Abstract
d-Galacturonic acid, the main monomer of pectin, is an attractive substrate for bioconversions, since pectin-rich biomass is abundantly available and pectin is easily hydrolyzed. l-Galactonic acid is an intermediate in the eukaryotic pathway for d-galacturonic acid catabolism, but extracellular accumulation of l-galactonic acid has not been reported. By deleting the gene encoding l-galactonic acid dehydratase (lgd1 or gaaB) in two filamentous fungi, strains were obtained that converted d-galacturonic acid to l-galactonic acid. Both Trichoderma reesei Δlgd1 and Aspergillus niger ΔgaaB strains produced l-galactonate at yields of 0.6 to 0.9 g per g of substrate consumed. Although T. reesei Δlgd1 could produce l-galactonate at pH 5.5, a lower pH was necessary for A. niger ΔgaaB. Provision of a cosubstrate improved the production rate and titer in both strains. Intracellular accumulation of l-galactonate (40 to 70 mg g biomass−1) suggested that export may be limiting. Deletion of the l-galactonate dehydratase from A. niger was found to delay induction of d-galacturonate reductase and overexpression of the reductase improved initial production rates. Deletion of the l-galactonate dehydratase from A. niger also delayed or prevented induction of the putative d-galacturonate transporter An14g04280. In addition, A. niger ΔgaaB produced l-galactonate from polygalacturonate as efficiently as from the monomer.
INTRODUCTION
d-Galacturonic acid is the principal component of pectin, a major constituent of sugar beet pulp and citrus peel, which are abundant and inexpensive raw materials. The annual worldwide production of sugar beet and citrus fruit is about 250 × 106 and 115 × 106 metric tons, respectively. After beet processing, 5 to 10% of the sugar beet remains as dried sugar beet pulp. This pulp contains ca. 25% pectin (6). Citrus peel contains ca. 20% pectin on a dry mass basis. Sugar beet pulp and citrus peel are mainly used as cattle feed, or they are dumped. The use as cattle feed requires that the pulp and peel are dried since; otherwise, they rot rapidly. Disposal of the material is problematic because of the bad odor generated at the dumping sites. In the case of sugar beet pulp the energy consumption for drying and pelleting are 30 to 40% of the total energy used for beet processing (6). This process is only economical when done on a large scale and when energy costs are low. Other products, such as pectin and limonene, may be extracted from citrus peel. Pectin is used as a gelling agent in the food industry; limonene as a flavor compound. These are limited markets, and with increasing energy costs and alternative animal feed sources reducing the revenues from pectin-rich biomass for cattle feed sales, it is desirable to find new ways to convert this biomass to other useful products. This may be accomplished by microbial fermentation (16). Genetically modified bacteria have been used to produce ethanol from pectin-rich biomass (5, 7). Using genetically modified fungi, d-galacturonic acid has been converted to galactaric acid (14) or to 2-keto-3-deoxy-l-galactonic acid (20).
Using fungi to valorize d-galacturonic acid is attractive since many species can use d-galacturonic acid efficiently for growth, indicating that these species have efficient d-galacturonic acid uptake. Filamentous fungi, especially Aspergillus niger, may also efficiently produce pectinases, enabling simultaneous hydrolysis and conversion of the pectin rich biomass. Other advantages are that many fungi are robust, low-pH-tolerant organisms with simple nutritional requirements.
In fungi, d-galacturonic acid is catabolized through a pathway (Fig. 1) that includes reactions catalyzed by d-galacturonic acid reductase (10), l-galactonate dehydratase (9), 2-keto-3-deoxy galactonate aldolase (8), and l-glyceraldehyde reductase (11); the intermediates are l-galactonate, 2-keto-3-deoxy-l-galactonate (3-deoxy-l-threo-hex-2-ulosonate), and l-glyceraldehyde, and the products of the pathway are pyruvate and glycerol. d-Galacturonic acid can induce pectinolytic and d-galacturonic acid catabolic genes in A. niger, regardless of whether d-galacturonic acid is metabolized or not (4, 14).
Fig 1.
Fungal d-galacturonic acid pathway. The genes encoding the enzymes in T. reesei (Tr) and A. niger (An) are indicated. The deletion of lgd1 in T. reesei and gaaB in A. niger disrupted the pathway and generated strains that accumulated l-galactonate.
By disrupting the native d-galacturonic acid catabolic pathway, it is possible to engineer fungal strains for alternative d-galacturonic acid conversions (14, 20). In the case of galactaric acid production, the gene encoding d-galacturonic acid reductase was deleted and a gene encoding a d-galacturonic acid dehydrogenase expressed (14). Strains lacking the reductase were unable to grow on d-galacturonic acid, and the strains also expressing the dehydrogenase converted d-galacturonic acid to galactaric acid. To produce 2-keto-3-deoxy-l-galactonic acid, it was only necessary to delete the gene for the 2-keto-3-deoxy-l-galactonic acid aldolase (20). The resulting strain did not grow on d-galacturonic acid (8) but converted d-galacturonic acid to 2-keto-3-deoxy-l-galactonic acid. The pathway for d-galacturonic acid catabolism in fungi can also be interrupted at the l-galactonate dehydratase step. A strain of Trichoderma reesei (anamorph of Hypocrea jecorina) in which the l-galactonate dehydratase gene, lgd1, was deleted was unable to grow on d-galacturonic acid (9). In the present study we show that deletion of the gene encoding l-galactonate dehydratase, i.e., lgd1 in T. reesei and gaaB in A. niger, results in strains that convert d-galacturonic acid to l-galactonic acid, which is excreted into the medium.
l-Galactonic acid is currently expensive and not widely used, but has the potential to be used more widely once it is available at a low price. The physicochemical properties are similar to those of d-gluconic acid, which is widely used as a chelator, in the pharmaceutical, cosmetic, and other industrial (e.g., dyes, detergents, solvents, and paints) sectors and as an acidifier in food. l-Galactonic acid is also a precursor for l-ascorbic acid (vitamin C) synthesis. The l-galactono-1,4-lactone that forms from l-galactonic acid at acidic pH can be oxidized to l-ascorbic acid chemically (3) or in a fermentative process (17).
MATERIALS AND METHODS
Strains.
