877 | 176 | 19.1 | 9.0 | ATTACCGAGCTGGCCgATG | Minor tail protein | clP1_053 (P. damnosus phage clP1) (169/176; 96) | 1.0E−118 | 273 | YP_004934218 |
| ORF441 (L. plantarum phage phiJL-1) (47/101; 47) | 1.0E−23 | 441 | YP_223899 | ||||||||
| 17 | 12892 | 15390 | 832 | 90.7 | 5.0 | ATATAGATAGGAGTGATG | Prophage tail superfamily | clP1_052 (P. damnosus phage clP1) (653/839; 78) | 0 | 829 | YP_004934217 |
| ORF738 (L. plantarum phage phiJL-1) (270/455; 59) | 0 | 738 | YP_223900 | ||||||||
| 18 | 15371 | 17479 | 702 | 75.7 | 5.4 | CGACAGGAGGAGTTAaacaATG | Tail/host recognition | clP1_051 (P. damnosus phage clP1) (648/702, 92) | 0 | 702 | YP_004934216 |
| ORF749 (L. plantarum phage phiJL-1) (66/189; 35) | 3.0E−26 | 749 | YP_223901 | ||||||||
| 19 | 17491 | 17862 | 123 | 14.0 | 5.4 | AGTTAGGAGGCCGAAccATG | clP1_050 (P. damnosus phage clP1) (118/119, 99) | 2.0E−79 | 119 | YP_004934215 | |
| 21 | 18031 | 18363 | 110 | 12.3 | 6.7 | AAAAAGAATTAAAGGagtATG | Holin | clP1_048 (P. damnosus phage clP1) (99/106, 93) | 2.0E−64 | 106 | YP_004934213 |
| 22 | 18363 | 19604 | 413 | 45.5 | 9.6 | GATAACGAGGTACAAtaATG | Endolysin | clP1_047 (P. damnosus phage clP1) (399/413, 97) | 0 | 413 | YP_004934212 |
| ORF398 (L. plantarum phage phiJL-1) (316/393; 80) | 0 | 398 | YP_223905 | ||||||||
| 23 | 20279 | 20395 | 38 | 3.9 | 6.7 | ATAACGGCGTTAGTTatGTG | clP1_046 (P. damnosus phage clP1) (36/38, 95) | 8.0E−18 | 71 | YP_004934211 | |
| 24 | 20346 | 20810 | 154 | 17.6 | 5.2 | CCACATGTGGCTCGCtactgGTG | Endonuclease | clP1_045 (P. damnosus phage clP1) (138/140 (99) | 3.0E−99 | 140 | YP_004934210 |
| ORF134 (L. plantarum phage phiJL-1) (46/129; 36) | 2.0E−13 | 134 | YP_223908 | ||||||||
| 25 | 20813 | 21559 | 248 | 28.2 | 4.6 | AGTGAGGAGGACTAAacATG | clP1_044 (P. damnosus phage clP1) (248/248, 100) | 0 | 248 | YP_004934209 | |
| ORF246 (L. plantarum phage phiJL-1) (118/233; 51) | 4.0E−71 | 246 | YP_223909 | ||||||||
| 26 | 21950 | 22609 | 219 | 23.6 | 5.7 | CGAGGAGAGATAAGCATG | Helicase (NTPd binding) | clP1_043 (P. damnosus phage clP1) (219/219, 100) | 5.0E−157 | 219 | YP_004934208 |
| ORF224 (L. plantarum phage phiJL-1) (169/218; 78) | 3.0E−121 | 224 | YP_223910 | ||||||||
| 27 | 22616 | 24556 | 646 | 72.7 | 5.1 | AAAGGGGAAATAAAGcactATG | DNA primase | clP1_042 (P. damnosus phage clP1) (641/646, 99) | 0 | 646 | YP_004934207 |
| ORF637 (L. plantarum phage phiJL-1) (285/634; 45) | 7.0E−180 | 637 | YP_223911 | ||||||||
| 29 | 24921 | 25337 | 138 | 15.7 | 5.1 | ATTGAGGAGGAAATGtaATG | clP1_040 (P. damnosus phage clP1) (105/138, 76) | 2.0E−68 | 138 | YP_004934205 | |
| 30 | 25340 | 25639 | 99 | 11.3 | 7.8 | AAACGGGAGGATTATtaaatATG | clP1_039 (P. damnosus phage clP1) (64/99, 65) | 4.0E−39 | 99 | YP_004934204 | |
| 31 | 25600 | 26091 | 163 | 18.6 | 10.3 | ACTAAGGGGGTGAAAacATG | Replication protein | clP1_038 (P. damnosus phage clP1) (143/150, 95) | 4.0E−102 | 150 | YP_004934203 |
| 32 | 25959 | 26450 | 163 | 19.2 | 5.2 | CGCGGGCACGTCATCtATT | clP1_037 (P. damnosus phage clP1) (115/123, 93) | 8.0E−80 | 123 | YP_004934202 | |
| 33 | 26488 | 26685 | 65 | 9.5 | 6.2 | TCATAGGAGGTAATTatATG | clP1_036 (P. damnosus phage clP1) (62/65, 95) | 2.0E−37 | 66 | YP_004934201 | |
| 34 | 26687 | 27151 | 154 | 17.8 | 5.1 | AAAAAGGGGAATTATtaacATG | Replicase | clP1_035 (P. damnosus phage clP1) (153/154, 99) | 4.0E−106 | 154 | YP_004934200 |
| ORF153 (L. plantarum phage phiJL-1) (74/156; 47) | 3.0E−35 | 153 | YP_223913 | ||||||||
| 35 | 27167 | 27487 | 106 | 11.8 | 4.6 | AGTAAAGGGGTAAAAcgATG | DNA binding | clP1_034 (P. damnosus phage clP1) (100/106, 94) | 2.0E−67 | 106 | YP_004934199 |
| ORF97 (L. plantarum phage phiJL-1) (45/94; 48) | 1.0E−18 | 97 | YP_223877 | ||||||||
| 37 | 27725 | 29155 | 476 | 53.3 | 9.1 | TGTACGGAGGGATTGcaATG | Helicase | clP1_033 (P. damnosus phage clP1) (470/476, 99) | 0 | 476 | YP_004934198 |
| ORF467 (L. plantarum phage phiJL-1) (291/446; 65) | 0 | 467 | YP_223915 | ||||||||
| 38 | 29199 | 29471 | 90 | 10.2 | 4.8 | ATCAAGCAAGGGAGGtaATT | clP1_032 (P. damnosus phage clP1) (64/88, 73) | 2.0E−39 | 102 | YP_004934197 | |
| 39 | 29458 | 29832 | 124 | 13.8 | 4.4 | AGAAAAGGGGTATTTtgATG | clP1_031 (P. damnosus phage clP1) (119/124, 96) | 3.0E−71 | 125 | YP_004934196 | |
| 40 | 29834 | 30253 | 139 | 16.0 | 5.4 | TGAAAGGATTGATTAacATG | clP1_030 (P. damnosus phage clP1) (125/143, 87) | 3.0E−85 | 149 | YP_004934195 | |
| 41 | 30225 | 30659 | 144 | 16.4 | 5.3 | AAACTAAAAGTCACGaATG | clP1_029 (P. damnosus phage clP1) (98/139, 71) | 4.0E−68 | 139 | YP_004934194 | |
| 42 | 30659 | 31102 | 147 | 16.2 | 9.0 | AAAAAGGGGTAATTGaataATG | clP1_028 (P. damnosus phage clP1) (129/147, 88) | 5.0E−89 | 147 | YP_004934193 | |
| 43 | 31095 | 32072 | 325 | 36.9 | 5.3 | ATTATGGAGGTTGTGaaagATG | Structural protein | clP1_027 (P. damnosus phage clP1) (306/325, 94) | 0 | 324 | YP_004934192 |
| 44 | 32073 | 32468 | 131 | 14.3 | 4.5 | AAATCGGAGGTTATTtaaATG | clP1_026 (P. damnosus phage clP1) (129/131, 98) | 1.0E−89 | 131 | YP_004934191 | |
| 45 | 32601 | 32840 | 79 | 9.1 | 6.0 | CGAAAGGACGAGGGAtaaATG | clP1_025 (P. damnosus phage clP1) (78/79, 99) | 4.0E−50 | 79 | YP_004934190 | |