The deletion of the lgd1 in Trichoderma reesei (anamorph of Hypocrea jecorina) was described previously (9). Aspergillus niger ATCC 1015 (ΔpyrG), with the gene encoding the orotidine-5′-phosphate decarboxylase (i.e., the pyrG gene) deleted (14), was used to construct the gaaB deletion strain. The cassette for deletion of gaaB contained 1,550 bp from the A. niger gaaB promoter, 1,533 bp from the A. niger gaaB terminator, and a 1,920-bp fragment containing the pyrG gene flanked with its native promoter and terminator. These fragments were obtained by PCR of A. niger ATCC 1015 genomic DNA using the primers gaaB-5-F, gaaB-5-R, gaaB-3-F, gaaB-3-R, pyrG-del-F_n, and pyrG-del-R_n (Table 1), and the proofreading DNA polymerase Phusion (Finnzymes). Plasmid pRSET-A (Invitrogen) was digested with EcoRI and PvuII (both NEB) and the terminator fragment (gaaB-3) with EcoRI to produce an intermediary construct by ligation using T4 DNA ligase (NEB). This intermediary construct was digested with XhoI (NEB) and Ecl136II (Fermentas) and ligated to the XhoI-digested promoter fragment (gaaB-5). The resulting vector was digested with Ecl136II and treated with phosphatase. The pyrG DNA fragment, after digestion with SmaI, was inserted between the two gaaB flanking regions. The deletion cassette, 5,006 bp containing the gaaB flanking regions and the pyrG gene, was released by EcoRI+XhoI digestion and introduced into the A. niger ATCC 1015 ΔpyrG strain as described previously (14). Transformants were selected by ability to grow in the absence of uracil. Strains with a correct deletion were verified by PCR and tested for growth on d-galacturonate as a sole carbon source.
Table 1.
Primers used to generate vectors for the deletion of gaaB and the incorporation of gaaA in A. niger ATCC 1015 ΔpyrG in order to confirm integration and for quantitative PCR
| Primer | Sequence (5′–3′) |
|---|---|
| gaaB-5-F | TATACTCGAGAGTTCCTCGATCAGGAACGA |
| gaaB-5-R | TATAGAGCTCGCAATCTAGTTGCAATGC |
| gaaB-3-F | TATAGAGCTCGCATTACATTGGTTATGTGGG |
| gaaB-3-R | TATAGAATTCAGACATTAGTCCCCGAGAA |
| pyrG-del-F_n | TATACCCGGGTGATTGAGGTGATTGGCGAT |
| pyrG-del-R_n | TATACCCGGGTTATCACGCGACGGACAT |
| gaaB-ORF-F | AGATCACAAGTTTCACCACGA |
| gaaB-ORF-R | GCCCCTCCAGAATGGTCTT |
| gaaA-exp-F | ATGAATTCGAGCTCCACAATGGCTCCCCCAG |
| gaaA-exp-R | AGGCGCGCCCGGGCTACTTCAGCTCCCACTTTC |
| gpdA-F | AAGTGGAAAGGCTGGTGTGC |
| gaaA_qPCR_F | AGGACACGATTACTCTACTTGTG |
| gaaA_qPCR_R | GAGCCCATATAATGGAAGTACTG |
| act_qPCR_F | CAACATTGTCATGTCTGGTGG |
| act_qPCR_R | GGAGGAGCAATGATCTTGAC |
| An07g00780_qPCR_F | CTATCATCAATGCCGCCTCC |
| An07g00780_qPCR_R | CCACTGACGAAGCCATAGAC |
| An14g04280_qPCR_F | GTATGTGAGCGAGATCTTCCC |
| An14g04280_qPCR_R | TTTCCTTGGCGAAGACAATGAC |
| An03g01620_qPCR_F | GGAATACGAAGAAGTGCAGGA |
| An03g01620_qPCR_R | GGTGTTTCCAGACATGCCAG |
The cassette for the overexpression of A. niger d-galacturonate reductase (gaaA) contained the native gaaA gene between the gpdA promoter and trpC terminator from A. nidulans, following the hygromycin B phosphotransferase (hph) gene under the gpdA promoter. The gaaA fragment was obtained by PCR from ATCC 1015 genomic DNA using the primers gaaA-exp-F and gaaA-exp-R (Table 1). The plasmid (JKp1-hph) containing the gpdA-trpC-hph fragment was derived from pRS426 (ATCC). Both JKp1-hph and the PCR-amplified gaaA fragment were digested with SacI and XmaI (both NEB), followed by ligation using T4 DNA ligase to generate the intermediary construct JKp1-hph-gaaA. JKp1-hph-gaaA was digested with BspHI and PsiI (both NEB), and the fragment containing the gpdA-gaaA-trpC-hph cassette was introduced into the A. niger ATCC 1015 gaaBΔ strain by transformation. Transformants were screened for integration of the gpdA-gaaA-trpC-hph cassette by growth in the presence of 400 μg of hygromycin B (Calbiochem) ml−1. Integration of the transformed cassette into the genome was confirmed by PCR with the primers gpdA-F and gaaA-exp-R (Table 1).
Media.
The defined medium of Vogel (19), modified as described by Mojzita et al. (14), was used to assess l-galactonate production in flasks and bioreactors. d-Xylose (2 to 11 g liter−1) was provided as a carbon source, and ammonium sulfate (1.65 or 3.3 g liter−1) was provided as a nitrogen source. d-Galacturonate (∼10 g liter−1; prepared as sodium salt), or polygalacturonate (15 g liter−1; prepared as a sodium salt and containing 11 g of d-galacturonic acid liter−1 plus 1 g of combined d-xylose, d-galactose, and d-mannose liter−1 when hydrolyzed) were used as substrates in production media. Alternatively, the A. nidulans defined minimal medium of Barratt et al. (1) was used for A. niger cultures with 20 g of d-galacturonate liter−1 and 5 g of d-xylose liter−1. The pH of production medium was adjusted between 3.0 and 6.0 with NaOH.
Medium (modified from Vogel [19]) for precultures contained 20 g of d-xylose liter−1 and was supplemented with 1 g of Bacto peptone liter−1 to provide more rapid growth in this chemically defined medium. A. niger precultures also contained 4 g of agar liter−1 or 30 g of gelatin liter−1, so that growth would be more filamentous. Agar was used in precultures for bioreactor cultures, since it was not metabolized by A. niger, and thus the biomass received the same nutrients as the T. reesei precultures. For studies of gene expression, precultures of A. niger were grown in medium containing 10 g of yeast extract liter−1, 20 g of peptone liter−1, and 30 g of gelatin liter−1.
Cultural conditions.
Small-scale cultures were grown in 250-ml Erlenmeyer flasks containing 50 ml of medium and incubated at 30°C and 200 rpm. Preculture flasks were inoculated with conidial suspensions (final concentrations, 5.3 × 105 conidia ml−1), and production flasks were inoculated with mycelium from the precultures. T. reesei precultures were allowed to grow for approximately 24 h before being harvested by vacuum filtration through disks of sterile, disposable cleaning cloth (X-tra 100% viscose household cleaning cloth; Inex Partners Oy, Helsinki, Finland) and rinsed with sterile H2O (>2 volumes) to remove residual peptone and d-xylose. A. niger was grown for 24 h in preculture medium containing 4 g of agar liter−1 or 30 g of gelatin liter−1 to reduce formation of pellets. Mycelium (5 ml) from agar-containing precultures was transferred to fresh preculture medium lacking agar (50 ml) and incubated for 18 h to reduce the agar content in the cultures and provide an inoculum consisting of very small (<2-mm-diameter) pellets for d-galacturonate conversion, which could be filtered and washed in the same manner as the T. reesei precultures. Alternatively, gelatin-containing precultures were harvested by vacuum filtration and rinsed with sterile H2O warmed to 37°C to remove gelatin and then with cold H2O. Washed mycelium was aseptically transferred to production medium.
For larger-scale cultures, mycelium was grown in bioreactors in 500 ml (Multifors; maximum working volume, 500 ml; Infors HT, Switzerland). Cultures were maintained at 30°C and 800 rpm, with a 1.6 volume of gas volume culture−1 min−1 (vvm). The culture pH was kept constant at pH 4.5, 4.9, or 5.5 by the addition of sterile 1 M KOH or 1 M H3PO4. Polypropylene glycol (mixed molecular weight [21]) was added to control foam production. The initial biomass concentration in T. reesei cultures was 0.3 g liter−1, and in A. niger cultures the concentrations were 0.4 g liter−1 in bioreactors and 0.7 to 1.4 g liter−1 in flasks.
Chemical analyses.
Samples (1 to 60 ml, depending on the culture scale and density of biomass) were removed at intervals, and the mycelium was separated from the supernatant by filtration through cloth. For analysis of intracellular l-galactonate concentrations, biomass that had been washed first with an equal volume of 9 g of NaCl liter−1 and then with distilled water was frozen at −20°C and subjected to freeze-drying. After the sample was weighed, the l-galactonate in the dried biomass was extracted in 5 mM H2SO4 as described previously for the extraction of intracellular 2-keto-3-deoxy-l-galactonate (20). Intracellular amounts are given as mg per g of dry biomass, but the concentration may be estimated by assuming that the volume (in ml) of cytoplasm per g of dry biomass would be similar to that of Penicillium chrysogenum, which has been determined to be 2.86 ml per g of dry biomass (15).
The concentrations of d-xylose, d-galacturonate, and l-galactonate were determined by HPLC using a fast acid analysis column (100 by 7.8 mm; Bio-Rad Laboratories, Hercules, CA) linked to an Aminex HPX-87H organic acid analysis column (300 by 7.8 mm; Bio-Rad Laboratories) with 2.5 or 5.0 mM H2SO4 as the eluant and a flow rate of 0.5 ml min−1. The column was maintained at 55°C. Peaks were detected using a Waters 410 differential refractometer and a Waters 2487 dual-wavelength UV (210-nm) detector.
Expression analysis.
Samples (1 ml) were collected from flask cultures, and the mycelium was harvested by vacuum filtration. The filtered mycelium was immediately frozen with liquid nitrogen and stored at −80°C. RNA was extracted using the RNeasy plant minikit (Qiagen), and 1 μg of total RNA was used for cDNA synthesis with a Transcriptor high-fidelity cDNA synthesis kit (Roche) according to the manufacturer's instructions. cDNA samples were diluted 1:10 with RNase-free water (Roche), and 5 μl of diluted cDNA was used for quantitative PCR using a LightCycler II with the LightCycler SYBR green I Master mix (both Roche). The expression of gaaA, An03g01620, An07g00780, An14g04280, and actin were quantified using the corresponding primers listed in Table 1. The level of expression of gaaA and the genes encoding the putative transporters was normalized to actin by using the accompanying software (Advance Relative Quantification tool).
RESULTS
Conversion of d-galacturonate to l-galactonate by T. reesei and A. niger (at pH 5.5).
Deletion of T. reesei lgd1 (9) and A. niger gaaB resulted in drastically reduced growth of the corresponding strains on d-galacturonic acid when this was provided as the sole carbon source (data not shown). Preliminary experiments demonstrated that both T. reesei Δlgd1 (1.8 g liter−1) and A. niger ΔgaaB (5.9 ± 0.1 g liter−1) produced l-galactonate when incubated for 120 h in flasks initially containing 10 g of d-galacturonate liter−1 and 2 g of d-xylose liter−1 as cosubstrates (initial pH of 5.1). Less l-galactonate (2.0 ± 0.1 g liter−1) was produced by A. niger ΔgaaB when no d-xylose was provided, and thus d-xylose was included as a cosubstrate in all further experiments.
When T. reesei Δlgd1 was cultivated in a bioreactor, l-galactonate production and d-galacturonate utilization increased with the provision of increasing concentrations of d-xylose as cosubstrate (Fig. 2). Up to 7.2 g of l-galactonate liter−1 was produced in the culture provided 11 g of d-xylose liter−1. The initial production rate was 0.07 to 0.12 g of l-galactonate liter−1 h−1, and the final yields were 0.60 to 0.85 g of l-galactonate per g of d-galacturonate consumed (Fig. 2). Although initial yields of 0.9 to 1.0 g of l-galactonate per g of d-galacturonate were observed, the yield decreased during the production phase. The biomass concentration also increased with increasing provision of d-xylose (yield of 0.5 g biomass g d-xylose−1), and the specific l-galactonate production rate was lower when 11 g of d-xylose liter−1 was provided than with 3 g liter−1 (Fig. 2F).
Fig 2.