| 46 | 32827 | 33183 | 118 | 13.7 | 9.3 | ATCATGGAGGACGACaATG | clP1_024 (P. damnosus phage clP1) (117/118, 99) | 2.0E−81 | 118 | YP_004934189 | |
| ORF114 (L. plantarum phage phiJL-1) (54/104, 52) | 8.0E−31 | 114 | YP_223917 | ||||||||
| 48 | 33518 | 33706 | 62 | 7.6 | 5.2 | AGGAAAGTGGTAATAaaaATG | clP1_023 (P. damnosus phage clP1) (61/62, 98) | 3.0E−35 | 76 | YP_004934188 | |
| 49 | 33703 | 33831 | 42 | 4.7 | 8.5 | GGATATGAGGTGATCgaATG | clP1_022 (P. damnosus phage clP1) (39/42, 93) | 3.0E−6 | 42 | YP_004934187 | |
| 50 | 33857 | 34030 | 57 | 6.2 | 12.1 | AACAAAGGGGTCTTAtattATG | clP1_021 (P. damnosus phage clP1) (42/44, 95) | 6.0E−18 | 57 | YP_004934186 | |
| 51 | 34030 | 34275 | 81 | 9.3 | 5.6 | AAAAAGGGGGCCAAGtaATG | clP1_020 (P. damnosus phage clP1) (78/81, 96) | 6.0E−51 | 81 | YP_004934185 | |
| ORF77 (L. plantarum phage phiJL-1) (40/75, 53) | 2.0E−3 | 77 | YP_223874 | ||||||||
| 52 | 34341 | 34634 | 97 | 11.5 | 9.5 | AGAACGTCATGGGTCgATG | clP1_019 (P. damnosus phage clP1) (94/97, 97) | 1.0E−64 | 119 | YP_004934184 | |
| 53 | 34621 | 34920 | 99 | 11.1 | 9.8 | TAAAAGGCGGCGAGAttATG | clP1_018 (P. damnosus phage clP1) (99/99, 100) | 1.0E−65 | 99 | YP_004934183 | |
| 54 | 34917 | 35108 | 63 | 7.2 | 4.4 | ATAAAGGGGATAAAAgtATG | clP1_017 (P. damnosus phage clP1) (61/63, 97) | 4.0E−37 | 63 | YP_004934182 | |
| 55 | 35077 | 35238 | 53 | 6.1 | 6.0 | CGAAAAGGGGTTTTTaaATG | clP1_016 (P. damnosus phage clP1) (52/53, 98) | 1.0E−27 | 53 | YP_004934181 | |
| 56 | 35251 | 35685 | 144 | 16.8 | 5.0 | AATTAGGAGGGTTTTaccATG | clP1_015 (P. damnosus phage clP1) (136/144, 94) | 4.0E−95 | 144 | YP_004934180 | |
| 57 | 35678 | 36124 | 148 | 17.1 | 6.1 | ACGGAGGTTGAAATCaATG | clP1_014 (P. damnosus phage clP1) (132/148, 89) | 3.0E−94 | 148 | YP_004934179 | |
| 58 | 36117 | 36512 | 131 | 14.9 | 9.8 | ATCGAGGTGAAGCTAcATG | clP1_013 (P. damnosus phage clP1) (1126/131, 96) | 3.0E−87 | 131 | YP_004934178 | |
| 59 | 36514 | 37032 | 172 | 19.1 | 10.0 | TACTGGGAGGTGTTAtgacATG | Endonuclease | clP1_012 (P. damnosus phage clP1) (167/172, 97) | 1.0E−119 | 172 | YP_004934177 |
| 60 | 37025 | 37441 | 138 | 15.7 | 9.1 | TGAAAGGTGATAATAATG | clP1_011 (P. damnosus phage clP1) (136/138, 99) | 4.0E−93 | 141 | YP_004934176 | |
Only the ORFs with significant hits to those of other proteins in the database are included.
RBS, ribosome binding site. Underlined codons correspond to bases identical to the L. plantarum RBS consensus sequence; uppercase letters represent the RBS sequence; boldface indicates the starting codon; lowercase letters indicate spacer nucleotides between the RBS and start codon.
Number of identical amino acids/total number of amino acids.
NTP, nucleoside triphosphate.
Table 3.
Open reading frames deduced from the genome of L. plantarum phage B2 and their predicted proteins
| ORFa or tRNA | Start (bp) | Stop (bp) | Predicted protein |
Putative RBS and start codonb | Predicted function | Best matches (extentc; % amino acid identity) | E value | Aligned protein |
|||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Size (aa) | Molecular mass (kDa) | pl | Size (aa) | GenBank accession no. | |||||||
| 12 | 3401 | 2769 | 210 | 25.1 | 5.2 | TAAAAGGAGGAATAAaattATG | Fatty acid/phospholipid synthesis protein | PlsX gene product (Clostridium ljungdahlii DSM 13528) (29/154; 19) | 9.0E−2 | 337 | YP_003779451 |
| 15 | 4262 | 4393 | 43 | 4.8 | 3.9 | AAAAGGGAGGACTTAgcATG | C.D.d: transcription factor | EUBREC_3432 (Eubacterium rectale ATCC 33656) (15/43; 35) | 2.0E−3 | 209 | YP_002939292 |
| 16 | 4320 | 4802 | 160 | 18.6 | 9.3 | ACAAGAAAGATGATTATG | C.D.: HNH endonuclease | pls32_p096 (Bacillus subtilis subsp. natto) (54/122; 44) | 8.0E−26 | 135 | YP_004243694 |
| 17 | 4815 | 5321 | 168 | 18.7 | 5.1 | TCTAAGGGGGTGAACacATG | Terminase small subunit | pls32_p095 (Bacillus subtilis subsp. natto) (61/166; 37) | 3.0E−22 | 163 | YP_004243693 |
| 18 | 5476 | 5988 | 170 | 19.7 | 4.9 | AAAAGGTAGGTAAAGtcaaATG | gp089 (Lc. lactis phage 949) (33/114; 29) | 8.0E−11 | 171 | YP_004306249 | |
| tRNA | 6246 | 6317 | tRNA-Asn | ||||||||
| tRNA | 6925 | 6997 | tRNA-Leu | ||||||||
| tRNA | 6999 | 7072 | tRNA-Met | ||||||||
| 20 | 7143 | 7403 | 86 | 10.2 | 5.3 | GAAAGAGAGGAAAACtATG | ORF78 (L. plantarum phage LP65) (81/86; 95) | 1.0E−40 | 109 | YP_164713 | |
| tRNA | 7742 | 7814 | tRNA-Gly | ||||||||
| 21 | 7937 | 9646 | 569 | 65.0 | 5.5 | TATAACGAGGTGATAtgTTG | Terminase large subunit | ORF5 (L. delbrueckii phage c5) (211/522; 40) | 9.0E−131 | 559 | ACA63297 |
| 22 | 9830 | 11137 | 435 | 48.0 | 4.8 | ACAGAAGATACGGTTATG | Portal protein | ORF5 (L. delbrueckii phage LL-Ku) (106/384; 28) | 3.0E−37 | 404 | AAV30165 |
| 23 | 11133 | 12236 | 367 | 40.6 | 4.3 | TGTTAGGAGGTAATGacaATT | Major capsid protein/protease | pls32_p090 (Bacillus subtilis subsp. natto) (93/201; 46) | 1.0E−38 | 313 | YP_004243688 |
| 24 | 12240 | 13526 | 428 | 45.9 | 5.3 | GATTTGGAGGTCTAAttaATG | Major capsid protein | ORF7 (L. delbrueckii phage LL-Ku) (106/381; 28) | 1.0E−21 | 395 | AAV30167 |