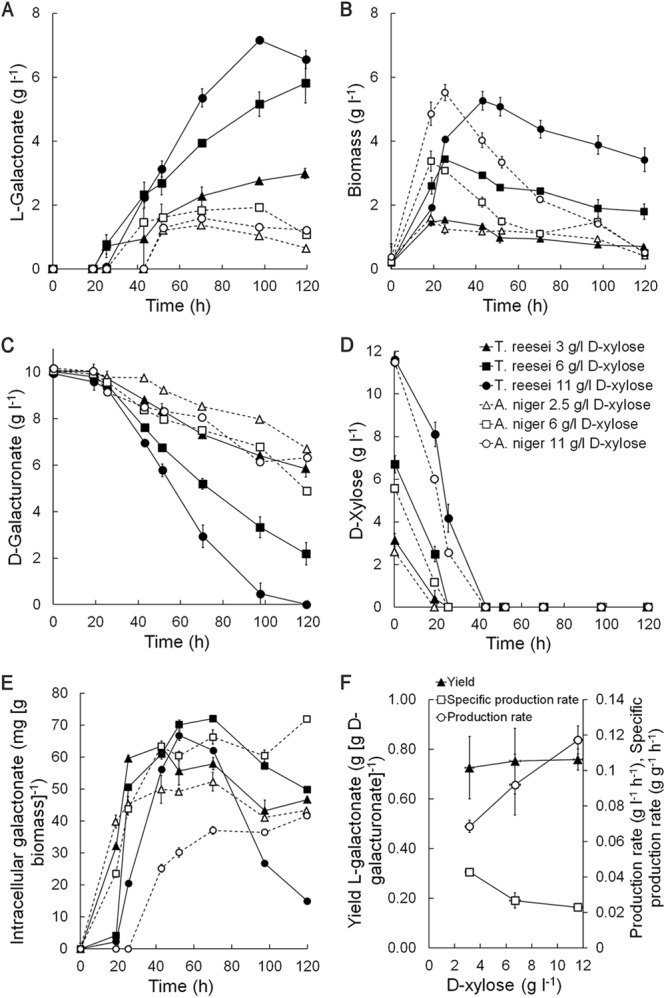
(A to E) Concentrations of extracellular l-galactonate (A), biomass (B), d-galacturonate (C), and d-xylose (D) and intracellular l-galactonate from T. reesei Δlgd1 (solid symbols) and A. niger ΔgaaB (open symbols) (E) in modified Vogel medium initially containing 10 g of d-galacturonate liter−1 and 2.5, 3, 6, or 11 g of d-xylose liter−1, as indicated, at pH 5.5, 800 rpm, 1.6-vvm aeration, and 30°C. (F) Effect of d-xylose concentration on yield of l-galactonate on d-galacturonate consumed, and volumetric production and specific production rates of l-galactonate for T. reesei Δlgd1. Error bars represent ± the standard error of the mean (SEM; n = 2).
Extracellular l-galactonate was not observed in T. reesei Δlgd1 until d-xylose had been consumed, but l-galactonate was present intracellularly prior to this (Fig. 2). During the production phase there was 40 to 70 mg of intracellular l-galactonate g biomass−1. Intracellular d-galacturonate remained <2 mg g biomass−1 (data not shown).
A. niger ΔgaaB produced only 1.4 to 1.9 g of l-galactonate liter−1 when cultivated in bioreactors at pH 5.5 (Fig. 2), although 5.9 g liter−1 had been produced in the preliminary flask experiment. The biomass concentrations were similar to those of T. reesei Δlgd1 (yield of 0.56 g biomass g d-xylose−1), as were the intracellular concentrations of l-galactonate (Fig. 2). d-Galacturonate (10 to 30 mg g biomass−1) was also detectable in mycelia from the cultures which received 6 or 11 g of d-xylose liter−1. An initial assessment indicated that gaaA expression in this strain was low (data not shown).
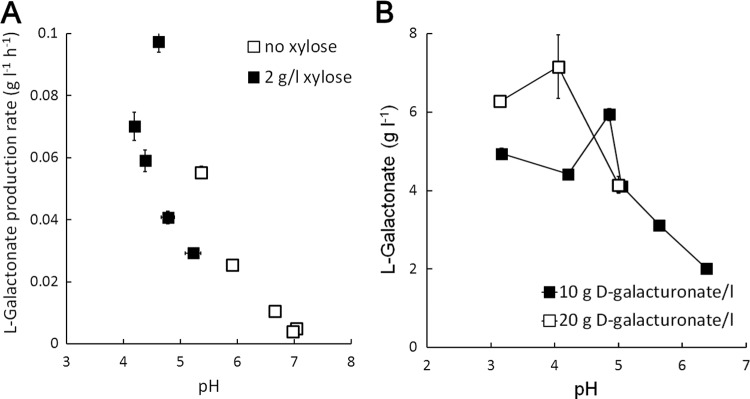
Production of l-galactonate by A. niger is sensitive to culture pH.
The modified Vogel medium used here is not well buffered, and thus the pH in flask cultures decreased as ammonium was consumed and increased when d-galacturonate was taken up from the medium without release of l-galactonate from the hyphae. The data from the preliminary A. niger flask cultures indicated that the highest l-galactonate production rates were observed when pH was low (Fig. 3) and suggested that pH 5.5 may be too high for l-galactonate production by A. niger. Indeed, l-galactonate production decreased with increasing pH above 5.0 in flask cultures but was generally high (5 to 6 g liter−1) at pH values below 5 (Fig. 3). l-Galactonate production was further improved at pH 3 to 4 by cultivating the strain in buffered medium with 20 g of d-galacturonate liter−1 and 5 g of d-xylose liter−1 (Fig. 3).
Fig 3.
l-Galactonate production by A. niger ΔgaaB in flasks. (A) l-Galactonate production rate as a function of pH for unbuffered cultures provided 10 g of d-galacturonate liter−1 at an initial pH of 5.2 with no d-xylose (open symbols) or 2 g of d-xylose liter−1 (solid symbols) provided for growth. Error bars represent ± the SEM (n = 3). (B) Concentration of l-galactonate produced in 120 to 144 h in unbuffered modified Vogel medium containing 10 g of d-galacturonate liter−1 and 2 g of d-xylose liter−1 (solid symbols) and in buffered A. nidulans medium containing 20 g of d-galacturonate liter−1 and 5 g of d-xylose liter−1 (open symbols). The pH of the medium was initially adjusted to 3, 4, 5, or 6, but the average culture pH is shown. Error bars represent ± the SEM for three to six replicate cultures and, where not visible, are smaller than the symbol.
When A. niger ΔgaaB was grown in a pH-controlled bioreactor at pH 4.8 with 10 g of d-galacturonate liter−1 and 6 to 7 g of d-xylose liter−1, 2.7 g of l-galactonate liter−1 was produced within 72 h at a rate of 0.04 g liter−1 h−1 (yield of 0.7 g of l-galactonate per g of d-galacturonate consumed; Fig. 4). An additional pulse of 8 g of d-xylose liter−1 was added after 127 h to compensate for the decreasing biomass, and a further 2.5 g of l-galactonate was produced at the same rate to give a final concentration of 5.4 g liter−1 (yield of 0.9 g per g of d-galacturonate consumed; Fig. 4) when the culture was harvested at 171 h. Intracellular l-galactonate accumulation (56 ± 2 mg g biomass−1; Fig. 4) was similar to that observed at pH 5.5 (Fig. 2) but decreased after the addition of d-xylose. d-Galacturonate (<1.6 mg g biomass−1) did not accumulate in the mycelia (data not shown).
Fig 4.