| 25 | 13657 | 14115 | 152 | 15.9 | 4.5 | TGATAGGAGGGAATActaTTG | Tail protein | SPC35_0138 (enterobacterial phage SPC35) (37/90; 41) | 5.0E−9 | 162 | YP_004306621 |
| 27 | 14453 | 14881 | 142 | 15.9 | 5.4 | TTTAGTGAGGTGAGAaaATG | Head-tail adaptor | HMPREF9104_01875 (Lactobacillus kisonensis F0435) (23/87; 26) | 1.0E−3 | 120 | ZP_09556164 |
| 28 | 14832 | 15323 | 163 | 18.4 | 6.2 | AAACGGCGGTTCATCtgGTG | Head-tail joining protein | LSL_0288 (Lactobacillus salivarius phage Sal2) (31/126; 25) | 7.6E−2 | 130 | YP_535185 |
| 29 | 15447 | 15704 | 85 | 9.7 | 4.8 | GTTCAGAATAAAATAGTG | Tail protein | ORF10 (L. casei phage phiAT3) (23/83; 28) | 5.0E−2 | 123 | YP_025035 |
| 30 | 15723 | 16334 | 203 | 21.5 | 4.7 | AAGAAAGAGGTAATTactaATG | Major tail protein | OF23 (L. plantarum phage Sha1) (61/209; 29) | 8.0E−06 | 212 | ADW01304 |
| 31 | 16442 | 16861 | 139 | 15.6 | 4.4 | AAAAATAAAATATTTagATG | LAR_1055 (Lactobacillus reuteri JCM 1112) (31/95; 33) | 1.0E−5 | 132 | YP_001842051 | |
| 33 | 17115 | 22778 | 1,887 | 199.5 | 10.1 | TGCAAGGAGGGTTTTaaATG | Tail tape measure protein | LSL_0794 (L. salivarius phage SalI) (243/686; 35) | 4.0E−93 | 1,274 | YP_535687 |
| 34 | 22802 | 24613 | 603 | 66.1 | 6.0 | ATACGAGGGGTAATCccctcGTG | Minor structural protein | ORF27 (L. plantarum phage Sha1) (321/596; 54) | 1.0E−177 | 590 | ADW01308 |
| 35 | 24669 | 27053 | 794 | 88.0 | 4.7 | GATTAGGAGGTAATGgaATG | Minor structural protein | ORF28 (L. plantarum phage Sha1) (339/768; 44) | 0 | 789 | ADW01309 |
| 36 | 27065 | 30406 | 1,113 | 118.8 | 4.9 | GAGTAGGAGGTTATCaaATG | Tail fiber/Host specificity protein | ORF29 (L. plantarum phage Sha1) (373/588; 63) | 0 | 929 | ADW01310 |
| 37 | 30403 | 30624 | 73 | 8.0 | 4.7 | CTGGAGGATAAAATCaaATG | ORF30 (L. plantarum phage Sha1) (61/71; 86) | 3.0E−34 | 84 | ADW01311 | |
| 38 | 30639 | 31061 | 140 | 16.1 | 4.7 | AAATAGGAGGAAATTaaactATG | ORF96 (L. plantarum phage LP65) (46/123; 37) | 5.0E−20 | 125 | YP_164731 | |
| 39 | 31078 | 32277 | 399 | 41.8 | 4.6 | AGAAAGGAATGATTTggtTTG | P53-like protein | ORF32 (L. plantarum phage Sha1) (115/179; 64) | 3.0E−65 | 294 | ADW01313 |
| 40 | 32351 | 33742 | 463 | 50.7 | 9.4 | TAAAAGGAGACAAAAagATG | Lysin | ORF88 (L. plantarum phage LP65) (388/45; 86) | 0 | 464 | YP_164723 |
| 41 | 33760 | 34098 | 112 | 12.9 | 5.3 | ATAAGGGAGGTTCACcacATG | phig1ep16 (L. plantarum phage phig1e) (26/92; 28) | 4.0E−5 | 118 | YP_003084354 | |
| 42 | 34085 | 34693 | 202 | 21.1 | 4.4 | AGGGGAGAAATAAACATG | ORF35 (L. plantarum phage Sha1) (53/95; 56) | 5.0E−29 | 176 | ADW01316 | |
| 43 | 34751 | 35725 | 324 | 38.3 | 7.7 | GGGAATGGTGAGATAcaATG | Recombinase/integrase | gp131 (Lc. lactis phage 949) (80/324; 25) | 8.0E−12 | 330 | YP_004306291 |
| 45 | 38528 | 36027 | 833 | 95.1 | 7.9 | ATTAGTCAGATGAAGatATA | DNA polymerase III alpha subunit | yorL (Bacillus phage SPBc2) (279/833; 34) | 7.0E−128 | 1,305 | NP_046685 |
| 47 | 40897 | 39467 | 476 | 54.9 | 5.7 | ATAGAGGAGGAAAAAtaATG | DNA polymerase III alpha subunit | yorL (Bacillus phage SPBc2) (171/472; 37) | 3.0E−72 | 1,305 | NP_046685 |
| tRNA | 42308 | 42237 | tRNA-Arg | ||||||||
| tRNA | 42595 | 42522 | tRNA-Arg | ||||||||
| 53 | 43264 | 42965 | 99 | 11.7 | 7.8 | TTATTGGAGGACATAttATG | ORF148 (L. plantarum phage LP65) (39/95; 41) | 2.0E−15 | 93 | YP_164783 | |
| 54 | 43897 | 43340 | 185 | 21.4 | 9.2 | ATTAAGAACATTACCATG | ORF145 (L. plantarum phage LP65) (76/121; 63) | 6.0E−46 | 157 | YP_164780 | |
| 55 | 44582 | 44277 | 101 | 11.7 | 5.0 | TGATTGGAGCAGTGAataATG | ORF21 (L. plantarum phage LP65) (32/86; 38) | 2.0E−10 | 95 | YP_164656 | |
| 57 | 45726 | 45028 | 232 | 24.3 | 5.2 | AGAAAGAGGTTTATTttaaATG | Endolysin | ORF121 (L. plantarum phage LP65) (92/204; 46) | 1.0E−33 | 193 | YP_164756 |
| 58 | 46337 | 45804 | 177 | 20.7 | 8.8 | AGGGAGAAATTAAATcATG | LRATCC53608_1805 (L. reuteri ATCC 53608) (30/97; 31) | 5.0E−3 | 174 | CCC04558 | |
| 60 | 46931 | 46671 | 86 | 9.7 | 5.8 | AGCGAGGAAAACGGCcgGTG | Growth inhibitor | ORF6 (L. reuteri) (33/87; 38) | 1.0E−10 | 94 | CAC03499 |
| 63 | 47855 | 47460 | 131 | 15.9 | 7.8 | GAAAGAGAGGTAAATaatgATG | ORF32 (L. plantarum phage LP65) (81/124; 66) | 1.0E−52 | 130 | YP_164667 | |
| 65 | 48770 | 48306 | 154 | 17.1 | 4.4 | AAGACGGAGGTAAAAtaATG | Nucleoside deoxyribosyltransferase | lb338_phage_72 (L. paracasei phage Lb338-1) (56/156; 36) | 5.0E−27 | 164 | YP_002790751 |
| 66 | 49087 | 48770 | 105 | 12.0 | 7.7 | AGATGGAGAGTGCTAaagATG | Glutaredoxin | nrdH (L. plantarum JDM1) (31/86; 36) | 5.0E−10 | 76 | YP_003062153 |
| 68 | 49575 | 49234 | 113 | 13.2 | 4.1 | GGAAAAGTGATTGTAATG | ORF127 (L. plantarum phage LP65) (41/64; 65) | 3.0E−16 | 74 | YP_164762 | |
| 69 | 50369 | 49665 | 234 | 26.5 | 9.4 | CAGAGTCAGTTAGTCggGTG | Nicotinamide mononucleotide transporter | ORF125 (L. plantarum phage LP65) (78/229; 34) | 3.0E−34 | 259 | YP_164760 |
| 70 | 51724 | 50492 | 410 | 46.2 | 5.7 | TACTAGAGGGAGAACttaATG | DNA polymerase | ORF63 (L. plantarum phage LP65) (90/323; 28) | 1.0E−13 | 434 | YP_164698 |
| 71 | 52780 | 51737 | 347 | 39.2 | 5.9 | AGGAGTGAGAGTATAaaaATG | ATP/GTP binding protein | yorG (Bacillus phage SPBc2) (81/335; 24) | 3.0E−18 | 323 | NP_046680 |