Concentrations of l-galactonate, biomass, and intracellular l-galactonate in A. niger ΔgaaB cultures in modified Vogel medium with 5 g of d-xylose liter−1 and containing 10 g of d-galacturonate liter−1 (open symbols, pH 4.8) or 15 g of polygalacturonate liter−1 (solid symbols pH 4.5). The cultures were maintained at 30°C, 800 rpm, and 1.6-vvm aeration and were given an additional 9 g of d-xylose liter−1 at 127.8 h. Error bars represent ± the SEM (n = 2) and, where not visible, are smaller than the symbol.
Bioconversion of polygalacturonate to l-galactonate.
A. niger ΔgaaB converted polygalacturonate to l-galactonate at a similar rate (initial rate of 0.04 g liter−1 h−1, increasing to 0.07 g liter−1 h−1 after the addition of extra d-xylose) and titer (2.5 g of l-galactonate liter−1 within 72 h) as it converted the monomer d-galacturonate (Fig. 4). l-Galactonate (1.2 g liter−1) was present in the culture supernatant after 26 h but did not accumulate above 2.8 g liter−1 at any time during the cultivation. The addition of d-xylose after 127 h resulted in a total of 6.5 g of l-galactonate liter−1 (yield of 0.85 g of l-galactonate per g of d-galacturonate consumed−1) after 171 h, increasing to 7.6 g liter−1 after 195 h. The intracellular concentration of l-galactonate (52 ± 4 mg g biomass−1) was similar to that observed in other l-galactonate-producing cultures and also decreased after the addition of d-xylose (Fig. 4). Low concentrations of d-galacturonate (0.2 to 4.3 mg g biomass−1) were also extracted from mycelia incubated in polygalacturonate (data not shown).
Overexpression of A. niger gaaA.
Since gaaA expression appeared to be low in the ΔgaaB strain, the galacturonate reductase coding gene, gaaA, was overexpressed in A. niger ΔgaaB. A. niger ATCC 1015, the ΔgaaB strain, and the overexpression strain (ΔgaaB-gaaA) were grown in modified Vogel medium with 10 g of d-galacturonate liter−1 and 2 g of d-xylose liter−1 at an initial pH of 3 in flasks. The expression of gaaA in A. niger ΔgaaB was considerably lower compared to the wild type after 6 h (Table 2). In contrast, in A. niger ΔgaaB-gaaA expression of gaaA was much higher at 0 and 6 h, as expected (Table 2). After 24 h, gaaA expression levels in A. niger ΔgaaB and A. niger ΔgaaB-gaaA were similar, whereas its expression in the wild type had decreased (Table 2), probably due to d-galacturonate depletion.
Table 2.
Relative expression of gaaA in A. niger ATCC 1015, ΔgaaB, and ΔgaaB-gaaA strainsa
| Time (h) | Relative expression of gaaA (avg ± SEM [n = 3]) |
||
|---|---|---|---|
| ATCC 1015 | ΔgaaB strain | ΔgaaB-gaaA strain | |
| 0 | 0.2 ± 0.0 | 0.4 ± 0.0 | 14.0 ± 0.0 |
| 3 | 3.6 ± 0.6 | 0.1 ± 0.0 | 16.6 ± 0.6 |
| 6 | 2.6 ± 0.1 | 0.1 ± 0.0 | 9.5 ± 0.6 |
| 24 | 0.2 ± 0.1 | 1.5 ± 0.1 | 1.5 ± 0.2 |
The relative expressions of gaaA in A. niger ATCC 1015, ΔgaaB, and ΔgaaB-gaaA strains grown in flasks in modified Vogel medium with 10 g of d-galacturonate liter−1 and 2 g of d-xylose liter−1 at an initial pH 3.0 are presented.
Approximate l-galactonate production rates were determined for the flask cultures. During the first 24 h after inoculation, A. niger ΔgaaB-gaaA produced l-galactonate at a significantly (P < 0.05) higher rate (0.070 g of l-galactonate liter−1 h−1) than A. niger ΔgaaB (0.048 g of l-galactonate liter−1 h−1; Table 3). After 24 h, the difference in the production rates of the ΔgaaB and ΔgaaB-gaaA strains decreased, and after 48 h, when l-galactonate production by both strains was decreasing, their production rates were similar (P > 0.05; 0.046 and 0.054 g of l-galactonate liter−1 h−1, respectively; Table 3).
Table 3.
l-Galactonate production rates for A. niger ΔgaaB and ΔgaaB-gaaA strainsa
| Time interval (h) | Avg l-galactonate production rate (g liter−1 h−1) ± SEM (n = 3)b |
|
|---|---|---|
| ΔgaaB strain | ΔgaaB-gaaA strain | |
| 0–24 | 0.048 ± 0.001A | 0.070 ± 0.002B |
| 24–48 | 0.064 ± 0.001A | 0.075 ± 0.002B |
| 48–78 | 0.046 ± 0.000A | 0.054 ± 0.002A |
The l-galactonate production rates were determined for A. niger ΔgaaB and ΔgaaB-gaaA strains grown in flasks in modified Vogel medium with 10 g of d-galacturonate liter−1 and 2 g of d-xylose liter−1 at an initial pH of 3.0.
Values in the same row with different superscript letters differed significantly (P < 0.05).
The final l-galactonate titers of the ΔgaaB and ΔgaaB-gaaA strains were compared in both modified Vogel medium and A. nidulans minimal medium in flasks (Table 4). Both the l-galactonate titer and the yield were generally higher for A. niger ΔgaaB-gaaA than for A. niger ΔgaaB when grown at pH 3 or 4 in either medium (Table 4). At pH 5 in A. nidulans minimal medium, the final l-galactonate titer was notably lower than at pH 4 for both strains, and there was no difference between the strains. However, the yield of l-galactonate on d-galacturonate for A. niger ΔgaaB-gaaA was higher than for A. niger ΔgaaB also at pH 5 (Table 4).
Table 4.
l-Galactonate production at 144 h by A. niger ΔgaaB and the ΔgaaB strain overexpressing gaaA (ΔgaaB-gaaA)a
| Medium | Initial pH | Strain | Mean amt of l-GalA (g liter−1) ± SEM | Conversion (g g−1) of l-GalA/d-GalUAinitial | Yield (g g−1) of l-GalA/d-GalUAconsumed |
|---|---|---|---|---|---|
| A. nidulans MM | 5 | ΔgaaB | 4.1 ± 0.2 | 0.20 | 0.82 |
| 5 | ΔgaaB-gaaA | 4.1 ± 0.3 | 0.20 | 0.97 | |
| 4 | ΔgaaB | 7.2 ± 0.8 | 0.35 | 0.95 | |
| 4 | ΔgaaB-gaaA | 7.8 ± 0.4 | 0.38 | 0.97 | |
| 3 | ΔgaaB | 6.3 ± 0.1 | 0.31 | 0.86 | |
| 3 | ΔgaaB-gaaA | 8.7 ± 0.2 | 0.43 | 1.00 | |
| Modified Vogel medium | 4 | ΔgaaB | 4.2 ± 0.1 | 0.41 | 0.70 |
| 4 | ΔgaaB-gaaA | 5.0 ± 0.1 | 0.49 | 0.75 | |
| 3 | ΔgaaB | 4.9 ± 0.1 | 0.47 | 0.70 | |
| 3 | ΔgaaB-gaaA | 6.2 ± 0.3 | 0.59 | 0.82 |
l-Galactonate (l-GalA) production at 144 h by A. niger ΔgaaB and the ΔgaaB strain overexpressing gaaA (ΔgaaB-gaaA) was determined in buffered A. nidulans minimal medium (MM) with 20 g of d-galacturonate liter−1 and 5 g of d-xylose liter−1 and in modified Vogel medium with 10 g of d-galacturonate liter−1 and 2 g of d-xylose liter−1 in flasks at an initial pH of 3, 4, or 5. Mean values are shown (n = 3). The conversion and yield on d-galacturonate (d-GalUA) are also shown.
Transcription of putative transporter genes in A. niger ΔgaaB.
The relative transcript levels of three genes which have been identified as possible transporters of d-galacturonate (An07g00780, An14g04280, and An03g01620, [12]) were assessed in A. niger ATCC 1015 and A. niger ΔgaaB 3, 6, and 24 h after transfer to d-galacturonic acid-containing medium at pH 3 (Table 5). Both An14g04280 and An03g01620 were strongly induced in ATCC 1015 within 3 h of the transfer, whereas the induction of An07g00780 was only seen 24 h after the transfer. In contrast, no induction of An14g04280 was observed in A. niger ΔgaaB. Transcription of An03g1620 and An07g00780 in A. niger ΔgaaB was similar to that observed in the control strain.
Table 5.
Relative expression of putative transporters An07g00780, An14g04280, and An03g01620 in A. niger ATCC 1015 and ΔgaaB strainsa
| Putative transporter | Time (h) | Avg relative expression ± SEM (n = 3) |
|
|---|---|---|---|
| ATCC 1015 | ΔgaaB strain | ||
| An07g00780 | 0 | 0.4 ± 0.0 | 0.1 ± 0.0 |
| 3 | 0.1 ± 0.0 | ND | |
| 6 | 0.3 ± 0.0 | 0.2 ± 0.1 | |
| 24 | 1.1 ± 0.3 | 1.9 ± 1.0 | |
| An14g04280 | 0 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| 3 | 2.1 ± 0.2 | 0.1 ± 0.0 | |
| 6 | 0.9 ± 0.0 | 0.1 ± 0.0 | |
| 24 | 0.1 ± 0.0 | 0.1 ± 0.0 | |
| An03g01620 | 0 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| 3 | 2.0 ± 0.5 | 3.4 ± 0.4 | |
| 6 | 0.3 ± 0.0 | 0.2 ± 0.1 | |
| 24 | 0.0 ± 0.0 | 0.0 ± 0.0 | |
The relative expression of putative transporters An07g00780, An14g04280, and An03g01620 in A. niger ATCC 1015 and the ΔgaaB strain grown in flasks in modified Vogel medium with 10 g of d-galacturonate liter−1 and 2 g of d-xylose liter−1 at an initial pH of 3. ND, no data.
DISCUSSION
Deletion of the gene for the l-galactonate dehydratase, lgd1 in T. reesei or gaaB in A. niger, resulted in strains that converted d-galacturonate to l-galactonate, which was secreted to the culture supernatant (Fig. 2 to 4). This confirmed that d-galacturonate was still taken up in the deletion strains, as was also the case when either the d-galacturonate reductase genes (gar1 or gaaA in T. reesei and A. niger, respectively [14]) or the 2-keto-3-deoxy-l-galactonate aldolase genes, lga1 or gaaC (20), were deleted. In T. reesei, the conversion of d-galacturonate to l-galactonate occurred at similar rates (0.07 to 0.12 g of l-galactonate liter−1 h−1) as previously reported for the conversion to keto-deoxy-l-galactonate (0.10 to 0.14 g liter−1 h−1 [20]) but was faster than the conversion to galactarate (0.024 to 0.046 g liter−1 h−1 [14]). In A. niger, on the other hand, the conversion of d-galacturonate to l-galactonate (0.04 to 0.07 g of l-galactonate liter−1 h−1) was much slower than the conversion to keto-deoxy-l-galactonate (0.27 to 0.33 g liter−1 h−1 [20]), suggesting that the disruption of the pathway at the earlier step created additional constraints in this strain.
The yield of l-galactonate from d-galacturonate was 0.6 to 0.8 g g−1 for T. reesei Δlgd1 and 0.7 to 0.9 g g−1 for A. niger ΔgaaB. Thus, the yields were only slightly lower than the theoretical yield (1.0 g of l-galactonate g of d-galacturonate−1) but still indicated that some of either the d-galacturonate or the produced l-galactonate was consumed in unidentified metabolic reaction(s). Futile consumption of d-galacturonate has been observed previously in strains deleted of gaaA/gar1 or gaaC/lga1 (14, 20), but the fate of the carbon remains unclear since there is no measurable production of biomass from d-galacturonate in these strains.
Although the production of both l-galactonate and keto-deoxy-l-galactonate require NADPH as a cofactor for the d-galacturonate reductase, l-galactonate production was more dependent on the addition of d-xylose as a cosubstrate (Fig. 2) to obtain good production than was the production of the keto-deoxy derivative. This may reflect a greater need for energy in the export of l-galactonate, since we observed that the intracellular concentration of l-galactonate (40 to 70 mg of l-galactonate g biomass−1 in both T. reesei and A. niger) was higher than the maximum intracellular concentrations of keto-deoxy-l-galactonate (35 to 45 mg of l-galactonate g biomass−1) in the corresponding strains (20). After provision of additional cosubstrate to A. niger ΔgaaB cultures at pH 4.5 to 4.8 the intracellular l-galactonate concentration decreased to around 23 mg g biomass−1 (Fig. 4), supporting the hypothesis that energy is needed for export.