| 73 | 54148 | 53165 | 327 | 36.0 | 4.8 | AACTAGGAGGAATTTgtaATG | Replication protein | ORF29 (L. casei phage phiAT3) (25/64; 39) | 2.4E−2 | 185 | YP_25056 |
| 74 | 54555 | 54220 | 111 | 13.1 | 4.4 | CACGATAATGTGAATtATG | BsubsN3_22549 (Bacillus subtilis sNCIB 3610) (27/92; 30) | 7.3E−2 | 344 | ZP_03598300 | |
| 75 | 55051 | 54527 | 174 | 20.4 | 9.2 | TAAAATTAAAAATACaaATG | LRU_02117 (Lactobacillus ruminis SPM0211) (68/143; 48) | 1.0E−38 | 151 | ZP_08564332 | |
| 82 | 57966 | 58499 | 177 | 20.5 | 6.3 | GAAGTGGAGTTGAGCgaatATG | Sca_0483 (Streptococcus carnosus TM300) (32/67; 48) | 1.0E−9 | 158 | YP_002633582 | |
| 83 | 58517 | 59224 | 235 | 27.2 | 4.8 | AATTAGGAGGAAAAAtaTTG | Deoxyguanosine kinase | ORF73 (L. paracasei phage Lb338-1) (80/239; 33) | 2.0E−42 | 240 | YP_002790752 |
| 85 | 59491 | 59961 | 156 | 18.2 | 9.1 | AAACAGGAGGTTAAAaccaATG | yorH (Bacillus subtilis subsp. natto) (49/162; 31) | 3.0E−12 | 162 | YP_004243622 | |
| 86 | 59954 | 61543 | 529 | 60.3 | 4.9 | TTAGTGGAGATGATTtacTTG | DNA helicase | yorI (Bacillus subtilis subsp. natto) (186/517; 36) | 2.0E−89 | 530 | YP_004243623 |
| 87 | 61745 | 62590 | 281 | 32.1 | 8.6 | CTCAAACTGTGGTTCaATG | DNA primase | ORF61 (Lc. lactis phage 949) (49/162; 31) | 2.0E−6 | 330 | ADM73619 |
| 88 | 62587 | 64332 | 581 | 65.6 | 5.3 | ACAAGGAAGGTAATGtcTTG | Single-stranded DNA exonuclease | ORF62 (Lc. lactis phage 949) (141/572; 25) | 2.0E−18 | 593 | YP_004306222 |
| 91 | 64909 | 65292 | 127 | 14.4 | 6.6 | CATTAGGAGGAAAAAgcgATG | ORF14 (L. plantarum phage LP65) (53/118; 45) | 2.0E−22 | 120 | YP_164649 | |
| 98 | 67529 | 67873 | 114 | 13.5 | 4.8 | TAAAATGACGAAAGAactaATG | ORF13 (L. plantarum phage LP65) (33/106; 31) | 7.0E−9 | 124 | YP_164648 | |
| 99 | 67870 | 68175 | 101 | 11.7 | 5.3 | TGGAATGGAGAGAGCATA | DNA binding protein | ORF97 (L. plantarum phage phiJL-1) (66/97; 68) | 3.0E−41 | 97 | YP_223877 |
| 100 | 68172 | 68573 | 133 | 15.6 | 4.4 | AAAGAGGAGGATAAGaagctATG | ORF5 (L. plantarum phage LP65) (26/71; 37) | 1.0E−1 | 182 | YP_164640 | |
| 101 | 68896 | 69588 | 230 | 26.4 | 6.7 | GGAGAAGAGGAGTTTaaatATG | ORF93 (L. plantarum phage phiJL-1) (35/85; 41) | 1.0E−08 | 93 | YP_223879 | |
| 102 | 69578 | 70021 | 147 | 16.9 | 5.9 | AGAAAGGTGACAACGATG | ORF157 (L. plantarum phage LP65) (55/156; 35) | 4.0E−10 | 146 | YP_164792 | |
| ORF142 (L. plantarum phage phiJL-1) (52/149; 35) | 2.0E−10 | 142 | YP_223880 | ||||||||
| 105 | 70682 | 71113 | 143 | 16.3 | 4.4 | AAAACGGAGGTGGCAacgATG | DNA replication protein | ORF15 (L. plantarum phage LP65) (55/161; 34) | 2.0E−07 | 149 | YP_164650 |
| ORF115 (L. plantarum phage phig1e) (49/144; 34) | 2.0E−10 | 115 | NP_695176 | ||||||||
| 108 | 71911 | 72354 | 147 | 16.6 | 4.9 | TCGATGGTAGTGACGatATG | ORF8 (L. plantarum phage Sha1) (49/106; 46) | 3.0E−18 | 140 | ADW01289 | |
| 110 | 72510 | 72953 | 147 | 17.4 | 5.7 | AGGAAGGCAGTGGTAatcATG | ORF93 (L. plantarum phage phiJL-1) (54/91; 59) | 4.0E−21 | 93 | YP_223879 | |
| 111 | 72964 | 73206 | 80 | 9.5 | 4.7 | TGTTAGGGGGAATAAtATG | phig1ep44 (L. plantarum phage phig1e) (16/50; 32) | 2.0E−3 | 73 | NP_695175 | |
| 114 | 73964 | 74617 | 217 | 25.0 | 5.0 | AGTAAGAAGGGAAAAaATG | Thymidine kinase | tk (enterobacterial phage RB69) (56/196; 29) | 1.0E−12 | 193 | NP_861801 |
| 123 | 77549 | 77731 | 60 | 7.0 | 9.3 | TAAAAGGGGGTGTTGagATG | ORF40 (Staphylococcus phage 2638A) (33/56; 59) | 9.0E−12 | 93 | YP_239845 | |
| 124 | 77757 | 78140 | 127 | 14.5 | 10.6 | AGAATAGAGGCTTATtaaaATG | ORF165 (L. plantarum phage LP65) (85/122; 70) | 5.0E−58 | 135 | YP_164800 | |
| 127 | 79214 | 79630 | 138 | 15.8 | 4.3 | AAATAAGGGTTGCAAttaaGTG | gp24 (Brochothrix phage A9) (37/125; 30) | 2.0E−2 | 198 | YP_004301357 | |
Fig 2.
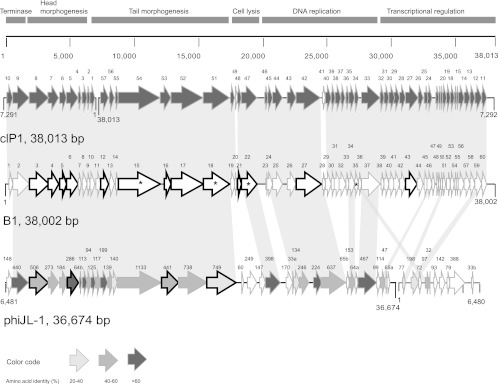
Genomic organizations of L. plantarum phages B1 and phiJL-1 as well as P. damnosus phage clP1. The scales above the genomes are in base pairs. Each arrow represents an ORF, and the numbering refers to Table 2 (for B1) and to the locus tags from phiJL-1 (accession number AY236756) and clP1 (accession number JN051154). The modules were based on the B1 organization. Genes coding for structural proteins experimentally determined by SDS-PAGE are indicated by thick outlines. Products of ORFs from phiJL-1 and clP1 sharing amino acid identity with those from B1 were drawn in a shade of gray according to the color code, and were linked by a shadow. White arrows represent products of ORFs sharing no identity. Phage phiJL-1 and clP1 genomes were split and reorganized in order to facilitate the alignment. ORFs sharing identity (>20%) with those of phage B2 are indicated by asterisks.
Fig 3.
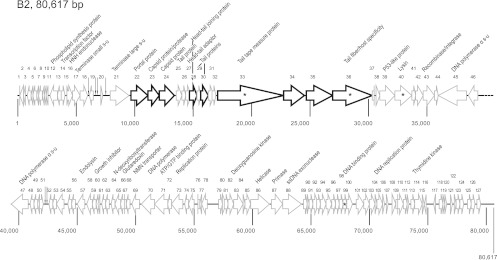
Genomic organization of L. plantarum phage B2. The scale under the genome is in base pairs. Each arrow represents an ORF, with its putative function, and the numbering refers to Table 3. Genes coding for structural proteins experimentally determined by SDS-PAGE are indicated by thick outlines. tRNAs are indicated by vertical arrows. ORFs sharing identity (>20%) with those of phage B2 are indicated by asterisks.
Presence of tRNA in the B2 genome.