Assuming the volume of cytoplasm to be ∼2.86 times the dry biomass (10), the average intracellular concentration of l-galactonate was ∼20 g liter−1 and was much higher than the l-galactonate concentration in the medium. This also suggests that export may be a bottleneck in extracellular production. In addition, the high intracellular concentration of l-galactonate may limit the rate of d-galacturonate conversion by feedback inhibition and/or providing substrate for the reverse reaction, which has been shown to occur with both the T. reesei gar1 (10) and the A. niger gaaA (13) d-galacturonate reductases. The Km for l-galactonate of T. reesei gar1 is 4 mM (0.8 g liter−1) (10), which is much lower than the intracellular l-galactonate concentrations observed. Thus, the accumulation of l-galactonate may limit the reaction more than accumulation of keto-deoxy-l-galactonate, since the action of the l-galactonate dehydratase is irreversible (9). Generation of intracellular d-galacturonate may also have affected the uptake of the substrate, about which little is known in filamentous fungi. Intracellular d-galacturonate was, however, only observed in A. niger and not in T. reesei.
In contrast to keto-deoxy-l-galactonate production (20), l-galactonate production was more efficient in T. reesei than in A. niger at pH 5.5, producing higher titers at higher rates (Fig. 2). T. reesei was also found to be more effective than A. niger in the production of galactarate (14), and these results confirm that T. reesei is an interesting and useful host for organic acid production, even though it is not known as a high producer of organic acids, nor is it tolerant to a very low culture pH.
Low galactarate production by A. niger ΔgaaA-udh was attributed to subsequent metabolism of the galactarate (14). The metabolism of l-galactonate appeared negligible (Fig. 4) or limited (Fig. 2) in A. niger ΔgaaB; instead, l-galactonate production by A. niger was found to be pH dependent, with the highest production rates and titers observed at pH values below 5.0 and no reduction in production even at pH 3.0 (Fig. 3). At pH 4.5 to 4.8, the production of l-galactonate by A. niger ΔgaaB was as good as that of T. reesei Δlgd1 at pH 5.5. At a low extracellular pH, more of the product is protonated to l-galactonic acid (pKa ∼3.5), creating a greater difference in concentration between the dissociated intra- and extracellular l-galactonate pools. If the protonated organic acid is not reimported into the cytoplasm, then a low extracellular pH can provide the dominant driving force for organic acid export, as has been predicted for citrate export from A. niger (2). Further, low extracellular pH may influence the transport of d-galacturonic acid (pKa 3.51). However, A. niger transported d-galacturonate at much higher rates when producing keto-deoxy-l-galactonate at pH 5.5 (0.12 to 0.56 g liter−1 h−1 [20]) or galactarate at pH 5.0 (0.21 to 0.46 g liter−1 h−1 [14]) than were observed during l-galactonate production at any pH (0.04 to 0.15 g liter−1 h−1; Fig. 2 and data not shown). Thus, improved uptake at low pH is unlikely to explain the improved l-galactonate production observed.
d-Galacturonate is an inducer of the d-galacturonate pathway genes gaaA, gaaB, and gaaC in the A. niger ATCC 1015, CBS120.49, and ΔgaaA strains (12, 14). In ATCC 1015, the transcription of these three genes was induced simultaneously within 4 h of transfer to d-galacturonate, and the induction of gaaB and gaaC remained similar in A. niger ΔgaaA compared to ATCC 1015 (14). In the present study, we observed that gaaA was not induced in A. niger ΔgaaB even 6 h after exposure to d-galacturonate (Table 2), although transcription had increased after 24 h. In ATCC 1015, gaaA expression was already reduced after 24 h of incubation due to d-galacturonate depletion. Induction of the gene encoding the third enzyme of the pathway, gaaC, was similarly delayed in A. niger ΔgaaB (J. Kuivanen, unpublished data), suggesting that the induction of the entire pathway was affected by the deletion of gaaB. The similar transcriptional responses of gaaA and gaaC might be expected since these genes share a bidirectional promoter (13). The altered transcription profiles of the genes in the ΔgaaB strain suggest that l-galactonate, keto-deoxy-l-galactonate, or l-galactonate dehydratase itself may have roles in transcriptional regulation of the d-galacturonate pathway genes. Regardless of the regulatory mechanism, the delayed induction of gaaA in the ΔgaaB strain would account for low initial rates of d-galacturonate conversion.
In order to eliminate gaaA induction as a rate-limiting factor for l-galactonate production, gaaA was overexpressed under the gpdA promoter in A. niger ΔgaaB. The l-galactonate production rate was initially significantly (P < 0.002) higher in A. niger ΔgaaB-gaaA compared to A. niger ΔgaaB in flasks at pH 3 (Table 3), indicating that low gaaA expression was indeed a rate-limiting factor. However, gaaA was expressed under the gpdA promoter, which gives less induction in the absence of a metabolizable carbon source (in this case, d-xylose), even though it is generally described as constitutive. Thus, the expression of gaaA decreased during the expression studies. After 24 h, when gaaA expression had been induced in the gaaB deletion strain, the production rates of the A. niger ΔgaaB-gaaA and A. niger ΔgaaB strains were similar (Table 3). The initial improved production resulted in 24 to 39% more l-galactonate being produced at pH 3 when gaaA was overexpressed than when it was not, with corresponding improvements in the conversion efficiency and yield (Table 4). Interestingly, the benefit of overexpression of gaaA was pH dependent even though the gaaA expression was not (Kuivanen, unpublished), with the greatest benefit at pH 3, although smaller improvements in yield were also observed at higher pH values (Table 4).
d-Xylose was previously found to be a good cosubstrate in the production of keto-deoxy-l-galactonate (20), but d-galacturonate did not appear to be taken up while d-xylose was being consumed (Fig. 2). Limited d-galacturonate uptake while gaaA expression was high in A. niger ΔgaaB-gaaA probably limited the improvement in l-galactonate production that could be achieved by this strain. In addition, only two of the three putative d-galacturonate transporters (12) were induced in the ΔgaaB strain (Table 5). The roles of these putative transporters is not known, but the limited d-galacturonate transport in A. niger ΔgaaB and A. niger ΔgaaB-gaaA may indicate that the protein encoded by An14g04280 has a dominant role.