Six tRNAs were found in genome of phage B2 (Table 3) but none in B1. These six tRNAs deliver the amino acids asparagine (Asn, AAC), leucine (Leu, CTA), methionine (Met, ATG), glycine (Gly, GGA), and arginine (Arg, AGG and AGA). They were located in two genomic regions (6246 to 7814 and 42308 to 42522) of phage B2. Among L. plantarum phages for which the genomes are available, only the myophage LP65 contained tRNAs (14 tRNAs). The presence of tRNAs is often linked to large phage genomes (62).
The frequency of codon usage was then investigated for phages B1 and B2 (Table 4). The anticodons of some tRNAs found in the genome of phage B2 did not correspond to the codons most frequently used by the phage. For example, one tRNA matched the CTA codon, encoded a leucine, and had a frequency of 21.1% in the whole genome, whereas the most frequently used leucine codon was TTA, which had a frequency of 42.5%. However, the CCT and TCT codons, which encoded arginine, were used more by phage B2 than other possible codons.
Table 4.
Codon usage of L. plantarum strains and phage B2 for amino acids encoded by the B2 tRNAsa
| Amino acid | Anticodon | Codon | Frequency of codon usage (%) for: |
||
|---|---|---|---|---|---|
| Phage B1 | Phage B2 | L. plantarum WCSF1 | |||
| Asn | GTT | AAC | 16.9 | 19.3 | 17.5 |
| AAT | 36.8 | 25.9 | 26.6 | ||
| Leu | TAG | CTA | 14.3 | 21.1 | 11.5 |
| TTA | 17.6 | 42.5 | 33.4 | ||
| TTG | 14.1 | 41.0 | 25.3 | ||
| CTT | 9.4 | 13.6 | 8.9 | ||
| CTC | 5.1 | 5.3 | 8.7 | ||
| CTG | 11.2 | 21.9 | 12.3 | ||
| Met | CAT | ATG | 32.0 | 38.4 | 26.1 |
| Gly | TCC | GGA | 12.9 | 8.5 | 10.0 |
| GGT | 22.7 | 12.4 | 26.4 | ||
| GGC | 25.2 | 5.3 | 17.3 | ||
| GGG | 11.9 | 6.8 | 12.3 | ||
| Arg | TCT | AGA | 3.7 | 20.7 | 1.7 |
| CCT | AGG | 2.9 | 11.3 | 0.8 | |
| CGT | 12.6 | 8.7 | 11.7 | ||
| CGC | 12.3 | 4.1 | 8.8 | ||
| CGA | 6.6 | 6.6 | 7.1 | ||
| CGG | 11.2 | 7.1 | 12.9 | ||
Codons indicated in boldface are those encoded by the tRNAs in the phage B2 genome.
The codon usage of phage B2 was also compared to that of L. plantarum WCFS1 because no bacterial host strain for phage B2 has been sequenced yet (Table 4). Our results agreed with others (3) who suggested that phages encode tRNAs corresponding to codons that are less used by the host bacteria to increase specific phage protein expression (Table 4). The presence of tRNAs was reported for some Lactococcus phages: P087 (5 tRNAs) (69), KSY1 (3 tRNAs) (11), and 949 (6 tRNAs) (62). In contrast to the results observed here, the frequencies of codon usage by phage 949 tRNAs were similar for the phage and its host Lactococcus lactis IL1403.
Function assignment and genomic organization of phages B1 and B2.
The ORF functions were assigned based on comparison with sequences in public databases (NCBI, InterProScan). Only the ORFs with the highest identity with those encoding other proteins in the database are shown in Tables 2 and 3. Although phages B1 and B2, isolated from corn silage and anaerobic sewage sludge, respectively, were similar according to morphological observations, genome sequencing confirmed wide differences between the phages. Diversity among Lactobacillus phages, due possibly to the high number of species in the Lactobacillus genus, was reported previously (74). However, a relatively conserved genome organization among them was evidenced (74). Yet, L. plantarum phages appear to be among the most diverse Lactobacillus phages. Distinct ecological niches and unrelated host strains may explain such diversity.
As for many siphophages, the genome of phage B1 is organized into the following functional clusters: DNA packaging, morphogenesis, lysis, and DNA replication (Fig. 2). No genes/proteins related to lysogeny were found, confirming its virulent nature. Interestingly, a high level of identity (97%) with the genome of phage clP1, infecting Pediococcus damnosus, followed by 77% identity with the genome of L. plantarum phage JL-1, was found. Of note, the genome of phage clP1 showed a GC content of 47.6%, which is much higher than those reported for pediococci (37.8 to 41.2%) (35). When each ORF was analyzed, high levels of identity with phage clP1 deduced proteins (65 to 100%) were also observed, while the levels of identity with proteins of phage JL-1 were always lower (29 to 80%) (Table 2). Pediococcus and L. plantarum strains are often found in the same ecological niches (cucumber fermentation, silage inoculants) (34, 73); thus, these comparative analyses support the notion that coexistence in the same environment can lead to the exchange of genetic elements (45). Others have shown that phages of L. plantarum were able to infect strains of other bacterial species isolated from the same habitat (10, 20, 45), although this was not tested here. L. plantarum myophage LP65 unexpectedly infected Carnobacterium strains associated with fermented meat (10), and some L. plantarum phages isolated from silage and sauerkraut were able to infect Lactobacillus pentosus and Lactobacillus brevis strains (20, 45). On the other hand, phages B1 and B2 have a narrow host range, as reported for other L. plantarum phages (11, 44, 69).
The genomic organization of phage B2 was also similar to those of other siphophages (Fig. 3). Some ORFs exhibited homology with L. plantarum myophage LP65. However, most were similar to ORFs of Bacillus and Lactobacillus strains and their phages (Table 3). Few proteins (Orf39, Orf43, and Orf105) were linked to prophage proteins, but phage B2 had the growth characteristics of a virulent phage. This observation was also reported for L. plantarum phages LP65 (10), g1e (70), and Sha1 (72). Overall, the genome assemblage of phage B2 was rather unique and appears to be made from parts of other characterized phages.
Phage DNA packaging.
The deduced B1 proteins Orf1 and Orf2 share high similarity with the putative small and large terminase subunits from various phages, including P. damnosus clP1 and L. plantarum g1e and phiJL-1. Phage B2 Orf17 and Orf21 exhibited sequence similarities to the small and large subunits of the terminases from Bacillus subtilis subsp. natto and Lactobacillus delbrueckii phage c5, respectively. Of note, this B2 genomic region was interrupted by 4 tRNAs. In tailed phages, the small terminase subunit is responsible for specific DNA binding whereas the large terminase subunit mediates the cleavage of concatameric phage DNA into genome units as well as prohead binding (26). In particular, the large subunit usually provides the endonuclease and ATPase activities for packaging (38).
The Orf59 gene product of phage B1 was associated with endonuclease function due to its homology with Orf12 of Pediococcus phage clP1 and Orf51 of Lactobacillus casei phage phiAT3. Taking into account the position of the gene in the phage B1 genome, this protein might also be involved in the DNA packaging or replication (43). In phage B2, Orf16 was identified as an HNH endonuclease, which could be involved in DNA packaging since it precedes the small terminase subunit. The HNH family of proteins is associated with DNA binding and cutting functions and includes some phage packaging proteins (47).
Phage DNA replication.
Orf24 and Orf26 of phage B1 have several characteristics in common with endonucleases and helicases (NTP binding). Orf27 exhibited homology to DNA primases, Orf31 to replication proteins, Orf34 to replicases, and Orf35 to DNA binding proteins. A helicase function was also attributed to Orf37 since it shared 99% identity with the putative helicase from phage clP1 (P. damnosus). These seven proteins may be involved in DNA replication. The phage B2 proteins Orf45 and Orf47 exhibited similarities to the DNA polymerase III protein (α subunit) from Bacillus phage SPBc2 (42). A DNA polymerase function was also attributed to Orf70. It is tempting to speculate that phage B2 encodes its own DNA polymerase instead of relying on its host. Helicase and DNA primase functions were attributed to Orf86 and Orf87, respectively. The protein product of ORF88 may be an exonuclease, and Orf71 may be linked to ATP/GTP binding proteins. Other B2 proteins may have roles in nucleotide modification (Orf65, Orf69, Orf83, and Orf114).
Host lysis.
A key step of the phage infection process is the release of new virions at the end of the lytic cycle. Orf21 of phage B1 has similarities with the holins of P. damnosus phage clP1 and of L. casei phage AT3. It has a transmembrane domain in the N-terminal part similar to holins of Lactobacillus rhamnosus phages Lc-Nu and Lmr1 (24, 66). Orf22 exhibited sequence similarity to the endolysins from various phages and was classified an endo-N-acetylmuramidase (muramidase). For phage B2, the endolysin function was attributed to ORF40 (muramidase-like endolysin) as well as Orf57 (transglycosylase). Similarly, two endolysins were encoded by the L. plantarum myophage LP65 genome (Orf88 and Orf121) (10). No recognizable gene encoding a holin was found for phage B2. Of the four classes of bacterial endolysins recognized (muramidase, tranglycosylase, amidase, and peptidase), two are commonly found in Lactobacillus phages (muramidase and amidase) (70). Moreover, similarities found among lysins of phages infecting several bacterial species could suggest a common evolutionary origin. Endolysins from phages LL-H (Lactobacillus delbrueckii subsp. lactis) and 0303 (Lactobacillus helveticus) were able to hydrolyze the cell walls of some species from Lactobacillus and Pediococcus (17, 67).
Structural proteins of phages B1 and B2.
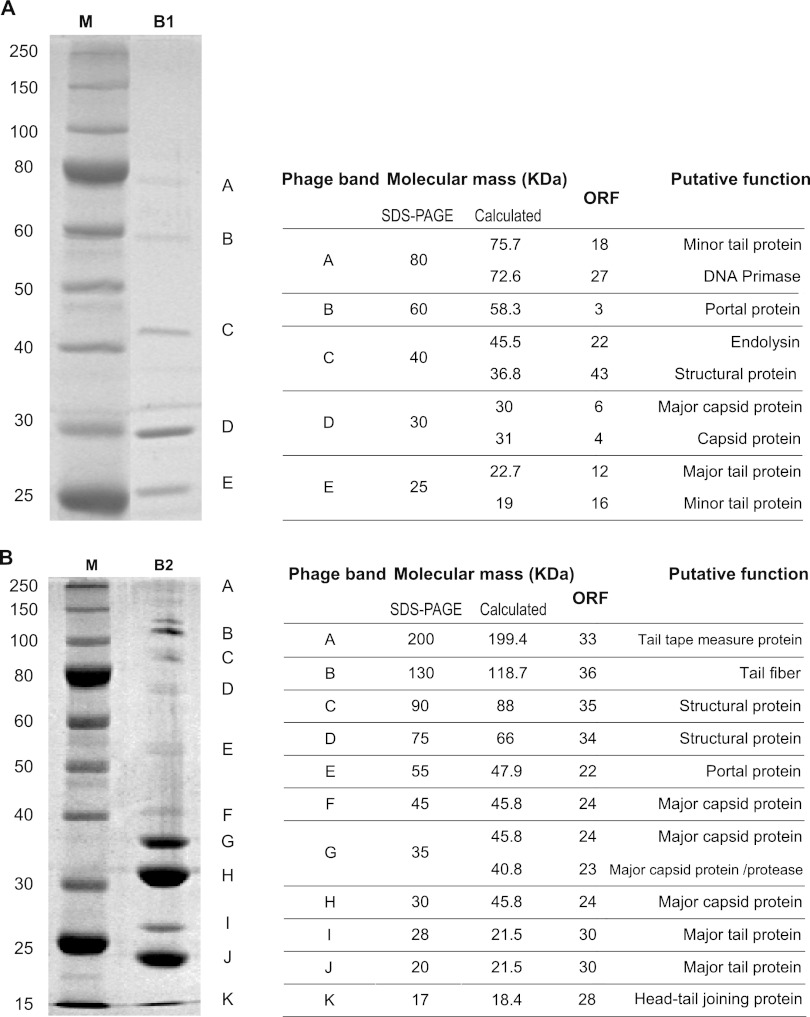
Analysis of phage B1 using SDS-PAGE revealed at least five protein bands (Fig. 4A). Band B was associated with one phage protein (Orf3, portal), whereas two phage proteins were identified in the other four bands. Band A contained a minor tail protein (Orf18) and, surprisingly, a putative DNA primase (Orf27). Band C was made of Orf43 and Orf22 (endolysin). Band D contained two capsid proteins (Orf4 and Orf6). Finally, bands B and E contained two tail proteins, Orf12 and Orf16. Orf27 (primase) and Orf22 (endolysin) are likely nonstructural proteins that were carried over despite the phage purification procedure. Overall, the observed molecular masses of the phage proteins matched the theoretical values (Fig. 4A). Proteomic analysis of the complete phage particle revealed four other proteins (Orf5, Orf15, Orf17, and Orf21). Orf5 and Orf15 likely correspond to the scaffold and the tape measure proteins, respectively.
Fig 4.
Migration of the phage B1 (A) and B2 (B) proteins on a 12% SDS-PAGE gel followed by Coomassie blue staining. The numbers on the left indicate the molecular masses of the ladder (protein ladder, 10 to 250 kDa; New England BioLabs). Letters on the right indicate bands cut out of the gel and identified by LC-MS/MS. Tables show the analysis of phage B1 and B2 structural proteins by LC-MS/MS.
For phage B2, significantly more protein bands were observed by SDS-PAGE (Fig. 4B). Except for protein band G, which contained two phage capsid proteins (Orf23 and Orf24), all Coomassie-stained bands contained only one phage protein. Orf24 (major capsid protein), with a calculated molecular mass of 45.8 kDa, was associated with three protein bands (F, G, and H), with estimated molecular masses of 45, 35, and 30 kDa, respectively. In fact, when the peptides from Orf24 in bands G and H were analyzed, it was found that the N-terminal peptides of the protein were missing. This suggested that the B2 major capsid protein was processed, a phenomenon observed for other phages (28, 40). Orf23, found in band G, shared homology with a major capsid protein from Bacillus and peptidase U35, which can be fused with capsid proteins (28). This putative peptidase activity may be involved in cleavage of Orf24. Orf36, associated with band B, showed homology with the tail fiber protein of phage Sha1 (L. plantarum). However, tail fibers were not observed in the morphology of phage B2 by electron microscopy (72). In total, nine structural proteins were identified for phage B2 (Fig. 4B). Analysis of the complete phage B2 particles did not reveal any additional structural proteins.
Conclusions.
Lactobacillus phages are understudied compared to other industrially relevant lactic acid bacteria (18, 29). One possible reason is that there are fewer reports of Lactobacillus phage infections than of Lactococcus lactis and Streptococcus thermophilus infections in the food industry. It is unclear if this lack of reported Lactobacillus phage infections is due their specific uses or due to their intrinsic properties. Understanding this paucity of Lactobacillus phage infections in industrial settings may provide novel tools to control phage populations in other susceptible environments. Still, phages infecting several Lactobacillus species represent a risk for industrial users (6, 7, 10, 58, 70). Knowledge of their diversity is necessary to devise adapted control strategies. L. plantarum phages seem to have a relatively narrow host range, suggesting that strain rotation could be, whenever possible, an approach to limit phage multiplication. Moreover, some L. plantarum strains carry phage resistance mechanisms, which may be taken into account during the strain selection process. Comparative analysis of the phage B1 genome indicated that it is related to that of L. plantarum phage JL-1, suggesting that they form a phage group. On the other hand, analysis of the phage B2 genome suggested that this phage is currently unique among L. plantarum phages. The ever-increasing number of complete phage genome sequences has greatly improved our knowledge about phage diversity. The characterization of additional L. plantarum phages will help to determine the extent of their diversity.
ACKNOWLEDGMENTS
We are grateful to Willem de Vos and Michiel Kleerebezem for strain WCFS1. We thank Barb Conway for editorial assistance.
M.B.M. was the recipient of a doctoral international fellowship awarded by American Society for Microbiology. S.M. acknowledges funding from the Natural Sciences and Engineering Research Council of Canada. S.M. holds a Tier 1 Canada Research Chair on Bacteriophages.