Despite the fact that production of l-galactonate with A. niger ΔgaaB required more investigation and additional strain development than with T. reesei Δlgd1, A. niger is more suitable for development of a consolidated l-galactonate production process, which would use less processed polymeric substrates, such as polygalacturonate, pectin, or even raw, untreated biomass. A. niger produces a more complex spectrum of pectinases than does T. reesei, which is unable to degrade pectin (20). Using the current A. niger ΔgaaB strain, the production of l-galactonate from polygalacturonate was found to be as efficient as production from the d-galacturonate monomer (Fig. 4). Thus, a high concentration of extracellular d-galacturonate was not necessary to sustain its uptake, and the slow release of monomer may be beneficial in providing continual induction of the native gaaA gene. Polygalacturonate was used as a substrate here, but these results suggest that l-galactonate could also be produced directly from pectin, which would require less processing and would also provide the cosubstrates (e.g., d-galactose, d-xylose, and l-arabinose) for the initial production of biomass and NADPH. A more gradual provision of cosubstrate in a fed-batch or continuous process may also be useful, since this would ensure that production rates did not decrease as a result of cell lysis after the cosubstrate was consumed and for the ΔgaaB-gaaA strain would sustain higher expression levels of gaaA.
d-Galactonate has been produced in high concentration from d-galactose using Gluconobacter oxydans (18), but this is the first report of extracellular production of l-galactonate in gram quantities from d-galacturonic and polygalacturonic acids. Its production has led to further insights into d-galacturonate metabolism in A. niger, while further enhancement in production by both strain engineering and process development may provide an efficient source of l-galactonate for, e.g., microbial ascorbic acid production and other applications.
ACKNOWLEDGMENTS
This study was supported by the Academy of Finland under the following research programs: Finish Centre of Excellence in White Biotechnology-Green Chemistry (grant 118573), Cadfiss (grant 122131), and Sustainable Energy (grant 131869).
We thank Tarja Laakso and Toni Paasikallio for technical assistance.
Footnotes
Published ahead of print 5 October 2012
REFERENCES
- 1. Barratt RW, Johnson GB, Ogata WN. 1965. Wild-type and mutant stocks of Aspergillus nidulans. Genetics 52:233–246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Burgstaller W. 2006. Thermodynamic boundary conditions suggest that a passive transport step suffices for citrate excretion in Aspergillus and Penicillium. Microbiology 152:887–893 [DOI] [PubMed] [Google Scholar]
- 3. Csiba M, Cleophax J, Petit S, Gero SD. 1993. An expedient and practical three-step synthesis of vitamin C from a byproduct of the sugar industry: the l-galactono-1,4-lactone pathway. J. Org. Chem. 58:7281–7282 [Google Scholar]
- 4. de Vries RP, et al. 2002. Expression profiling of pectinolytic genes from Aspergillus niger. FEBS Lett. 530:41–47 [DOI] [PubMed] [Google Scholar]
- 5. Doran JB, Cripe J, Sutton M, Foster B. 2000. Fermentations of pectin-rich biomass with recombinant bacteria to produce fuel ethanol. Appl. Biochem. Biotechnol. 84–86:141–152 [DOI] [PubMed] [Google Scholar]
- 6. Doran-Peterson J, Cook DM, Brandon SK. 2008. Microbial conversion of sugars from plant biomass to lactic acid or ethanol. Plant J. 54:582–592 [DOI] [PubMed] [Google Scholar]
- 7. Grohmann K, Manthey JA, Cameron RG, Busling BS. 1998. Fermentation of galacturonic acid and pectin rich materials to ethanol by genetically modified strains of Erwinia. Biotechnol. Lett. 20:195–200 [Google Scholar]
- 8. Hilditch S, Berghäll S, Kalkkinen N, Penttilä M, Richard P. 2007. The missing link in the fungal d-galacturonate pathway: identification of the l-threo-3-deoxy-hexulosonate aldolase. J. Biol. Chem. 282:26195–26201 [DOI] [PubMed] [Google Scholar]
- 9. Kuorelahti S, Jouhten P, Maaheimo H, Penttilä M, Richard P. 2006. l-Galactonate dehydratase is part of the fungal path for d-galacturonic acid catabolism. Mol. Microbiol. 61:1060–1068 [DOI] [PubMed] [Google Scholar]
- 10. Kuorelahti S, Kalkkinen N, Penttilä M, Londesborough J, Richard P. 2005. Identification in the mold Hypocrea jecorina of the first fungal d-galacturonic acid reductase. Biochemistry 44:11234–11240 [DOI] [PubMed] [Google Scholar]
- 11. Liepins J, Kuorelahti S, Penttilä M, Richard P. 2006. Enzymes for the NADPH-dependent reduction of dihydroxyacetone and d-glyceraldehyde and l-glyceraldehyde in the mould Hypocrea jecorina. FEBS J. 273:4229–4235 [DOI] [PubMed] [Google Scholar]
- 12. Martens-Uzunova E. 2008. Assessment of the pectinolytic network of Aspergillus niger by functional genomics: insight from the transcriptome. Ph.D. thesis University of Wageningen, Wageningen, Netherlands [Google Scholar]
- 13. Martens-Uzunova ES, Schaap PJ. 2008. An evolutionary conserved d-galacturonic acid metabolic pathway operates across filamentous fungi capable of pectin degradation. Fungal Genet. Biol. 45:1449–1457 [DOI] [PubMed] [Google Scholar]
- 14. Mojzita D, et al. 2010. Metabolic engineering of fungal strains for conversion of d-galacturonate to meso-galactarate. Appl. Environ. Microbiol. 76:169–175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nestaas E, Wang DIC. 1981. A new sensor, the “filtration probe”, for quantitative characterization of the penicillin fermentation. I. Mycelial morphology and culture activity. Biotechnol. Bioeng. 23:2803–2813 [DOI] [PubMed] [Google Scholar]
- 16. Richard P, Hilditch S. 2009. d-Galacturonic acid catabolism in microorganisms and its biotechnological relevance. Appl. Microbiol. Biotechnol. 82:597–604 [DOI] [PubMed] [Google Scholar]
- 17. Roland JF, Cayle T, Dinwoodie RC, Mehnert DW. October 1983. Fermentation production of ascorbic acid from l-galactonic substrate. US patent 4,595,659
- 18. Švitel J, Šturdik E. 1994. d-Galactose transformation to d-galactonic acid by Gluconobacter oxydans. J. Biotechnol. 37:85–88 [Google Scholar]
- 19. Vogel HJ. 1956. Convenient growth medium for Neurospora (Medium N). Microb. Genet. Bull. 243:112–119 [Google Scholar]
- 20. Wiebe MG, Mojzita D, Hilditch S, Ruohonen L, Penttilä M. 2010. Bioconversion of d-galacturonate to keto-deoxy-l-galactonate (3-deoxy-l-threo-hex-2-ulosonate) using filamentous fungi. BMC Biotechnol. 10:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wiebe MG, Robson GD, Shuster J, Trinci APJ. 2001. Evolution of a recombinant (glucoamylase-producing) strain of Fusarium venenatum A3/5 in chemostat cultures. Biotechnol. Bioeng. 73:146–156 [DOI] [PubMed] [Google Scholar]