Footnotes
Published ahead of print 5 October 2012
REFERENCES
- 1. Ackermann HW. 2009. Phage classification and characterization. Methods Mol. Biol. 501:127–140 [DOI] [PubMed] [Google Scholar]
- 2. Awad S, Ahmed N, El Soda M. 2010. Influence of microfiltration and adjunct culture on quality of Domiati cheese. J. Dairy Sci. 93:1807–1814 [DOI] [PubMed] [Google Scholar]
- 3. Bailly-Bechet M, Vergassola M, Rocha E. 2007. Causes for the intriguing presence of tRNAs in phages. Genome Res. 17:1486–1495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Binetti AG, Suárez VB, Tailliez P, Reinheimer JA. 2007. Characterization of spontaneous phage-resistant variants of Streptococcus thermophilus by randomly amplified polymorphic DNA analysis and identification of phage-resistance mechanisms. Int. Dairy J. 17:343–349 [Google Scholar]
- 5. Bringel F, Frey L, Boivin S, Hubert J-C. 1997. Arginine biosynthesis and regulation in Lactobacillus plantarum: the carA gene and the argCJBDF cluster are divergently transcribed. J. Bacteriol. 179:2697–2706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Capra ML, Binetti AG, Mercanti DJ, Quiberoni A, Reinheimer JA. 2009. Diversity among Lactobacillus paracasesi phages isolated from a probiotic dairy product plant. J. Appl. Microbiol. 107:1350–1357 [DOI] [PubMed] [Google Scholar]
- 7. Capra ML, Quiberoni A, Ackermann HW, Moineau S, Reinheimer JA. 2006. Characterization of a new virulent phage (MLC-A) of Lactobacillus paracasei. J. Dairy Sci. 89:2414–2423 [DOI] [PubMed] [Google Scholar]
- 8. Capra ML, Quiberoni A, Reinheimer J. 2006. Phages of Lactobacillus casei/paracasei: response to environmental factors and interaction with collection and commercial strains. J. Appl. Microbiol. 100:334–342 [DOI] [PubMed] [Google Scholar]
- 9. Caso JL, et al. 1995. Isolation and characterization of temperate and virulent bacteriophage of Lactobacillus plantarum. J. Dairy Sci. 78:741–750 [Google Scholar]
- 10. Chibani-Chennoufi S, Dillmann M-L, Marvin-Guy L, Rami-Shojaei S, Brüssow H. 2004. Lactobacillus plantarum LP65: a new member of the SPO1-like genus of the family Myoviridae. J. Bacteriol. 186:7069–7083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chopin A, Deveau H, Ehrlich SD, Moineau M, Chopin MC. 2007. KSY1, a lactococcal phage with a T7-like transcription. Virology 365:1–9 [DOI] [PubMed] [Google Scholar]
- 12. Corsetti A, Valmorri S. 2011. Lactobacillus spp.: Lactobacillus plantarum, p 114–118 In Fuquay J, Fox P, McSweeney P. (ed), Encyclopedia of dairy science, 2nd ed, vol 3 Academic Press, Elsevier Science, San Diego, CA [Google Scholar]
- 13. Craig R, Beavis RC. 2003. A method for reducing the time required to match protein sequences with tandem mass spectra. Rapid Commun. Mass Spectrom. 17:2310–2316 [DOI] [PubMed] [Google Scholar]
- 14. De Angelis M, et al. 2008. Selection and use of autochthonous multiple strain cultures for the manufacture of high moisture traditional Mozzarella cheese. Int. J. Food Microbiol. 125:123–132 [DOI] [PubMed] [Google Scholar]
- 15. De Angelis M, Gobbetti M. 2011. Lactobacillus spp.: general characteristics, p 78–90 In Fuquay J, Fox P, McSweeney P. (ed), Encyclopedia of dairy science, 2nd ed, vol 3 Academic Press, Elsevier Science, San Diego, CA [Google Scholar]
- 16. De Antoni G, et al. 2010. Lactobacillus plantarum bacteriophages isolated from Kefir grains: phenotypic and molecular characterization. J. Dairy Res. 77:7–12 [DOI] [PubMed] [Google Scholar]
- 17. Deutsch S-M, Guezenec S, Piot M, Foster S, Lortal S. 2004. Mur-LH, the broad-spectrum endolysin of Lactobacillus helveticus temperate bacteriophage Φ-0303. Appl. Environ. Microbiol. 70:96–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Deveau H, Labrie SJ, Chopin Moineau MCS. 2006. Biodiversity and classification of lactococcal phages. Appl. Environ. Microbiol. 72:4338–4346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Deveau H, van Calsteren MR, Moineau S. 2002. Effect of exopolysaccharides on phage-host interactions in Lactococcus lactis. Appl. Environ. Microbiol. 68:4364–4369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Doi K, et al. 2003. A comparative study and phage typing of silage-making Lactobacillus bacteriophages. J. Biosci. Bioeng. 95:518–525 [DOI] [PubMed] [Google Scholar]
- 21. Douglas LJ, Wolin MJ. 1971. Cell wall polymers and phage lysis of Lactobacillus plantarum. Biochemistry 10:1551–1555 [DOI] [PubMed] [Google Scholar]
- 22. Duplessis M, Moineau S. 2001. Identification of a genetic determinant responsible for host specificity in Streptococcus thermophilus bacteriophages. Mol. Microbiol. 41:325–336 [DOI] [PubMed] [Google Scholar]
- 23. Dupuis M-E, Moineau S. 2010. Genome organization and characterization of the virulent lactococcal phage 1358 and its similarities with Listeria phages. Appl. Environ. Microbiol. 76:1623–1632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Durmaz E, Miller MJ, Azcarate-Peril MA, Toon SP, Klaenhammer TR. 2008. Genome sequence and characteristics of Lrm1, a prophage from industrial Lactobacillus rhamnosus strain M1. Appl. Environ. Microbiol. 74:4601–4609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Emond E, Moineau S. 2007. Bacteriophages and food fermentations, p 93–124 In McGrath S, van Sinderen D. (ed), Bacteriophage: genetics and molecular biology. Horizon Scientific Press/Caister Academic Press, Norfolk, United Kingdom [Google Scholar]
- 26. Fujisawa H, Morita M. 1997. Phage DNA sequencing. Genes Cells 2:537–545 [DOI] [PubMed] [Google Scholar]
- 27. Garneau J, Moineau S. 2011. Bacteriophages of lactic acid bacteria and their impact on milk fermentations. Microb. Cell Fact. 10(Suppl. 1):S20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Garneau J, Tremblay DM, Moineau S. 2008. Characterization of 1706, a virulent phage from Lactococcus lactis with similarities to prophages from other Firmicutes. Virology 373:298–309 [DOI] [PubMed] [Google Scholar]
- 29. Guglielmotti D, et al. 2009. Genome analysis of two virulent Streptococcus thermophilus phages isolated in Argentina. Int. J. Food Microbiol. 136:101–109 [DOI] [PubMed] [Google Scholar]
- 30. Hall TA. 1999. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. (Oxf.). 41:95–98 [Google Scholar]
- 31. Hendrix RW. 2002. Bacteriophages: evolution of the majority. Theor. Popul. Biol. 61:371–480 [DOI] [PubMed] [Google Scholar]
- 32. Hultberg A, et al. 2007. Lactobacilli expressing llama VHH fragments neutralise Lactococcus phages. BMC Biotechnol. 7:58 doi:10.1186/1472-6750-7-58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Keller A, Nesvizhskii AI, Kolker E, Aebersold R. 2002. Empirical statistical model to estimate the accuracy of peptide identifications made by MS/MS and database search. Anal. Chem. 74:5383–5392 [DOI] [PubMed] [Google Scholar]
- 34. Kelly D, et al. 2011. Isolation and characterization of bacteriophages that inhibit strains of Pediococcus damnosus, Lactobacillus brevis, and Lactobacillus paraplantarum that cause beer spoilage. J. Am. Soc. Brew. Chem. 69:8–12 [Google Scholar]
- 35. Kelly D, et al. 4 May 2012. Genome sequence of the phage c1P1, which infects the beer spoilage bacterium Pediococcus damnosus. Gene doi:10.1016/j.gene.2012.04.085 [DOI] [PubMed] [Google Scholar]
- 36. Kleerebezem M, et al. 2003. Complete genome sequence of Lactobacillus plantarum WCFS1. Proc. Natl. Acad. Sci. U. S. A. 100:1990–1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kodaira KI, et al. 1997. Genome structure of the Lactobacillus temperate phage wg1e: the whole genome sequence and the putative promoter/repressor system. Gene 187:45–53 [DOI] [PubMed] [Google Scholar]
- 38. Kutter E, Sulakvelidze A. 2005. Bacteriophages: biology and applications. CRC Press, Boca Raton, FL [Google Scholar]
- 39. Labrie SJ, Samson JE, Moineau S. 2010. Bacteriophage resistance mechanisms. Nat. Rev. Microbiol. 8:317–327 [DOI] [PubMed] [Google Scholar]
- 40. Labrie SJ, et al. 2012. Involvement of the major capsid protein and two early-expressed phage genes in the activity of the lactococcal abortive infection mechanism AbiT. Appl. Environ. Microbiol. 78:6890–6899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Laslett D, Canback B. 2004. ARAGORN, a program to detect tRNA genes and tmRNA genes in nucleotide sequences. Nucleic Acids Res. 32:11–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lazarevic V, et al. 1999. Nucleotide sequence of the Bacillus subtilis temperate bacteriophage SPbetac2. Microbiology 145:1055–1067 [DOI] [PubMed] [Google Scholar]
- 43. Lu Z, et al. 2005. Sequence analysis of the Lactobacillus plantarum bacteriophge ΦJL-1. Gene 348:45–54 [DOI] [PubMed] [Google Scholar]
- 44. Lu Z, Breidt F, Fleming HP, Altermann E, Klaenhammer TR. 2003. Isolation and characterization of a Lactobacillus plantarum bacteriophage, JL-1, from a cucumber fermentation. Int. J. Food Microbiol. 84:225–235 [DOI] [PubMed] [Google Scholar]
- 45. Lu Z, Breidt F, Plengvidhya V, Fleming HP. 2003. Bacteriophage ecology in commercial sauerkraut fermentations. Appl. Environ. Microbiol. 69:3192–3203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lukashin AV, Borodovsky M. 1998. GeneMark.hmm: new solutions for gene finding. Nucleic Acids Res. 26:1107–1115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Mehta P, Katta K, Krishnaswamy S. 2004. HNH family subclassification leads to identification of commonality in the His-Me endonuclease superfamily. Protein Sci. 13:295–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Milesi MM, McSweeney PLH, Hynes ER. 2008. Impact of chymosin- and plasmin-mediated primary proteolysis on the growth and biochemical activities of lactobacilli in miniature cheddar-type cheeses. J. Dairy Sci. 91:3277–3290 [DOI] [PubMed] [Google Scholar]
- 49. Milesi MM, McSweeney PLH, Hynes ER. 2008b. Viability and contribution to proteolysis of an adjunct culture of Lactobacillus plantarum in two model cheese systems: cheddar cheese type and soft-cheese type. J. Appl. Microbiol. 105:884–892 [DOI] [PubMed] [Google Scholar]
- 50. Miller A, Sandine WE, Elliker PR. 1970. Deoxyribonucleic acid base composition of lactobacilli determined by thermal denaturation. J. Bacteriol. 102:278–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Moineau S, Lévesque C. 2005. Control of bacteriophages in industrial fermentations, p 285–296 In Kutter E, Sulakvelidze A. (ed), Bacteriophages: biology and applications. CRC Press, Boca Raton, FL [Google Scholar]
- 52. Moineau S, Walker SA, Vedamuthu ER, Vandenbergh PA. 1995. Cloning and sequencing of LlaDCHI restriction/modification genes from Lactococcus lactis and relatedness of this system to the Streptococcus pneumoniae DpnII system. Appl. Environ. Microbiol. 61:2193–2203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Molin G. 2008. Lactobacillus plantarum: the role in foods and in human health, p 353–394 In Farnworth E. (ed), Handbook of fermented functional foods. CRC Press, Boca Raton, FL [Google Scholar]
- 54. Nes IF, Brendehaug J, von Husby KO. 1988. Characterization of the bacteriophage B2 of Lactobacillus plantarum ATCC 8014. Biochemistry 70:423–427 [DOI] [PubMed] [Google Scholar]
- 55. Nesvizhskii AI, Keller A, Kolker E, Aebersold R. 2003. A statistical model for identifying proteins by tandem mass spectrometry. Anal. Chem. 75:4646–4658 [DOI] [PubMed] [Google Scholar]
- 56. Neviani E, Carminati D, Giraffa G. 1992. Selection of some bacteriophage- and lysozyme-resistant variants of Lactobacillus helveticus CNRZ 892. J. Dairy Sci. 75:905–913 [Google Scholar]
- 57. Reference deleted.
- 58. Quiberoni A, Guglielmotti DM, Reinheimer JA. 2003. Inactivation of Lactobacillus delbrueckii bacteriophages by heat and biocides. Int. J. Food Microbiol. 84:51–62 [DOI] [PubMed] [Google Scholar]
- 59. Quiberoni A, Moineau S, Rousseau GM, Reinheimer JA, Ackermann H-W. 2010. Streptococcus thermophilus bacteriophages. Int. Dairy J. 20:657–664 [Google Scholar]
- 60. Quiberoni A, Suárez VB, Binetti AG, Reinheimer JA. 2011. Bacteriophage, p 430–438 In Fuquay J, Fox P, McSweeney P. (ed), Enclyclopedia of dairy science, 2nd ed, vol 1 Academic Press, Elsevier Science, San Diego, CA [Google Scholar]
- 61. Sambrook J, Russell DW. 2001. Molecular cloning: a laboratory manual, 3rd ed Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- 62. Samson J, Moineau S. 2010. Characterization of Lactococcus lactis phage 949 and comparison with other lactococcal phages. Appl. Environ. Microbiol. 76:6843–6852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Siezen RJ, et al. 2012. Complete resequencing and reannotation of the Lactobacillus plantarum WCFS1 genome. J. Bacteriol. 194:195–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Svensson U, Christiansson A. 1991. Methods for phage monitoring. FIL-IDF Bull. 263:29–36 [Google Scholar]
- 65. Trevors KE, Holley RA, Kempton AG. 1983. Isolation and characterization of a Lactobacillus plantarum bacteriophage isolated from a meat starter culture. J. Appl. Bacteriol. 54:281–288 [Google Scholar]
- 66. Tuohimaa A, Riipinen KA, Brandt K, Alatossava T. 2006. The genome of the virulent phage Lc-Nu of probiotic Lactobacillus rhamnosus, and comparative genomics with Lactobacillus casei phages. Arch. Virol. 151:947–965 [DOI] [PubMed] [Google Scholar]
- 67. Vasala A, Välkkilä M, Caldentey J, Alatossava T. 1995. Genetic and biochemical characterization of the Lactobacillus delbrueckii subsp. lactis bacteriophage LL-H lysine. Appl. Environ. Microbiol. 61:4004–4011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Vignolo GM, Fontana C, Cocconcelly PS. 2010. New approaches for the study of lactic acid bacteria biodiversity: a focus on meat ecosystems, p 251–271 In Mozzi F, Raya RR, Vignolo GM. (ed), Biotechnology of lactic acid bacteria. Blackwell Publishing, Ames, IA [Google Scholar]
- 69. Villion M, et al. 2009. P087, a lactococal phage with a morphogenesis module similar to an Enterococcus faecalis prophage. Virology 388:49–56 [DOI] [PubMed] [Google Scholar]
- 70. Villion M, Moineau S. 2009. Bacteriophages of Lactobacillus. Front. Biosci. 14:1661–1683 [DOI] [PubMed] [Google Scholar]
- 71. Wang Y, et al. 2011. Complete genome sequence of the probiotic Lactobacillus plantarum ST-III. J. Bacteriol. 193:313–314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Yoon BH, Jang SH, Chang HI. 2011. Sequence analysis of the Lactobacillus temperate phage Sha1. Arch. Virol. 156:1681–1684 [DOI] [PubMed] [Google Scholar]
- 73. Yoon SS, Barrangou-Poueys R, Breidt F, Jr, Fleming H. 2007. Detection and characterization of a lytic Pediococcus bacteriophage from the fermenting cucumber brine. J. Microbiol. Biotechnol. 17:262–270 [PubMed] [Google Scholar]
- 74. Yoon SS, Kim J-W, Breidt F, Fleming HP. 2001. Characterization of a lytic Lactobacillus plantarum bacteriophage and molecular cloning of a lysin gene in Escherichia coli. Int. J. Food Microbiol. 65:63–74 [DOI] [PubMed] [Google Scholar]
- 75. Zhang ZY, et al. 2009. Complete genome sequence of Lactobacillus plantarum JDM1. J. Bacteriol. 191:5020–5021 [DOI] [PMC free article] [PubMed] [Google Scholar]